Abstract
Background
Transforming growth factor-α (TGF-α), a stimulatory growth factor and member of the epidermal growth factor family, is a mediator of oncogenesis and malignant progression in colorectal carcinogenesis. Limited evidence suggests its utility as a growth-related biomarker of risk for colorectal cancer.
Methods
We measured expression of TGF-α in biopsies of normal-appearing colorectal mucosa using automated immunohistochemistry and quantitative image analysis in a subsample of 29 cases and 31 controls from a colonoscopy-based case-control study (n = 203) of biomarkers of risk for incident sporadic colorectal adenoma. Diet, lifestyle, and medical history were assessed with validated questionnaires.
Results
TGF-α expression in the rectum was 51% higher in cases compared with controls (P = 0.05) and statistically significantly associated with accepted risk factors for colorectal neoplasms (36% lower among nonsteroidal anti-inflammatory drug users, 49% lower among women using hormone replacement therapy, 79% higher among persons with a family history of colorectal cancer).
Conclusions
TGF-α expression in the normal-appearing rectal mucosa shows promise as an early, potentially modifiable biomarker of risk for colorectal cancer.
Introduction
Cancers of the colon and rectum take many years to develop and begin when a few epithelial cells lining the colon and rectum begin to exhibit abnormal properties (1). Nearly 90% of colorectal cancers arise from polyps, which reoccur over a period of years in nearly half the people who have them removed, suggesting that normal-appearing tissue may retain components of risk (1–4). The long, latent, precancerous state characteristic of colorectal cancer and the prevalence of adenomatous polyps make it well suited to mass screening and present opportunities to derail the disease before it starts or to treat it in its earliest detectable stages (4). Better understanding the complexities of colorectal carcinogenesis coupled with advancements in the measurement of early risk are needed to progress research and practice in the prevention of colorectal cancer (3, 5).
The earliest phases of colorectal carcinogenesis likely begin in normal mucosa with a disorder of cell replication and renewal, followed by the subsequent appearance of clusters of enlarged crypts showing proliferative, biochemical, and biomolecular abnormalities (2, 6, 7). Transforming growth factor-α (TGF-α), a stimulatory growth factor and member of the epidermal growth factor family, is an important mediator of oncogenesis and malignant progression (8–11). In the gut mucosa environment, TGF-α plays a role in multiple pathways, including stimulating cell proliferation, assurance of cell survival, maintenance of cellular integrity, and response to injury or inflammation (12–14).
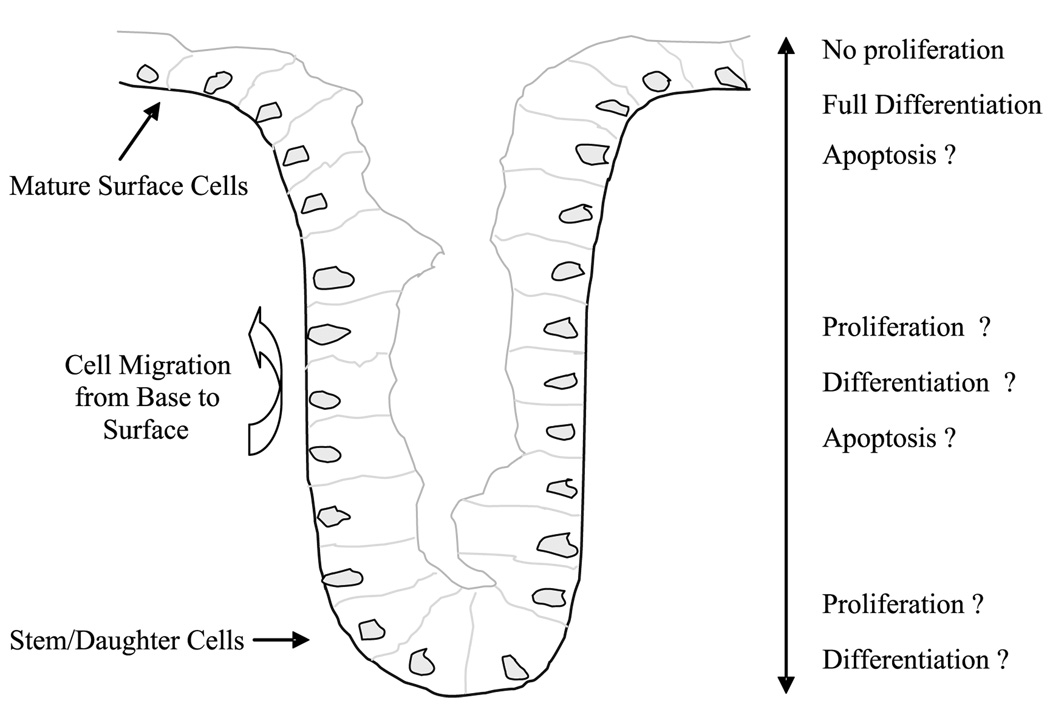
Within colon crypts, TGF-α expression is correlated with the distribution of proliferating cells and mediated by its interaction with the epidermal growth factor receptor. Under normal conditions, stem and progenitor cells capable of cell proliferation remain located toward the base of the crypt (zone of proliferation), whereas daughter cells of newly divided cells migrate up the crypt wall cease cell proliferation and differentiate into functionally mature cells as they move toward the crypt mouth (Fig. 1; refs. 15, 16). The zone of cell proliferation may change in response to various exogenous factors (17).
Figure 1.
Colon crypt model.
Dietary and lifestyle modifications have been shown to cause adaptive changes in crypt cell proliferation within the rapidly renewing colon and rectal mucosal crypt epithelium. These exogenous factors work by modulating multiple endogenous factors involved in adaptive crypt changes (8, 18–22). To date, limited in vivo evidence is available to suggest that TGF-α may be a potential marker of colorectal cancer risk (13, 23) or that it may be modifiable through aspects of diet and lifestyle (24, 25). Mechanisms whereby changes in TGF-α expression within the colon may modulate risk remain unclear. To begin to address these issues, we characterized the expression of TGF-α protein within the normal-appearing colorectal mucosa and assessed its association with adenoma and other risk factors for colorectal cancer in a pilot case-control study.
