Abstract
The MOS4-associated complex (MAC) is a highly conserved nuclear protein complex associated with the spliceosome. We recently purified the MAC from Arabidopsis (Arabidopsis thaliana) nuclei, identified its potential components by mass spectrometry, and showed that at least five core proteins in the MAC are required for defense responses in plants. Here, we report the characterization of a putative RNA-binding protein identified in the MAC named MAC5A and its close homolog MAC5B. We confirmed that MAC5A is a component of the MAC through coimmunoprecipitation with the previously described MAC protein CELL DIVISION CYCLE5 from Arabidopsis. In addition, like all other characterized MAC proteins, MAC5A fused to the Green Fluorescent Protein localizes to the nucleus. Double mutant analysis revealed that MAC5A and MAC5B are unequally redundant and that a double mac5a mac5b mutant results in lethality. Probably due to this partial redundancy, mac5a and mac5b single mutants do not exhibit enhanced susceptibility to virulent or avirulent pathogen infection. However, like other MAC mutations, mac5a-1 partially suppresses the autoimmune phenotypes of suppressor of npr1-1, constitutive1 (snc1), a gain-of-function mutant that expresses a deregulated Resistance protein. Our results suggest that MAC5A is a component of the MAC that contributes to snc1- mediated autoimmunity.
Higher plants have evolved a sophisticated innate immune system against microbial pathogen infection. Pattern recognition receptors and Resistance (R) proteins represent two major classes of receptors in plants that recognize the presence of pathogens and trigger cell-autonomous defense responses (Jones and Dangl, 2006). Many pattern recognition receptors are transmembrane receptor-like kinases that contain extracellular leucine-rich repeats (LRRs) and are involved in the perception of nonself epitopes common to whole groups of microbes called microbe-associated molecular patterns (Zipfel, 2009). Most cloned R genes encode intracellular proteins containing a central nucleotide-binding (NB) site and C-terminal LRRs and are involved in the recognition of effector proteins secreted by pathogens during infection. The activation of NB-LRRs is often associated with the onset of a cell death program known as the hypersensitive response, in which the plant cell kills itself to restrict pathogen growth. Most mutants with deregulated NB-LRR proteins exhibit seedling lethality, suggesting that NB-LRRs are under tight negative regulation to avoid unnecessary cell death (Lukasik and Takken, 2009). SUPPRESSOR OF NPR1-1, CONSTITUTIVE1 (SNC1) encodes a NB-LRR with an N-terminal Toll/Interleukin1 Receptor (TIR) domain (Li et al., 2001b; Zhang et al., 2003). The unique gain-of-function mutant snc1 exhibits constitutive defense responses in the absence of cell death, representing an autoimmune model amenable to genetic analysis. A forward genetic screen for suppressors of snc1 revealed that snc1-mediated defense includes components involved in nucleocytoplasmic partitioning (Palma et al., 2005; Zhang and Li, 2005; Cheng et al., 2009), protein modification (Goritschnig et al., 2007, 2008), and RNA processing (Zhang et al., 2005; Palma et al., 2007). Importantly, like most other TIR-NB-LRRs, snc1 signaling requires the function of PHYTOALEXIN DEFICIENT4 and ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1; Li et al., 2001b; Zhang et al., 2003).
MODIFIER OF SNC1, 4 (MOS4) is the founding member of the MOS4-associated complex (MAC; Palma et al., 2007), a highly conserved spliceosome-associated complex homologous to the Prp19 complex (NTC) in yeast (Tarn et al., 1994) and the CDC5L complex in human (Ajuh et al., 2000). The MAC is composed of over 20 proteins in Arabidopsis (Arabidopsis thaliana), many of which have predicted roles in RNA processing and/or splicing (Monaghan et al., 2009). Accordingly, several MAC proteins are encoded by essential genes, including AtSKIP, EMB2765, CLO/VAJ/GFA/MEE5, EMB1507, ESP3/EMB2733, and SUS2/EMB177 (Tzafrir et al., 2004; Pagnussat et al., 2005; Herr et al., 2006; Moll et al., 2008; Liu et al., 2009; Yagi et al., 2009; Lim et al., 2010; Supplemental Table S1). We previously demonstrated that the MAC core proteins MOS4, CELL DIVISION CYCLE5 (AtCDC5), PRL1, and MAC3A/3B associate in planta and are all required for plant immunity, as loss-of-function mutations in the genes encoding these proteins result in plants exhibiting enhanced susceptibility to infection by virulent pathogens (Palma et al., 2007; Monaghan et al., 2009). In addition, these loci are broadly required for R protein-mediated defense pathways and snc1 autoimmune signaling.