Materials and Methods
The Markers of Adenomatous Polyps II (MAP II; 2002) study was a community- and colonoscopy-based case-control study of incident sporadic colorectal adenomas designed to investigate whether the expression patterns of various genes and cell cycle markers in normal-seeming rectal mucosa are associated with adenomas and thus can be possible biomarkers of risk for colorectal neoplasms. Participants in the study were recruited upon referral for routine outpatient elective colonoscopy at Consultants in Gastroenterology, PA, a large private practice gastroenterology group in Columbia, SC. English-speaking adults, with ages 30 to 74 y and capable of informed consent, were eligible to participate. Subjects were excluded if they had previous adenomatous polyps, familial adenomatous polyposis, inflammatory bowel disease, incident colorectal cancer, or prevalent cancer other than nonmelanoma skin cancer. Of the 351 patients identified over a 5-mo period, 305 (86.6%) were eligible to participate upon initial recruitment screening. Of these, 232 (76%) were successfully contacted and provided informed consent before colonoscopy. Of the 203 (87.5%) who met final eligibility criteria, 87 (42.8%) had polyps, of whom 49 (56.3%) had adenomas (overall adenoma prevalence, 24.1%). Thus, the final sample size of 203 was composed of 49 cases (24.1%) and 154 controls (75.9%). Five participants were later excluded because of missing questionnaire data or implausibly low (<600 kcal/d) or high (>5,000 kcal/d) self-reported total energy intake. Hyperplastic polyps were not considered in the criteria for study inclusion or exclusion or in the assignment of case-control status. The final sample for the main study included 198 participants of whom 49 were incident sporadic adenoma cases. Because of limited tissue availability for the multiple biomarkers under investigation, from the entire study population, a subsample consisting of the first 31 cases and 29 controls with adequate slide samples and staining was selected for TGF-α analysis.
Data Collection
Before undergoing colonoscopy, participants completed mailed questionnaires eliciting self-reported demographics, medical history, anthropometrics (26), diet, and lifestyle characteristics. Information collected included family history of polyps or colon cancer, reproductive and hormonal history, alcohol and tobacco use, use of medications such as aspirin and other nonsteroidal anti-inflammatory drugs (NSAID), medical conditions, and reasons for and the sequence of events leading to colonoscopy. Physical activity was assessed using a modified Paffenbarger (27) questionnaire. Diet and nutritional supplement information was obtained using a modified Food Frequency Questionnaire (28, 29).
Colonoscopy, Pathology, and Tissue Collection
Colonoscopy of all participants was done in the usual manner following a 12-h fast and polyethylene glycol bowel cleansing preparation. All participants had six “pinch” biopsies taken from the normal-appearing rectal mucosa (10 cm above the anus). On 20% of the participants, biopsies from the midsigmoid and proximal ascending colon were also collected. No biopsies were taken within 4 cm of a polyp or tumor. For any polyp removed at colonoscopy, colon site, in vivo size, and shape were recorded and histologic information was further reviewed by the study index pathologist according to the protocol developed by the National Polyp Study (30).
Laboratory Methods
All biopsy specimens were fixed with 10% normal buffered formalin for 24 h then stored in 70% ethanol. Within a week, the specimens were processed and embedded in paraffin blocks with three biopsies per colon site per block. The paraffin blocks were then cut into 3-µm-thick sections, with each level 40 µm apart. Five slides with four biopsy levels each were processed and stained within 7 d of being cut, yielding a total of 20 biopsy levels per patient. The slides underwent immunohistochemical processing using a DAKO Automated Immunostainer (DAKO Corp.), Leica H&E Autostainer, and Leica CV500 Coverslipper (Leica Microsystems, Inc.). First, TGF-α antigen was unmasked via a heat-induced epitope retrieval procedure by placing the slides in a preheated Pretreatment Module (Lab Vision Corp.) with 100× citrate buffer (pH 6.0, DAKO S1699) and steamed for 40 min. Then, the slides were immunohistochemically processed using an anti–TGF-α antibody (Neomarkers MS1000P) in a 1:50 dilution, a DAKO LSAB2 detection kit (DAKO K065), and 3,3′-diaminobenzidine (DAKO K3466) as the chromogen. Slides were not counterstained. Positive and negative control tissue (tonsil) slides were processed and stained with each batch of patient samples and treated identically, except that antibody diluent was used rather than primary antibody on the negative control slide.
Image Analysis
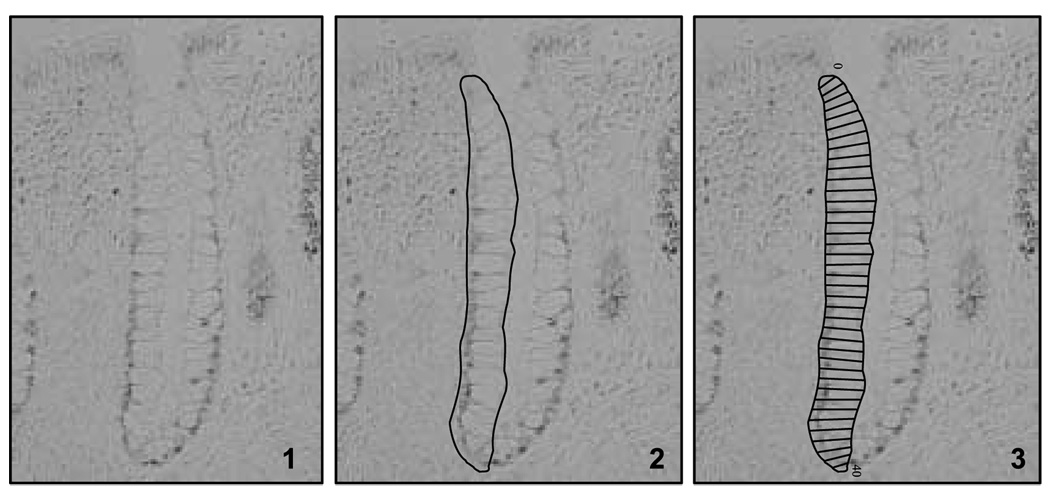
The basic scoring method (Fig. 2) used to describe and quantify various characteristics of the labeled antigens in the colon crypts is an image analysis scoring procedure for antigens that are labeled with a wide range of intensities in gradient distributions along the crypt axis (31). Methods and staining protocols are fine-tuned to achieve ideal staining quality within the tissue for quantification using our custom-designed plug-in into ImagePro Plus image analysis software (Media Cybernetics, Inc.), a light microscope, digital camera, and drawing board. The scoring and analysis unit is a hemicrypt, defined as one side of a colonic crypt bisected from its base to its gut luminal surface. Intact i.e., crypts (that is, extending from the muscularis mucosae to the gut lumen) are analyzed in a systematic and reliable process by a trained technician (intrarater reliability; intraclass correlation r ≥ 0.9).
Figure 2.
Molecular phenotyping is conducted by detecting the expression of various genes using automated immunohistochemistry (1). The detected expression of the genes in the tissues is quantified using custom-designed quantitative image analysis software, which measures and quantifies total expression, as well as the architecture or tissue distribution of the expression. Guided by the program, the technician selects and traces a hemicrypt (2),then the automated system divides the selection into a number of sections representing the width of an average colonocyte from the crypt base to the apex (3) and records the staining optical density.
In brief, the technician reviews the slides and selects 2 of 3 biopsies on which 16 to 20 “scorable” hemicrypts per biopsy (32 per patient) can be obtained. The technician is guided through the scoring protocol by the computer software with background correction images obtained for each slide. Hemicrypts are manually traced by the technician and divided by the software into a number of segments corresponding in width to an average normal crypt epithelial cell (Fig. 2). Overall hemicrypt- and segment-specific optical signal densities are then calculated by the software and stored into a Microsoft Access database along with various dimensional parameters of the hemicrypt. All images are obtained at 200× magnification and stored as 16-bit grayscale 1,600 × 1,200 pixel images.
Statistical Analysis
Cases included participants with pathology-confirmed, incident, sporadic colorectal adenomas, regardless of their number, shape, type, degree of dysplasia, or location. Participants free of adenoma upon colonoscopy were considered controls. Baseline characteristics for the MAP II study population (49 cases and 149 controls) and the subsample of participants for whom slides were immunohistochemically processed for TGF-α (31 cases and 29 controls) were examined to assess the potential for confounding and comparability between the main study and biomarker subpopulation. Analysis of covariance was used for continuous variables and adjusted for age and total energy intake (both continuous), as appropriate. Fisher’s exact test or χ2 test was used for categorical variables as appropriate. Nutrients were adjusted for energy according to the residual regression method of Willett and Stampfer (32). All statistical analyses were conducted using SAS version 9.1 software (SAS Institute).
The primary biomarker variable of interest was the staining optical density for TGF-α expression within crypts of the rectal mucosa and its association with adenoma. Although the immunohistochemical procedures were fully automated, there was some variability between staining runs or batches. Therefore, biomarker values were standardized for staining batch by taking the value in each individual divided by the mean of the staining batch in which the individual’s sample was processed. Densities from control tissue (tonsil) slides run with each batch to ensure staining quality were considered when combining batches (as little as possible) to create more balanced subgroups. The batch-standardized primary biomarker variables adequately met multiple regression assumptions as evaluated by residual linear regression plots and diagnostics. No influential observations were detected (33). In sensitivity analyses, other methods for batch adjustment (e.g., adjusting for staining batch as a fixed covariate in statistical models) were investigated. Because these other methods did not yield materially different results and simulation analyses indicated they introduce more potential bias, only the results based on the batch standardization procedure described above are reported.
We investigated the distribution of TGF-α expression graphically along the axis of colon and rectal crypts. Crypt divisions or cells (Fig. 1) were first standardized to 50 divisions per hemicrypt, averaging measurements within each division across all crypts separately for each patient, and adjusted for possible batch effects as described previously. Descriptive plots were created using nonparametric smoothed (locally weighted scatter plot smoothing) procedures separately in cases and controls to depict TGF-α distribution from crypt base to apex.
The mean marker amount refers to the overall expression across rectal crypts for each patient and was calculated by summing the staining densities from all analyzed crypts from the biopsy specimens and dividing by the number of crypts analyzed. Measures of within-crypt expression or distribution of the marker (e.g., expression in sections from the lower 60% or upper 40% of the crypt) were also calculated for each patient by taking the mean of the biomarker densities from sections in the lower 60% of crypts or in the upper 40% of crypts and constructing ratios of these means.
We used analysis of covariance methods to determine adjusted proportional differences in mean marker amount, standardized for batch, in cases versus controls. All estimates were adjusted for age and sex as fixed covariates. In secondary analyses, we also examined means in a subgroup of patients (10 cases and 10 controls) with biopsy samples from the ascending and sigmoid colon in an equivalent manner.
Diet and lifestyle covariates considered in analyses were selected following a literature review of potential risk factors for colorectal neoplasms. Continuous exposures were converted to a dichotomous (high, low) variable based on the median value (sex-specific for nutrients) in all 149 controls from the main MAP II study.
Bivariate analyses to evaluate TGF-α expression in the rectum according to diet and lifestyle characteristics was conducted in all 60 individuals, as well as among cases and controls separately. We used analysis of covariance to compare adjusted means for TGF-α expression according to high versus low levels of risk factors. All values were standardized for staining batch as described previously and adjusted for age, sex, energy, and case-control status as appropriate. Diet and lifestyle risk factors with notable differences in bivariate analyses were further evaluated as confounders in analyses for investigating adjusted associations between biomarker levels and adenoma.
The odds ratio with 95% confidence interval (95% CI) was calculated as a measure of association of incident sporadic adenoma with continuous batch-standardized biomarker expression using standard logistic regression modeling methods for case-control studies. Covariates in small multivariate logistic regression models of the association of incident sporadic adenoma with TGF-α expression in the rectum were carefully selected based on biological plausibility, strength of confounding, and consideration of highly correlated variables.
Results
Selected characteristics of cases and controls are presented in Table 1. On average, cases were more likely to be smokers, less likely to take NSAIDs regularly (once per week or more), and had higher total energy intake than controls. Unexpectedly, controls had lower physical activity levels and were more likely to have a first degree relative with a history of colorectal cancer. Female cases were more likely to use hormone replacement therapy. None of these differences were statistically significant. Baseline characteristics in all cases and controls (not shown) were comparable with those of this subpopulation.
Table 1.
Selected characteristics of patients with (cases) and without (controls) incident sporadic colorectal adenoma, MAP II
| Characteristic* | Cases (n = 31) | Controls (n = 29) | P† |
|---|---|---|---|
| Age (y) | 55.0 ± 1.4 | 54.7 ± 1.5 | 0.89 |
| Male (%) | 41.9 | 44.8 | 1.00 |
| Caucasian (%) | 93.5 | 96.6 | 1.00 |
| Current smoker (%) | 16.1 | 10.3 | 0.14 |
| College graduate (%) | 35.5 | 27.6 | 0.83 |
| HRT use among women (%) | 87.5 | 66.7 | 0.22 |
| Consumes alcohol currently (%) | 64.5 | 65.5 | 1.00 |
| Regular NSAID use (%) | 32.3 | 55.2 | 0.12 |
| Regular aspirin use (%) | 32.3 | 37.9 | 0.79 |
| 1st degree relative with CRC (%) | 10.0 | 25.9 | 0.17 |
| Recreational physical activity (METs/d) | 27.9 ± 3.5 | 21.6 ± 3.6 | 0.22 |
| Body mass index (kg/m2) | 30.5 ± 1.4 | 32.3 ± 1.4 | 0.37 |
| Daily intakes | |||
| Total energy intake (kcal) | 1,943.8 ± 121.3 | 1,624.3 ± 125.5 | 0.07 |
| Total fiber intake‡ (g) | 14.1 ± 4.9 | 15.3 ± 6.6 | 0.42 |
| Total calcium intake‡ (mg) | 928.8 ± 85.3 | 867.9 ± 88.3 | 0.63 |
| Total vitamin D intake‡ (IU) | 335.7 ± 52.1 | 321.0 ± 54.0 | 0.85 |
| Total folate intake‡ (µg) | 471.9 ± 45.8 | 491.0 ± 47.4 | 0.78 |
| Sucrose intake (g) | 40.3 ± 2.9 | 35.8 ± 3.0 | 0.30 |
| Whole fruit and vegetable servings/d | 3.3 ± 0.30 | 3.4 ± 0.31 | 0.57 |
| Weekly intakes | |||
| Processed meat servings/wk | 2.2 ± 0.30 | 2.6 ± 0.37 | 0.37 |
| Red meat servings/wk | 6.2 ± 2.5 | 8.2 ± 2.5 | 0.58 |
| Low-fat dairy servings/wk | 4.5 ± 1.0 | 4.1 ± 1.0 | 0.79 |
Abbreviations: HRT, hormone replacement therapy; CRC, colorectal cancer; MET, metabolic equivalents.