Here, we characterize two unequally redundant putative RNA-binding proteins, MAC5A and MAC5B, with sequence similarity to the human RNA-binding motif protein RBM22. MAC5A was previously isolated as a component of the MAC (Monaghan et al., 2009) but has otherwise not yet been studied in plants. We show that MAC5A localizes to the nucleus and interacts with AtCDC5 in planta, confirming its association with the MAC. In addition, we show that MAC5A and its close homolog MAC5B are partially redundant in a dosage-dependent manner and that a double mac5a mac5b mutant is lethal. Although single mac5a and mac5b mutants do not exhibit obvious enhanced susceptibility to pathogen infection, we found that mac5a-1 suppresses snc1-associated phenotypes. Overall, our results indicate that MAC5A is a component of the MAC that functions in the snc1 signaling pathway.
RESULTS
Isolation of mac5a, mac5b, and mac5c T-DNA Insertion Mutants
We used the MAC5A protein sequence as a query in BLAST and identified two additional proteins with significant sequence similarity to MAC5A (At1g07360) encoded in the Arabidopsis genome. We named these proteins MAC5B (At2g29580) and MAC5C (At5g07060). MAC5A and MAC5B are proteins of approximately 480 amino acids in length that are 82% identical and contain a CCCH-type zinc-finger domain and an RNA recognition motif (RRM; Fig. 1A; Supplemental Fig. S1). Conversely, MAC5C is a truncated protein of 363 amino acids that contains only a zinc-finger domain and no RRM (Fig. 1A; Supplemental Fig. S1). The phylogenetic relationship between these proteins indicates that MAC5A and MAC5B are more closely related to each other than to MAC5C (Addepalli and Hunt, 2008; Wang et al., 2008). In addition, according to publicly available microarray data (Toufighi et al., 2005; Winter et al., 2007), MAC5A and MAC5B are expressed in similar tissue types, although MAC5A is expressed at a much higher level (Supplemental Fig. S2). Conversely, MAC5C is expressed at very low levels in dry seeds, senescent leaves, and floral organs but not at all in any other tissues (Toufighi et al., 2005; Winter et al., 2007; Supplemental Fig. S2). MAC5A, MAC5B, and MAC5C share homology with the human protein RBM22/hECM2/fSAP47 (42%–50% identity at the amino acid level) and share very weak homology with the yeast protein Ecm2p/Slt11p (13%–16% identity; Supplemental Fig. S1). These proteins have been repeatedly isolated as components of the NTC/MAC in several eukaryotes (Ohi et al., 2002; Deckert et al., 2006; Gavin et al., 2006; Bessonov et al., 2008; Herold et al., 2009; Monaghan et al., 2009).
Figure 1.
Isolation of mac5a, mac5b, and mac5c loss-of-function mutants. A, Gene structures of MAC5A (At1g07360), MAC5B (At2g29580), and MAC5C (At5g07060) showing the positions of T-DNA insertions. Lines indicate introns, and boxes indicate exons. The locations of translation start (ATG) and stop (TGA) codons are indicated. The predicted protein domain structures are shown to the right of the gene structures. aa, Amino acids. The phylogenetic relationship between the encoded proteins is indicated to the left, as predicted by Addepalli and Hunt (2008) and Wang et al. (2008). B, Morphology of homozygous mac5a-1, mac5b-1, and mac5c-1 mutants compared with Col-0. Soil-grown plants were photographed 3 weeks after germination. Bar = 1 cm. [See online article for color version of this figure.]
To study the biological functions of MAC5A, MAC5B, and MAC5C, we obtained T-DNA insertion alleles from Arabidopsis seed centers. Salk_132881 (mac5a-1) carries an insertion in the second exon of MAC5A, whereas Salk_142085 (mac5a-2) and Salk_072670 (mac5a-3) carry insertions in the first intron of MAC5A (Fig. 1A). Semiquantitative reverse transcription (RT)-PCR indicated that all alleles were genuine mac5a knockout mutants, as no MAC5A transcript could be detected compared with ecotype Columbia (Col-0; Supplemental Fig. S3B). The leaves of these mac5a mutants appeared slightly serrated, twisty, and often exhibited elongated petioles (Fig. 1B; Supplemental Fig. S3A). In addition, mac5a mutants displayed shortened roots compared with Col-0 plants (Supplemental Fig. S3C), were early flowering (Supplemental Fig. S3A), and had reduced seed yield (data not shown). Only plants homozygous for the mac5a mutations displayed the same morphological phenotypes, indicating that the alleles are recessive and that the phenotypes cosegregate with the mutations. Importantly, F1 progeny from crosses between these mutants did not exhibit phenotypic complementation (Supplemental Fig. S3A), confirming that they are allelic.
GK419F11 carries an insertion in the first exon of MAC5B (Fig. 1A). This particular T-DNA insertion line is annotated as also being in the promoter region of PCNA2, a locus that neighbors MAC5B in the opposite orientation on chromosome II (Supplemental Fig. S4A). Because of this, we examined the expression levels of both genes in GK419F11 compared with Col-0 using semiquantitative RT-PCR. We found that expression of MAC5B was strongly reduced in this line, whereas the expression of PCNA2 was not affected (Supplemental Fig. S4B). This indicated that GK419F11 is a null allele of MAC5B, so we named it mac5b-1. Plants homozygous for the insertion did not exhibit any aberrant phenotypes and appeared morphologically similar to wild-type Col-0 plants (Fig. 1B). Although our in silico analysis indicated that MAC5C is barely expressed in Arabidopsis, we sought to test the biological function of this locus using a knockout mutant as well. For this, we obtained WiscDsLox262F09, which carries an insertion in the fourth intron of MAC5C, very close to an intron-exon junction (Fig. 1A). Like mac5b-1 mutant plants, these mac5c-1 plants also appeared indistinguishable from Col-0 (Fig. 1B).
MAC5A and MAC5B Are Unequally Redundant
Because of the sequence similarity between MAC5A, MAC5B, and MAC5C, we were interested to test for possible genetic redundancy between these loci. To do this, we crossed homozygous mutants together to create mac5a-1 mac5b-1, mac5a-1 mac5c-1, and mac5b-1 mac5c-1 double mutants. The mac5a-1 mac5c-1 double mutants appeared morphologically similar to mac5a-1 single mutants (Supplemental Fig. S5A), and the mac5b-1 mac5c-1 double mutants appeared morphologically similar to Col-0 wild-type plants (Supplemental Fig. S5B). These data suggest that there are no redundant functions shared between MAC5C and MAC5A or MAC5B. In contrast, MAC5A and MAC5B seem to perform some redundant functions in plants, as a double mac5a-1 mac5b-1 mutant is lethal. Out of 127 randomly chosen progeny from a parent that was homozygous for mac5b-1 but heterozygous for mac5a-1, 52 were wild type for MAC5A, 75 were heterozygous for mac5a-1, and none was homozygous for mac5a-1, indicating a 1:2:0 ratio rather than a normal 1:2:1 ratio. A χ2 test using these values indicates that a combination of mac5a-1 and mac5b-1 is lethal (χ2 = 3.263; P = 0.07). From another segregating population in which the parent was heterozygous for both mac5a-1 and mac5b-1, we recovered very few plants (typically one or two out of approximately 100 plants) that were homozygous for mac5a-1 and heterozygous for mac5b-1. These plants, in which there was only one functional copy of MAC5B and no functional copies of MAC5A, displayed severe developmental defects, including dwarfism, delayed growth, abnormal floral organs, and sterility (Fig. 2A). Terminal outgrowths known as enations were also observed extending from the leaf margins of these plants (Fig. 2B). To further investigate the redundant roles of MAC5A and MAC5B, we tested if overexpression of MAC5B can compensate for the loss of MAC5A in mac5a-1 mutants. For this, we stably expressed MAC5B under the control of the constitutive cauliflower mosaic virus P35S promoter in homozygous mac5a-1 plants by Agrobacterium tumefaciens-mediated transformation. Analysis of transgenic plants revealed that expression of P35S-MAC5B was indeed able to complement mac5a-1 (Fig. 2A). Overexpression of MAC5B in the transgenics was confirmed by semiquantitative RT-PCR (Fig. 2C). These results further supported redundant functions between MAC5A and MAC5B and suggested that the major difference between these loci is linked to their steady-state expression levels. Thus, because a double mac5a-1 mac5b-1 mutant is lethal and overexpression of MAC5B can compensate for the loss of MAC5A, we conclude that MAC5A and MAC5B are unequally redundant, as defined by Briggs et al. (2006), and that MAC5A is the dominant contributor of the pair.