Mean ± SE presented unless otherwise specified.
Fisher’s exact for categorical variables; Pdiff (ANCOVA) for continuous variables; adjusted for age and total energy intake as appropriate.
Total intake (diet + supplements) adjusted for total energy intake.
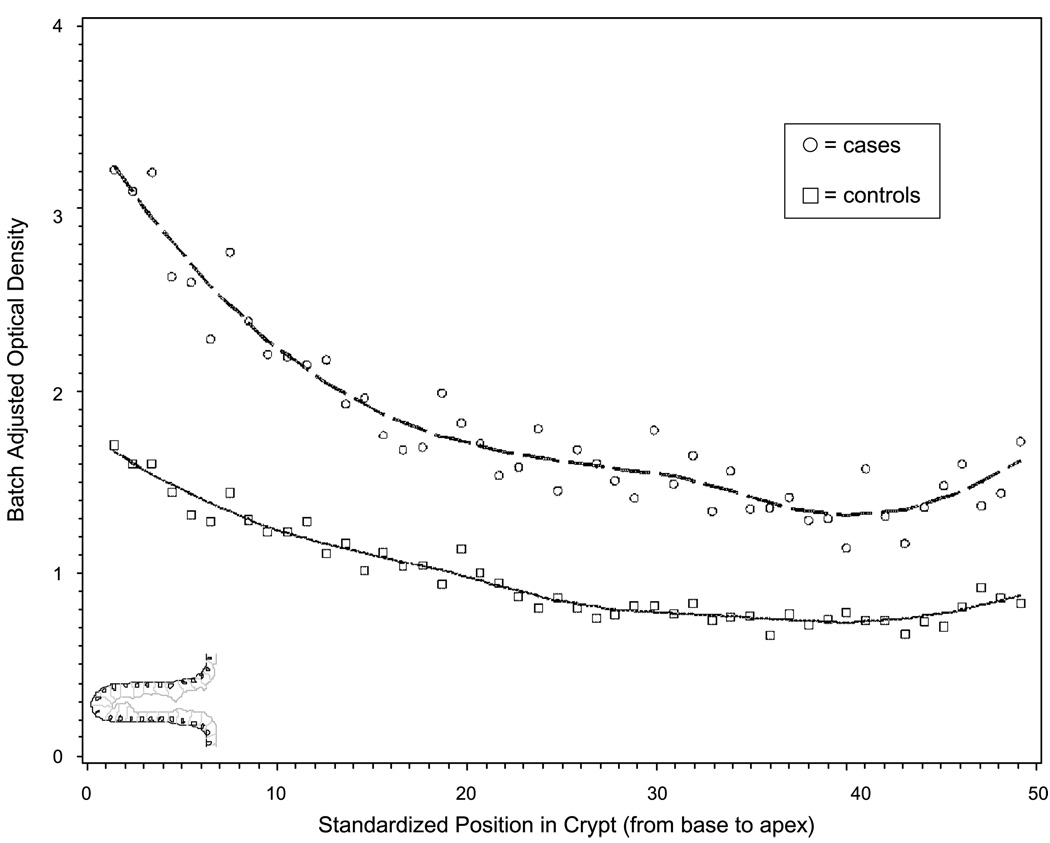
The distribution of TGF-α expression (staining optical density) within normal-appearing rectal crypts for cases and controls is presented in Fig. 3. TGF-α expression appeared to be highest at the base of the colorectal crypt and appeared to decrease moving up the crypt toward the colon lumen (Fig. 1). Throughout the crypt, expression of TGF-α appeared to be higher in cases than controls.
Figure 3.
Distribution of TGF-α expression throughout colon crypts in incident sporadic colorectal adenoma cases and controls, MAP II. Cells from the base to the opening of the crypt (proliferative zone to zone of differentiation) are presented on the X axis; Y axis, batch-standardized staining optical density; circles, measures in cases; squares, measures in controls; points, the average of all patients’ hemicrypts at a standardized crypt section or location; smoothed lines, the best fit for the data.
Average TGF-α expression throughout the rectal crypts (Table 2) was 51% higher in cases than controls (age- and sex-adjusted proportional difference; P = 0.05). There was a greater difference in total TGF-α expression among female cases and controls than among males, but associations in both sexes were in the same direction (P interaction not significant, data not shown). There was also some evidence of differential risks by NSAID use, but the test for interaction was not statistically significant (P = 0.28). Among persons not using NSAIDs, TGF-α expression was 57% higher in cases than controls (P diff = 0.12), whereas among persons using NSAIDs once per week or more, there was no difference between cases and controls (P diff = 0.98; data not shown). Strong, statistically significant case-control differences were apparent in the lower 60% and in the upper 40% zones of the crypt (Table 2). TGF-α expression in the normal rectal epithelium was directly associated with case-control status (adenoma) in the entire crypt (odds ratio, 2.23; 95% CI, 0.98–5.07), as well as in the lower 60% (odds ratio, 2.16; 95% CI, 1.11–4.17) and upper 40% (odds ratio, 2.12; 95% CI, 1.03–4.38) of the crypts.
Table 2.
TGF-α expression (staining optical density) in the normal-appearing rectal mucosa of incident sporadic colorectal adenoma cases and controls, MAP II
| Crypt section* | Case† (n = 29) | Control† (n = 31) | Prop diff (%)‡ | Pdiff§ | Odds ratio‖ (95% CI) |
|---|---|---|---|---|---|
| FC | 1.18 (0.14) | 0.78 (0.14) | 51.2 | 0.05 | 2.23 (0.98–5.07) |
| L60% | 2.12 (0.32) | 1.01 (0.31) | 109.9 | 0.02 | 2.16 (1.11–4.17) |
| U40% | 2.31 (0.41) | 0.98 (0.39) | 135.7 | 0.02 | 2.12 (1.03–4.38) |
| L60%/FC | 1.66 (0.11) | 1.34 (0.11) | 23.9 | 0.04 | 3.75 (1.01–13.91) |
| U40%/FC | 1.81 (0.22) | 1.40 (0.22) | 29.2 | 0.19 | 1.52 (0.80–2.90) |
NOTE: Batch standardized as individual value divided by batch mean; all estimates adjusted for age and sex.
Abbreviations: FC, full crypt; L60%, lower 60%; U40%, upper 40%; Prop diff, proportional difference.
Full crypt = average expression throughout entire crypt; lower = sections from base of crypt; upper = sections from crypt apex or mouth; ratios of section-specific expression.
Mean staining densities and SEs.
Difference between means (cases − controls) divided by mean in controls × 100%.
Difference P value for comparison of means (analysis of covariance).
Association between TGF-α expression (as a continuous variable) and adenoma (relative odds of being a case) adjusted for age and sex.
We were unable to replicate differences seen in the rectal tissue among the small subset of ascending and sigmoid colon samples (data not shown). For both colon sites, cases had slightly lower TGF-α expression (10–20%) than controls, but these findings were not statistically significant (P > 0.60).