Figure 2.
MAC5A and MAC5B are unequally redundant. A, Morphology of soil-grown plants photographed 4 weeks after planting. Bar = 1 cm. B, Closeup of a plant with the genotype mac5a-1/mac5a-1 MAC5B/mac5b-1 taken approximately 6 weeks after germination. Arrows indicate leaf enations. Bar = 1 cm. C, Expression analysis of MAC5A and MAC5B compared with ACTIN1 in the respective genotypes, as indicated by equal cycles of semiquantitative RT-PCR. Primers used to amplify the MAC5A and MAC5B transcripts are described in “Materials and Methods”; the MAC5A fragment shown above is 525 bp, and the MAC5B fragment is 232 bp. This experiment was repeated using cDNA isolated from four independent experiments with similar results. [See online article for color version of this figure.]
mac5a-1 Suppresses snc1 Autoimmunity
MOS4, AtCDC5, and MAC3A/3B are required for snc1-mediated immunity, as deduced from double mutant analysis between snc1 and mos4, Atcdc5, or mac3a mac3b mutants (Palma et al., 2007; Monaghan et al., 2009). To test if MAC5A, MAC5B, or MAC5C is likewise required for snc1 signaling, we created snc1 mac5a-1, snc1 mac5b-1, and snc1 mac5c-1 double mutants and conducted suppression analysis of snc1-related phenotypes. The loss of MAC5A (Fig. 3), but not MAC5B or MAC5C (Supplemental Fig. S6), suppressed snc1-associated dwarfism, as snc1 mac5a-1 plants were more than twice the size of snc1 (Fig. 3A). Heightened resistance to Hyaloperonospora arabidopsidis (H.a.) isolate Noco2 and Pseudomonas syringae pathovar tomato (P.s.t.) strain DC3000 was also suppressed in snc1 mac5a-1, as indicated by quantification of pathogen growth in these genotypes compared with the Col-0 wild type. As shown in Figure 3B, whereas H.a. Noco2 was unable to colonize snc1 plants, snc1 mac5a-1 mutants were as susceptible as Col-0 to this pathogen. Infection with P.s.t. DC3000 indicated that snc1 mac5a-1 plants sustain 100-fold higher bacterial growth over 3 d compared with snc1 (Fig. 3C). Furthermore, quantitative RT-PCR showed that the constitutive expression of PR-1 and PR-2 in snc1 is partially suppressed by mac5a-1 (Fig. 3D). Importantly, accumulation of the SNC1 transcript did not differ between the snc1 and snc1 mac5a-1 mutants (Fig. 3D), indicating that altered SNC1 expression is not the likely cause for the observed suppression.
Figure 3.
The loss of MAC5A suppresses snc1 autoimmune phenotypes. A, Morphology of Col-0 compared with snc1, mac5a-1, and the double snc1 mac5a-1 mutant. Soil-grown plants were photographed 4 weeks after planting. Bar = 1 cm. B, Growth of H.a. isolate Noco2 at 7 d after infection with 4 × 104 spores mL−1 water. Values represent averages of two replicates of 20 seedlings each ± sd. Statistically significant groups were calculated using multiple unpaired Student’s t tests comparing the means of all samples; a indicates no significant difference compared with Col-0, and b indicates a statistically significant difference from group a (P < 0.008). This experiment was repeated three times with similar results. FW, Fresh weight. C, Growth of P.s.t. DC3000 at 0 and 3 d after infection. Values represent averages of four replicates ± sd. Statistically significant groups were calculated using multiple unpaired Student’s t tests comparing the means of all samples; a indicates no significant difference compared with Col-0, and b indicates a statistically significant difference from group a (P < 0.0008). This experiment was repeated six times with similar results. cfu, Colony-forming units. D, Steady-state expression analysis of PR-1, PR-2, and SNC1 relative to Col-0 and normalized against ACTIN1 using quantitative RT-PCR. Values represent averages of three experimental replicates ± sd. This experiment was repeated using cDNA isolated from five independent trials with similar results. [See online article for color version of this figure.]