When investigating the potential relationship between diet and lifestyle risk factors and TGF-α expression (Table 3), we found that TGF-α expression was 79% higher among subjects with a positive family history of colorectal cancer (P = 0.02), 36% lower in persons regularly taking NSAIDs (P = 0.05) and 49% lower among women who used hormone replacement therapy (P = 0.06). Among persons free of adenoma at colonoscopy (controls), TGF-α expression was 28% lower among persons with a high physical activity level (P = 0.22), 58% higher in persons with high sucrose intake (P = 0.07), and 31% lower in persons with high fruit and vegetable intake (P = 0.16). Differences across cases and controls, examined separately, seemed to be in the same direction as with cases and controls combined and adjusted for case-control status.
Table 3.
TGF-α expression (staining optical density) according to diet and lifestyle risk factors, MAP II
| Characteristic* | n | All† | Prop diff (%)‡ | Pdiff§ | n | Controls | Prop diff (%)‡ | Pdiff§ | n | Cases | Prop diff (%)‡ | Pdiff§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lifestyle | ||||||||||||
| Age < 55 y | 32 | 0.94 | 15 | 0.75 | 17 | 1.12 | ||||||
| Age ≥ 55 y | 28 | 1.06 | 12.8 | 0.55 | 14 | 0.84 | 12 | 0.68 | 14 | 1.28 | 14.3 | 0.66 |
| NSAID use never to <once/wk | 34 | 1.16 | 13 | 0.83 | 21 | 1.4 | ||||||
| NSAID use once/wk | 26 | 0.74 | −36.2 | 0.05 | 16 | 0.76 | −8.4 | 0.74 | 10 | 0.66 | −52.9 | 0.05 |
| HRT use never (women) | 7 | 1.68 | 5 | 1.11 | 2 | 2.72 | ||||||
| HRT use ever (women) | 24 | 0.86 | −48.8 | 0.06 | 10 | 0.66 | −4.1 | 0.23 | 14 | 1.07 | −60.7 | 0.07 |
| BMI< 30 kg/m2 | 31 | 1.06 | 13 | 0.78 | 18 | 1.34 | ||||||
| BMI ≥ 30 kg/m2 (obese) | 29 | 0.93 | −12.3 | 0.5 | 16 | 0.81 | 3.8 | 0.89 | 13 | 1 | −25.4 | 0.33 |
| Physical activity‖ < 22 METs/d | 37 | 1.05 | 20 | 0.85 | 17 | 1.24 | ||||||
| Physical activity > 22 METs/d | 23 | 0.99 | −5.7 | 0.75 | 9 | 0.69 | −18.8 | 0.46 | 14 | 1.27 | 2.4 | 0.92 |
| No family history of CRC | 47 | 0.82 | 20 | 0.72 | 27 | 0.97 | ||||||
| 1° relative with CRC | 10 | 1.47 | 79.2 | 0.02 | 7 | 0.78 | 8.3 | 0.78 | 3 | 2.53 | 160.8 | 0.01 |
| Dietary intakes¶ | ||||||||||||
| Total energy‖ low | 20 | 1.13 | 10 | 0.79 | 10 | 1.43 | ||||||
| Total energy high | 40 | 0.92 | −18.6 | 0.32 | 19 | 0.75 | −5.1 | 0.84 | 21 | 1.08 | −24.4 | 0.36 |
| Total vitamin D low | 33 | 1.02 | 16 | 0.82 | 17 | 1.24 | ||||||
| Total vitamin D high | 27 | 0.95 | −6.9 | 0.72 | 13 | 0.77 | −6.1 | 0.82 | 14 | 1.13 | −8.9 | 0.75 |
| Total calcium low | 33 | 1.02 | 17 | 0.79 | 16 | 1.28 | ||||||
| Total calcium high | 27 | 0.96 | −5.9 | 0.76 | 12 | 0.8 | 1.3 | 0.97 | 15 | 1.1 | −14.1 | 0.67 |
| Total fiber low | 35 | 0.98 | 16 | 0.78 | 19 | 1.17 | ||||||
| Total fiber high | 25 | 1.01 | 3.1 | 0.85 | 13 | 0.81 | 3.8 | 0.89 | 12 | 1.23 | 5.1 | 0.86 |
| Sucrose low | 28 | 0.89 | 15 | 0.62 | 13 | 1.15 | ||||||
| Sucrose high | 32 | 1.08 | 21.3 | 0.33 | 14 | 0.98 | 58.1 | 0.07 | 18 | 1.22 | 6.1 | 0.84 |
| Red and processed meat low | 31 | 1.11 | 15 | 0.82 | 16 | 1.41 | ||||||
| Red and processed meat high | 29 | 0.87 | −21.6 | 0.29 | 14 | 0.77 | −6.1 | 0.87 | 15 | 0.97 | −31.2 | 0.25 |
| Low fat dairy low | 34 | 1.07 | 16 | 0.88 | 18 | 1.26 | ||||||
| Low fat dairy high | 26 | 0.89 | −16.8 | 0.38 | 13 | 0.69 | −21.6 | 0.35 | 13 | 1.1 | −12.7 | 0.68 |
| Fruit and vegetables low | 26 | 1.07 | 12 | 0.97 | 14 | 1.17 | ||||||
| Fruit and vegetables high | 34 | 0.93 | −13.1 | 0.53 | 17 | 0.67 | −30.9 | 0.16 | 17 | 1.21 | 3.4 | 0.91 |
NOTE: Batch standardized as individual value divided by batch mean; full crypt values.
All estimates (except age) adjusted for age; cut points based on medians in all MAP II controls.
Adjusted for case-control status.
Proportional difference = difference between means [high − low (reference)] divided by low or mean in reference group × 100%.
Difference P value for comparison of means (analysis of covariance).
Physical activity and total energy intake mutually adjusted for each other.
Nutrient values residual energy-adjusted + total energy intake covariate; cut points based on sex-specific medians in all controls.
We investigated associations between TGF-α expression and risk for adenoma in multivariate models adjusted for risk factors that were suggested as potential confounders by the analyses presented in Tables 1 and 3. Case-control differences were attenuated when adjusted for NSAID use and strengthened when adjusted for total energy intake and/or family history of colorectal cancer (Table 4). Estimates were not strongly affected by inclusion of other risk factors in the models. In the multivariate-adjusted model, higher TGF-α expression in normal-appearing rectal tissue was associated with a 3- to 4-fold increased risk for adenoma (odds ratio, 3.75; 95% CI, 1.18–11.98).
Table 4.