We previously demonstrated that MAC5A and MAC5B are unequally redundant and that MAC5A is the dominant contributor. Thus, it is likely that the reason mac5b-1 was unable to suppress snc1 autoimmunity was simply due to normal expression of MAC5A in the double mutants. To investigate the role of MAC5B in snc1 autoimmunity, we stably expressed P35S-MAC5B in snc1 mac5a-1 plants and tested for restoration of the snc1 phenotype. As a control, we also stably expressed PMAC5A-MAC5A in snc1 mac5a-1. Out of 15 P35S-MAC5B transgenics, nine restored snc1 morphology (Supplemental Fig. S7). Similarly, out of 12 PMAC5A-MAC5A transgenics, nine restored snc1 morphology (Supplemental Fig. S7). These results indicate that MAC5B can function in the snc1 pathway and further demonstrate unequal genetic redundancy between MAC5A and MAC5B in Arabidopsis.
The Loss of MAC5A or MAC5B Does Not Result in Enhanced Disease Susceptibility
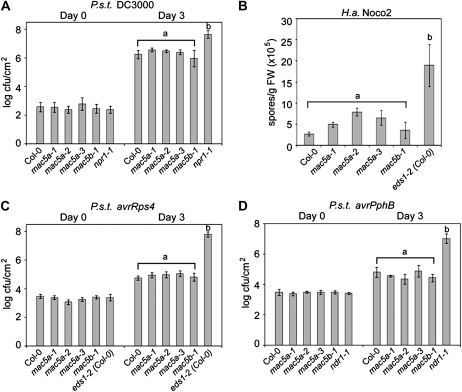
Mutations in MOS4, AtCDC5, PRL1, and MAC3A/3B lead to enhanced susceptibility to pathogen infection (Palma et al., 2007; Monaghan et al., 2009). To test if any of the three MAC5 homologs are necessary for the full expression of basal defense responses in Arabidopsis, mac5a, mac5b, and mac5c mutants were challenged with P.s.t. DC3000 and bacterial growth was assayed after 3 d. We observed similar bacterial growth in Col-0 and the mutants (Fig. 4A; Supplemental Fig. S8), whereas the well-known enhanced susceptibility mutant npr1-1 (Cao et al., 1994) harbored an over 10-fold higher titer of bacteria in all experiments. In addition, when we infected plants with H.a. Noco2, we consistently observed slightly higher oomycete colonization of the mac5a mutants compared with Col-0; however, this difference could not be repeatedly supported by statistical analysis (Fig. 4B).
Figure 4.
mac5a and mac5b mutants do not exhibit enhanced disease susceptibility. A, Growth of the bacterial pathogen P.s.t. DC3000 at 0 and 3 d postinoculation. Values represent averages of four replicates ± sd. Statistically significant groups were calculated using multiple unpaired Student’s t tests comparing the means of all samples; a indicates no significant difference compared with Col-0, and b indicates a statistically significant difference from group a (P < 0.0005). This experiment was repeated six times with similar results. cfu, Colony-forming units. B, Growth of H.a. isolate Noco2 at 7 d after infection with 2.5 × 104 spores mL−1 water. Values represent averages of four replicates of 20 seedlings each ± sd. Statistically significant groups were calculated using multiple unpaired Student’s t tests comparing the means of all samples; a indicates no significant difference compared with Col-0, and b indicates a statistically significant difference compared with group a (P < 0.005). This experiment was repeated three times with similar results. FW, Fresh weight. C and D, Growth of the bacterial pathogens P.s.t. avrRps4 and P.s.t. avrPphB at 0 and 3 d postinoculation. Values represent averages of four replicates ± sd. Statistically significant groups were calculated using multiple unpaired Student’s t tests comparing the means of all samples; a indicates no significant difference compared with Col-0, and b indicates a statistically significant difference from group a (P < 0.0001). These experiments were repeated six times with similar results.
To test if R protein-mediated defenses other than snc1 autoimmunity require MAC5A or MAC5B, we also infected plants with pathogens that express avirulence effectors specifically recognized by R proteins in Col-0. P.s.t. avrRps4 and P.s.t. avrPphB (recognized by RPS4 and RPS5, respectively) grew to similar levels in Col-0 and the mac5a and mac5b mutants (Fig. 4, C and D). In addition, the H.a. isolates Emwa1 and Cala2, expressing avirulence effectors recognized by RPP4 and RPP2, respectively, grew to similar levels in Col-0 and mac5a-1 (data not shown), and both genotypes were able to mount a hypersensitive response at infection sites, as indicated by lactophenol trypan blue staining on leaf tissue 7 d after inoculation (Supplemental Fig. S9). Together, these data reveal that although MAC5A is required for snc1-mediated defense, MAC5A and MAC5B may not be required for other R protein-mediated pathways or basal defense in plants. However, the involvement of MAC5A or MAC5B in these pathways may be masked by the partial redundancy between the two loci.
MAC5A Localizes to the Nucleus and Associates with AtCDC5 in Planta
To confirm that MAC5A is a MAC component, we tested whether MAC5A localizes to the nucleus and if it is capable of associating with the MAC protein AtCDC5 in a coimmunoprecipitation assay. For this, we stably transformed either PMAC5A-MAC5A-GFP or PMAC5A-MAC5A-HA into mac5a-1 plants by Agrobacterium-mediated transformation. The majority of transgenic lines (24 of 25 independent GFP lines and 21 of 22 independent hemagglutinin [HA] lines) complemented mac5a-1 phenotypes such as morphology (Fig. 5A) and root length (Fig. 5B). Because these fusion proteins complemented mac5a-1 phenotypes, they should function similarly to endogenous MAC5A. We confirmed that the fusion proteins were expressed in vivo by extracting total protein from representative transgenic lines followed by western-blot analysis using antibodies against GFP or HA (data not shown).
Figure 5.
Subcellular localization of MAC5A-GFP. A, Full-length genomic MAC5A-GFP driven by its native promoter complements mac5a-1 morphology. Soil-grown plants were photographed 4 weeks after germination. Bar = 1 cm. B, Root length assay demonstrating complementation of mac5a-1 by the MAC5A-GFP transgene. Plants were grown on MS medium supplemented with 0.5% Suc and 0.3% phytagel for 8 d. Values represent means of 10 replicates ± sd. Statistically significant groups were calculated using multiple unpaired Student’s t tests comparing the means of all samples against Col-0; a indicates no significant difference compared with Col-0, and b indicates a statistically significant difference from group a (P < 0.0001). This experiment was repeated three times with similar results. C, MAC5A-GFP localizes to the nucleus. Confocal microscopy was used to examine the localization of MAC5A-GFP in the transgenic line shown in A. Root cells from a representative line are shown. 4,6-Diamidino-2-phenylindole (DAPI) was used as a control for nuclear localization. DIC, Differential interference contrast.
All MAC5A-GFP complementing lines showed clear nuclear localization when viewed with a fluorescence microscope. Root cells from one representative line are shown in Figure 5C, but nuclear localization was also observed in other tissues, including leaves (data not shown). This localization was not unexpected, as the MAC5A homolog in zebrafish, RBM22, also localizes to the nucleus (Montaville et al., 2006), as do the MAC core proteins MOS4, AtCDC5, PRL1, and MAC3A/3B. To test if MAC5A associates with AtCDC5, we isolated total nuclear protein and immunoprecipitated MAC5A-HA using anti-HA microbeads. Western-blot analysis using an anti-AtCDC5 antibody revealed that AtCDC5 was present only in the eluted fraction from transgenic plants and not from control Col-0 plants (Fig. 6). Association between MAC5A and AtCDC5 has been shown in other eukaryotes as well (McDonald et al., 1999; Ohi et al., 2002; Deckert et al., 2006; Gavin et al., 2006; Bessonov et al., 2008). These data indicate that MAC5A localizes to the nucleus and associates with AtCDC5 in planta. Thus, we conclude that MAC5A is indeed a member of the MAC.
Figure 6.
MAC5A-HA and AtCDC5 coimmunoprecipitate in planta. Total nuclear extracts were isolated from a complementing mac5a-1 transgenic line expressing PMAC5A-MAC5A-HA (+) and Col-0 (−). MAC5A-HA was immunoprecipitated using anti-HA microbeads. MAC5A-HA and AtCDC5 were detected in the eluted fractions (20× concentrated) by western-blot analysis using antibodies against HA or AtCDC5.