Adjusted associations for TGF-α expression (staining optical density) in the normal-appearing rectal mucosa of incident sporadic colorectal adenoma cases and controls, MAP II
| Case* (n = 29) | Control* (n = 31) | Prop diff (%)† | Pdiff‡ | Odds ratio§ (95% CI) | Model covariates‖ |
|---|---|---|---|---|---|
| 1.10 (0.14) | 0.80 (0.14) | 37.5 | 0.14 | 2.26 (0.91–5.64) | NSAID use |
| 1.22 (0.14) | 0.74 (0.14) | 64.9 | 0.02 | 2.83 (1.11–7.24) | Total energy intake |
| 1.41 (0.17) | 0.88 (0.15) | 60.2 | 0.01 | 3.00 (1.17–7.70) | Family history of CRC |
| 1.39 (0.16) | 0.84 (0.15) | 65.5 | 0.01 | 3.75 (1.18–11.98) | NSAID use, energy, family history of CRC |
NOTE: Adjusted for risk factors or potential confounders (Table 1, Table 3). Batch standardized as individual value divided by batch mean; full crypt values.
Mean staining densities and SEs.
Difference between means (cases − controls) divided by mean in controls × 100%.
Difference P value for comparison of means (analysis of covariance).
Association between TGF-α expression (as a continuous variable) and adenoma (relative odds of being a case).
All estimates adjusted for age and sex and other covariates as specified; total energy intake modeled as a continuous variable.
Discussion
In the MAP II study, expression of TGF-α, an autocrine-paracrine growth factor of the epidermal growth factor family (8–11), was statistically significantly higher in normal-appearing rectal tissue from adenoma cases than from controls. These associations persisted following adjustment for potential confounders (that is, risk factors for colorectal neoplasms that also seemed to be associated with TGF-α levels). TGF-α expression varied significantly across key risk factors for colorectal neoplasms. Although our results are preliminary and require further validation, they suggest that higher TGF-α expression in the normal-appearing colorectal mucosa may indicate an at-risk phenotype, which may also be modulated by risk factors or behaviors believed to be important for the prevention of colorectal neoplasms. These findings support the potential for TGF-α as a modifiable biomarker of risk for colorectal cancer.
Basic science evidence strongly supports a progrowth and hyperproliferative mechanism in colon carcinogenesis with elevated TGF-α expression in colon adenoma and adenocarcinomas (13). Identifying at-risk molecular phenotypes is critical to advance the understanding of how colorectal cancer develops and how to target risk at a reversible stage. Early growth-related changes in the normal colorectal mucosal crypt epithelium appear to precede or at least accompany the development of polyps or cancer and thus may have value as a predictive or diagnostic marker (34–39), yet there are few human studies on TGF-α expression in normal-appearing colon or rectal crypts of persons at varied risk for colorectal neoplasms (24, 25). To begin to address these needs, we characterized and quantified phenotypic expression patterns of TGF-α in normal-appearing rectal tissue and detected significantly higher levels in persons at increased risk for colorectal cancer (persons with adenomatous polyps as compared with polyp-free controls). The clear difference in the magnitude of total TGF-α expression between cases and controls was reflected in the parallel distribution of expression within crypts in normal-appearing tissue from adenoma cases compared with adenoma-free controls. We found TGF-α staining in the cytoplasmic region of colonocytes in the rectal crypts to be slightly higher at the base of the crypt and throughout the proliferative zone with strong case-control differences in TGF-α expression maintained throughout the crypt, including the upper portion of the crypt, believed to be the zone of differentiation. Preliminary studies from other groups showed denser TGF-α staining expression in the upper one-third to two-thirds of colonic crypts (8, 24, 40). Discrepancies in characterization of the localization of TGF-α in colon crypts may be due to differences in tissue fixation, processing, staining, or quantification methods. We are the first to use our custom image analysis program, which allows us to quantify average expression and section (cell)–specific expression for each crypt analyzed (32 hemicrypts per patient × 60 patients), as well as measure subtle gradients not quantifiable by other methods. Our findings seem more plausible for TGF-α, an early stimulator of growth and proliferation, because we found the greatest level of expression near the base of the crypt or proliferative zone.
Little is known about the effects of diet and lifestyle behaviors on TGF-α signaling in the colon; therefore, we investigated whether the expression of TGF-α in normal-appearing rectal tissue varied across plausible risk factors for colorectal neoplasms. We found TGF-α expression to be associated with a family history of colorectal cancer or adenomatous polyps in a first-degree relative(s), NSAID use, and hormone replacement therapy use in postmenopausal women. Despite strong plausible evidence for these risk factors (41–43), the mechanisms whereby they modulate risk within the colon remain unclear. Animal and human studies have shown that manipulation of diet and other lifestyle exposures result in adaptive changes in the colon crypt epithelium and in TGF-α expression specifically (17, 21, 22, 24, 25). Our preliminary findings suggest that TGF-α levels may vary according to modifiable dietary risk factors (intake of sucrose, low-fat dairy, fruits, and vegetables), and although, in this small sample, few differences were statistically significant, many are consistent with current hypotheses about diet, growth, and colorectal cancer (7, 14, 17, 20, 24, 25, 35, 44–48). Furthermore, associations between TGF-α expression and risk for adenoma were strengthened following adjustment for risk factors, suggesting that a person with elevated TGF-α levels could further increase or decrease their probability of being a case (that is, risk for incident sporadic colorectal adenoma) based on their history of risk behaviors, such as use of NSAIDs or total energy intake. The efficacy of NSAIDs in preventing colorectal adenoma is well-documented (49–51), and other investigators found decreased staining for TGF-α in the rectal mucosa of patients with a history of adenoma treated with aspirin (25). Rodent studies also support total energy restriction to reduce cellular growth (52–54). Beyond modifiable risk factors, such as diet and lifestyle, elevated expression of TGF-α in normal-appearing tissue from persons with a family history of colorectal cancer emphasizes the importance of regular monitoring and screening for high-risk persons and the imminent need for early biomarkers of risk. Our group is currently evaluating the effect of supplemental calcium and/or vitamin D on TGF-α expression and other markers to further develop and validate our biomarkers of risk panel and determine whether the markers are treatable.
The MAP II project had several major strengths including of the use of new technologies and integration of laboratory, clinical, and epidemiologic methods. Moving beyond cell culture studies and gene arrays, expression of genes (phenotype) in colon tissue, particularly within the structure of colon epithelial crypts, provides more relevant information about early tissue-level changes and the progression toward carcinogenesis, which is the result not only of genotype but also of epigenetic influences, gene-gene interactions, gene-environment interactions, and complex multigene-multienvironment interactions. However, results of this novel but small pilot study should be viewed and interpreted with caution. We were not able to address multiple confounding effects or interactions, and some covariate categories were restricted to a small number of individuals. Larger samples, representative of the population at large, will be needed to validate these findings. Nor were we able to adequately address TGF-α expression in more proximal sites of the colon. However, the study size was sufficient to begin to accomplish our objectives of obtaining preliminary estimates to provide direction for future study. Other limitations included those inherent to case-control studies in general and colonoscopy-based case-control studies of adenomas in particular. Cases and controls may not be representative of the population at large because they were selected from among individuals referred for routine colonoscopy screening and thus may have been at potentially higher risk for colorectal neoplasms. Knowledge of the postulated diet and lifestyle associations with colorectal cancer are likely to have been available and of interest to this population. The most likely product of these biases is that controls were more similar to cases, leading to an underestimate of true risk. However, a major strength of this investigation is that controls were derived from the population that gave rise to cases, and the potential for differential reporting bias was limited by obtaining self-reports of exposure prior to colonoscopy and diagnosis of adenoma.
As a retrospective evaluation of risk factors and cross-sectional investigation of the biomarker-disease relationship, the design of our pilot study does not allow us to determine causal associations with diet or with adenoma i.e., progression (that is, which came first, the biomarker or the neoplasm). However, to learn whether or not the biomarker is associated with the presence of a neoplasm, temporality is irrelevant, and there is negligible misclassification of neoplasm status with a colonoscopy-based design. Although it is of relevance primarily to etiology and risk assessment, it is not unlikely that, considering that patients going to colonoscopy are likely at higher risk, some patients who received colonoscopies and are classified as controls will later develop neoplasms and therefore have biomarker profiles similar to those of cases. The latter limitation would tend to attenuate true associations; however, despite this potential limitation, our preliminary data suggest that there may be substantial, biologically plausible, and statistically significant differences between cases and controls. Although our results seem promising and informative of the potential mechanisms involved, further research is needed to clarify whether TGF-α expression in colorectal crypts is predictive of relevant precancerous changes leading to the appearance of colorectal neoplasms.
Results illuminating potential associations between TGF-α and diet and lifestyle risk factors suggest that larger studies of similar design to assess biomarkers of risk may elucidate poorly understood diet-cancer mechanisms. Although convincing human evidence is needed, large prospective studies are challenging and costly in cancer research due to the temporality of the exposure-disease relationship and the lack of biomarkers of risk to advance research in this area. In the future, a panel of biomarkers for colorectal cancer could serve as an end point in relatively short-term prospective studies and chemoprevention trials to investigate promising diet and lifestyle exposures and to make vital progress in colorectal research and prevention.
Acknowledgments
Grant support: Fullerton Foundation, Franklin Foundation, and Georgia Cancer Coalition Distinguished Scholar Award (R.M. Bostick).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Church J, Casey G. Molecular genetics and colorectal neoplasia. Kluwer Academic Publishers; 2004. [Google Scholar]
- 2.Srivastava S, Lippman SM, Hong WK, Mulshine JL, editors. Early detection of cancer: molecular markers. Armonk (NY): Futura Publishing Company, Inc.; 1994. [Google Scholar]
- 3.Schatzkin A, Gail M. The promise and peril of surrogate end points in cancer research. Nat Rev Cancer. 2002;2:19–27. doi: 10.1038/nrc702. [DOI] [PubMed] [Google Scholar]
- 4.Curry S, Byers T, Hewitt M, editors. Institute of Medicine National Research Council. Washington (DC): National Academy of Sciences; 2003. Fulfilling the potential of cancer prevention and early detection. [PubMed] [Google Scholar]
- 5.Branca F, Hanley AB, Pool-Zobel B, Verhagen H. Biomarkers in disease and health. Br J Nutr. 2001;86 Suppl 1:S55–S92. doi: 10.1079/bjn2001339. [DOI] [PubMed] [Google Scholar]
- 6.Ponz de Leon M. Colorectal cancer. New York: Springer-Verlag Berlin Heidelberg; 2002. [Google Scholar]
- 7.Bostick RM, Fosdick L, Grandits GA, et al. Colorectal epithelial cell proliferative kinetics and risk factors for colon cancer in sporadic adenoma patients. Cancer Epidemiol Biomarkers Prev. 1997;6:1011–1019. [PubMed] [Google Scholar]
- 8.Cameron IL, Hardman WE. Paracrine action of transforming growth factor-α in rectal crypt epithelium of humans. Cell Biol Int. 2002;26:1029–1034. doi: 10.1006/cbir.2002.0962. [DOI] [PubMed] [Google Scholar]
- 9.Awwad RA, Sergina N, Yang H, et al. The role of transforming growth factor α in determining growth factor independence. Cancer Res. 2003;63:4731–4738. [PubMed] [Google Scholar]
- 10.Srivastava S, Henson DE, Gazdar AF. Molecular pathology of early cancer. Amsterdam: IOS Press; 1998. [Google Scholar]
- 11.Markowitz SD, Molkentin K, Gerbic C, Jackson J, Stellato T, Willson JK. Growth stimulation by coexpression of transforming growth factor-α and epidermal growth factor-receptor in normal and adenomatous human colon epithelium. J Clin Invest. 1990;86:356–362. doi: 10.1172/JCI114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riese DJ, Kim ED, Elenius K, et al. The epidermal growth factor receptor couples transforming growth factor-α, heparin-binding epidermal growth factor-like factor, and amphiregulin to Neu, ErbB-3, and ErbB-4. J Biol Chem. 1996;271:20047–20052. doi: 10.1074/jbc.271.33.20047. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Imanishi K, Haruma K, et al. Immunoreactive transforming growth factor-A and epidermal growth factor in colorectal adenomas and carcinomas. Oncology. 1992;49:381–385. doi: 10.1159/000227077. [DOI] [PubMed] [Google Scholar]
- 14.Herlyn M, Kath R, Williams N, Valyi-Nagy I, Rodeck U. Growth-regulatory factors for normal, premalignant, and malignant human cells in vitro. Adv Cancer Res. 1990;54:213–234. doi: 10.1016/s0065-230x(08)60812-x. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox JN, Derynck R. Localization of cells synthesizing transforming growth factor-α mRNA in the mouse brain. J Neurosci. 1988;8:1901–1904. doi: 10.1523/JNEUROSCI.08-06-01901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipkin M, Deschner E. Early proliferative changes in intestinal cells. Cancer Res. 1976;36:2665–2668. [PubMed] [Google Scholar]
- 17.Cameron IL, Hardman WE, Heitman DW, Carter JW. Dietary fibre on cell proliferation in large bowel mucosal crypts near or away from lymphoid nodules and on mineral bioavailability. Cell Prolif. 2000;33:367–379. doi: 10.1046/j.1365-2184.2000.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger B, Carey HV, Procaccino F, et al. Reduced susceptibility of mice overexpressing transforming growth factor α to dextran sodium sulphate induced colitis. Gut. 1998;43:64–70. doi: 10.1136/gut.43.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. J Nutr. 2007;137:200S–204S. doi: 10.1093/jn/137.1.200S. [DOI] [PubMed] [Google Scholar]
- 20.Caderni G, Palli D, Lancioni L, et al. Dietary determinants of colorectal proliferation in the normal mucosa of subjects with previous colon adenomas. Cancer Epidemiol Biomarkers Prev. 1999;8:219–225. [PubMed] [Google Scholar]