Analysis of Constitutive Splicing in Higher Order MAC Mutants
Because of the close association between the MAC and the spliceosome, we previously tested if general splicing was impaired in the MAC mutants. For this, we monitored the presence of several known alternative transcripts in MAC mutants compared with Col-0 (Palma et al., 2007). We did not observe obvious differences in the presence of U1-70K, AtSRp34/SR1, or AtSRp30 alternative transcript variants (Palma et al., 2007), leading us to conclude that the MAC is not required for general splicing in Arabidopsis. However, although we have been unable to show aberrations in general/constitutive splicing, the MAC as a whole appears to be essential for plant viability, as all higher order MAC mutants so far described (mos4-1 Atcdc5-1, mos4-1 prl1-1, mos4-1 mac3a mac3b, and mac3a mac3b prl1-1) result in lethality (Palma et al., 2007; Monaghan et al., 2009). Probably because of the partial redundancy between MAC5A and MAC5B, we were able to successfully isolate double mutants when we crossed mos4-1, Atcdc5-1, prl1-2, or mac3a mac3b with mac5a-1. The morphological phenotypes of the double mutants looked like a combination of phenotypes between the respective loci (Fig. 7A). The most striking phenotype was observed with mac5a-1 prl1-2 double mutant plants, which exhibited severe developmental defects and often displayed leaf enations similar to the mac5a-1/mac5a-1 MAC5B/mac5b-1 mutant. Interestingly, when we infected plants with P.s.t. DC3000 and monitored growth 3 d later, we did not observe a further increase in bacterial titer in any of the combination mutants (Supplemental Fig. S10; data not shown), indicating that the additional loss of MAC5A does not further impair basal defenses. When we tested the fidelity of the splicing machinery in these higher order MAC mutants, we again did not observe any changes in the presence of U1-70K, AtSRp34/SR1, or AtSRp30 alternative transcript variants (Fig. 7B). We also did not observe any changes in these transcripts in the mac5a-1/mac5a-1 MAC5B/mac5b-1 mutant (data not shown). These data indicate that the spliceosome is functional in MAC single and higher order mutants and further demonstrate that, individually, MOS4, AtCDC5, PRL1, MAC3A/3B, and MAC5A/5B do not contribute key functions to general splicing in plants, even though the MAC is closely associated with proteins that form the spliceosome. It is important to keep in mind, however, that our analysis is not stringent enough to observe even severalfold differences in the abundance of splice variants, so we are not able to address splicing efficiency in the MAC mutants at this time. We also cannot rule out the possible involvement of the MAC in specific splicing events such as those that may be important during the plant immune response.
Figure 7.
Analysis of constitutive splicing in higher order MAC mutants. A, Morphology of soil-grown plants photographed 4 weeks after planting. Bar = 1 cm. B, The presence of alternative transcript variants for the genes UI-70K, AtSRp34/SR1, and AtSRp30 was monitored in Col-0 compared with the MAC mutants mos4-1, Atcdc5-1, prl1-2, mac3a mac3b, mac5a-1, and the higher order mutants. An equal amount of RNA from all genotypes was used to make cDNA for subsequent analysis by semiquantitative RT-PCR. TUBULIN was included as a control. This experiment was repeated four times from independent trials. The schematic to the right represents the nature of the alternative transcript variants as adapted from Palma et al. (2007). [See online article for color version of this figure.]
DISCUSSION
The MAC is a spliceosome-associated protein complex homologous to the well-studied NTC in yeast and the CDC5L complex in human (Palma et al., 2007; Monaghan et al., 2009). It contains proteins with diverse functions, including the scaffolding protein MOS4, the Myb transcription factor AtCDC5, the WD-40 protein PRL1, the E3 ubiquitin ligases MAC3A and MAC3B, as well as several nucleic acid-binding proteins and small nuclear ribonucleoprotein (snRNP) subunits that are predicted to be integral components of the spliceosome (Monaghan et al., 2009). Here, we present evidence suggesting that the putative RNA-binding protein MAC5A is unequally redundant with its close homolog MAC5B and functions in snc1-mediated autoimmunity as a component of the MAC.