- 21.Cameron IL, Ord VA, Hunter KE, Van Nguyen M, Padilla GM, Heitman DW. Quantitative contribution of factors regulating rat colonic crypt epithelium: role of parenteral and enteral feeding, caloric intake, dietary cellulose level and the colon carcinogen DMH. Cell Tissue Kinet. 1990;23:227–235. doi: 10.1111/j.1365-2184.1990.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 22.Deschner EE, Lytle JS, Wong G, Ruperto JF, Newmark HL. The effect of dietary omega-3 fatty acids (fish oil) on azoxymethanol-induced focal areas of dysplasia and colon tumor incidence. Cancer. 1990;66:2350–2356. doi: 10.1002/1097-0142(19901201)66:11<2350::aid-cncr2820661117>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Imanishi K, Yoshihara M, et al. Immunoreactive transforming growth factor α is commonly present in colorectal neoplasia. Am J Pathol. 1991;139:123–129. [PMC free article] [PubMed] [Google Scholar]
- 24.Hardman WE, Cameron IL, Beer WH, Speeg KV, Kadakia SC, Lang KA. Transforming growth factor α distribution in rectal crypts as a biomarker of decreased colon cancer risk in patients consuming cellulose. Cancer Epidemiol Biomarkers Prev. 1997;6:633–637. [PubMed] [Google Scholar]
- 25.Barnes CJ, Hamby-Mason RL, Hardman WE, Cameron IL, Speeg KV, Lee M. Effect of aspirin on prostaglandin E2 formation and transforming growth factor α expression in human rectal mucosa from individuals with a history of adenomatous polyps of the colon. Cancer Epidemiol Biomarkers Prev. 1999;8:311–315. [PubMed] [Google Scholar]
- 26.Kushi L, Kaye S, Folsom A, Soler J, Prineas R. Accuracy and reliability of self-measurement of body girths. Am J Epidemiol. 1988;128:740–748. doi: 10.1093/oxfordjournals.aje.a115027. [DOI] [PubMed] [Google Scholar]
- 27.Pereira M, FitzerGerald SJ, Gregg EW, et al. A collection of physical activity questionnaires for health related research. Med Sci Sports Exerc. 1997;29:S5–S88. [PubMed] [Google Scholar]
- 28.Willett W, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 29.Willett W, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127 doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien M, Winawer S, Zauber A, et al. The National Polyp Study Workgroup. The National Polyp Study: patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98:371–379. [PubMed] [Google Scholar]
- 31.Bostick RM, Kong KY, Ahearn TU, Chaudry Q, Cohen V, Wang MD. Detecting and quantifying biomarkers of risk for colorectal cancer using quantum dots and novel image analysis algorithms; Conf Proc IEEE Eng Med Biol Soc; 2006. pp. 3313–3316. [DOI] [PubMed] [Google Scholar]
- 32.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 33.Neter J, Kutner M, Nachtsheim C, Wasserman W. Applied linear statistical models. Chicago: Times Mirror Higher Education Group, Inc.; 1996. [Google Scholar]
- 34.Akedo I, Ishikawa H, Ioka T, et al. Evaluation of epithelial cell proliferation rate in normal-appearing colonic mucosa as a high-risk marker for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:925–930. [PubMed] [Google Scholar]
- 35.Bostick RM, Fosdick L, Wood JR, et al. Calcium and colorectal epithelial cell proliferation in sporadic adenoma patients: a randomized, double-blinded, placebo-controlled clinical trial. J Natl Cancer Inst. 1995;87:1307–1315. doi: 10.1093/jnci/87.17.1307. [DOI] [PubMed] [Google Scholar]
- 36.Einspahr JG, Alberts DS, Gapstur SM, Bostick RM, Emerson SS, Gerner EW. Surrogate end-point biomarkers as measures of colon cancer risk and their use in cancer chemoprevention trials. Cancer Epidemiol Biomarkers Prev. 1997;6:37–48. [PubMed] [Google Scholar]
- 37.Chen LC, Hao CY, Chiu YS, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64:3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 38.Hao CY, Moore DH, Chiu YS, et al. Altered gene expression in normal colonic mucosa of individuals with polyps of the colon. Dis Colon Rectum. 2005;48:2329–2335. doi: 10.1007/s10350-005-0153-2. [DOI] [PubMed] [Google Scholar]
- 39.Hao CY, Moore DH, Wong P, Bennington JL, Lee NM, Chen LC. Alteration of gene expression in macroscopically normal colonic mucosa from individuals with a family history of sporadic colon cancer. Clin Cancer Res. 2005;11:1400–1407. doi: 10.1158/1078-0432.CCR-04-1942. [DOI] [PubMed] [Google Scholar]
- 40.Thomas DM, Nasim MM, Gullick WJ, Alison MR. Immunoreactivity of transforming growth factor α in the normal adult gastrointestinal tract. Gut. 1992;33:628–631. doi: 10.1136/gut.33.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959–2967. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 42.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 43.Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 44.Caderni G, Luceri C, Lancioni L, Dolara P. Dietary sucrose, glucose, fructose, and starches affect colonic functions in rats. Nutr Cancer. 1996;25:179–186. doi: 10.1080/01635589609514440. [DOI] [PubMed] [Google Scholar]
- 45.Luceri C, Caderni G, Lancioni L, et al. Effects of repeated boluses of sucrose on proliferation and on AOM-induced aberrant crypt foci in rat colon. Nutr Cancer. 1996;25:187–196. doi: 10.1080/01635589609514441. [DOI] [PubMed] [Google Scholar]
- 46.Stamp D, Zhang XM, Medline A, Bruce WR, Archer MC. Sucrose enhancement of the early steps of colon carcinogenesis in mice. Carcinogenesis. 1993;14:777–779. doi: 10.1093/carcin/14.4.777. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Patil S, Li W, Humphrey LE, Brattain MG, Howell GM. Activation of the TGFα autocrine loop is downstream of IGF-I receptor activation during mitogenesis in growth factor dependent human colon carcinoma cells. Oncogene. 2002;21:2785–2796. doi: 10.1038/sj.onc.1205375. [DOI] [PubMed] [Google Scholar]
- 48.Yee YK, Chintalacharuvu SR, Lu J, Nagpal S. Vitamin D receptor modulators for inflammation and cancer. Mini Rev Med Chem. 2005;5:761–778. doi: 10.2174/1389557054553785. [DOI] [PubMed] [Google Scholar]
- 49.Arber N, Eagle CJ, Spicak J, et al. the PreSAP Trial Investigators. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 50.Bertagnolli MM, Eagle CJ, Zauber AG, et al. the APC Study Investigators. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 51.Baron JA, Cole BF, Mott L, et al. Neoplastic and antineoplastic effects of β-carotene on colorectal adenoma recurrence: results of a randomized trial. J Natl Cancer Inst. 2003;95:717–722. doi: 10.1093/jnci/95.10.717. [DOI] [PubMed] [Google Scholar]
- 52.Weindruch R, Albanes D, Kritchevsky D. The role of calories and caloric restriction in carcinogenesis. Hematol Oncol Clin North Am. 1991;5:79–89. [PubMed] [Google Scholar]
- 53.Albanes D, Salbe AD, Levander OA, Taylor PR, Nixon DW, Winick M. The effect of early caloric restriction on colonic cellular growth in rats. Nutr Cancer. 1990;13:73–80. doi: 10.1080/01635589009514047. [DOI] [PubMed] [Google Scholar]
- 54.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]