MAC5A and MAC5B are predicted to be RNA-binding proteins by virtue of containing both an RRM and a CCCH-type zinc-finger motif. Although the intrinsic ability of MAC5A/5B to bind RNA species has yet to be shown experimentally, proteins containing CCCH zinc fingers or RRMs have been demonstrated to bind RNA in vitro (Jessen et al., 1991; Burd and Dreyfuss, 1994; Wang et al., 2008). The most abundant RNA-binding domain in eukaryotes is the RRM (Lorković and Barta, 2002), often found in proteins also containing CCCH motifs, such as in MAC5A/5B. There are 196 RRM-containing proteins encoded in the Arabidopsis genome, including several predicted poly(A)-binding proteins, Arg-rich (SR) proteins, and snRNPs (Lorković and Barta, 2002). Importantly, it has been demonstrated that the RRM is necessary and sufficient for RNA binding (Jessen et al., 1991; Burd and Dreyfuss, 1994). In addition to the RRM, there are several other RNA-binding domains, such as the CCCH zinc-finger motif. This family is also fairly large in Arabidopsis, with 68 proteins encoded in the genome (Wang et al., 2008). Whereas most zinc fingers are involved in DNA binding, several zinc-finger-containing CCCH motifs have been shown to function posttranscriptionally and to have RNA-binding capability (Wang et al., 2008). For example, the Arabidopsis protein ENHANCER OF AG-4, 1 contains six tandem CCCH motifs and binds RNA in vitro (Li et al., 2001a). Likewise, the Arabidopsis cleavage and polyadenylation specificity factor AtCPSF30 contains three tandem CCCH motifs and has also been shown to bind RNA (Delaney et al., 2006; Wang et al., 2008). Interestingly, a recent study demonstrated that five CCCH zinc-finger proteins in Arabidopsis, representing proteins from diverse subfamilies, are capable of degrading RNA in vitro, uncovering the intriguing possibility that ribonuclease activity may be a common property of CCCH proteins in Arabidopsis (Addepalli and Hunt, 2008).
It is not hard to imagine roles for RNA-binding proteins and/or ribonucleases in plant defense and snc1 autoimmunity. A large number of genes are differentially regulated following the perception of pathogens, and this massive change in gene expression must be correlated with subsequent RNA processing and/or degradation events. A relevant example is the Gly-rich RRM-containing protein AtGRP7, which was recently shown to be targeted by HopU1, a Pseudomonas type III effector with mono-ADP-ribosyltransferase activity, to suppress the plant immune response and increase virulence (Fu et al., 2007). Loss-of-function Atgrp7 mutants were shown to display enhanced susceptibility to infection by Pseudomonas in the same study. In addition, MOS2, a protein containing RNA-binding G-patch and KOW motifs, is likewise required for innate immunity in Arabidopsis (Zhang et al., 2005), although the precise function of this protein is unclear. Our finding that MAC5A is partly required for snc1-mediated autoimmunity points to a possible role for this RNA-binding protein in plant defense. However, we did not observe enhanced susceptibility in mac5a or mac5b single mutants when we infected plants with a variety of pathogens, possibly complicated by the partial redundancy between MAC5A and MAC5B. The lethality of the mac5a-1 mac5b-1 double mutant prevented us from testing whether plants lacking both MAC5A and MAC5B exhibit enhanced disease susceptibility like the other MAC mutants characterized to date.
Importantly, whereas the MAC core components MOS4, AtCDC5, PRL1, and MAC3A/3B are not encoded by essential genes in Arabidopsis, both MAC5A and MAC5B are required for plant viability. Because of the physical association between the MAC and components of the spliceosome, it is very likely that the essential functions employed by MAC5A and MAC5B have something to do with RNA processing. That said, we have not yet been able to directly link the function of the MAC to splicing. We can only speculate that this association has to do with transcriptional coregulation or alternative splicing of currently unknown gene targets. The presence of transcript variants for three known alternatively spliced genes, U1-70K, AtSRp34/SR1, and AtSRp30, was not affected in mac5a-1, mac5a-1/mac5a-1 MAC5B/mac5b-1, or any other MAC mutants, broadly suggesting that the spliceosome is functional despite these lesions. And yet, the loss of any two MAC components in combination results in synthetic lethality, indicating that the MAC as a whole is required for an essential function, such as splicing or spliceosome assembly, as in yeast (Chan et al., 2003). In this respect, the identification of MAC substrates and downstream signaling components is of particular interest. Uncovering what genes are regulated by AtCDC5, what proteins are ubiquitinated by MAC3A/3B, and what RNA species are bound by MAC5A/5B will truly allow us to elucidate the molecular function of the MAC. A major hurdle, however, will be deciphering which downstream components are specific to the plant defense response. It is clear that MAC proteins are employed by several signaling networks, as indicated by the pleiotropic phenotypes observed for MAC mutants described to date. All MAC mutants, to one extent or another, show defects in flowering time, leaf development, fertility, root length, hormone signaling, sugar signaling, and innate immunity (Németh et al., 1998; Palma et al., 2007; Monaghan et al., 2009; J. Monaghan and X. Li, unpublished data). Based on these observations, it is possible that the MAC acts as a key regulatory node utilized by many signaling pathways in plants. A joint effort to study the molecular function of this complex across kingdoms could shed light on the role of the MAC/NTC in signal transduction relay and may lead to a better understanding of how plants utilize this complex to achieve immunity.
MATERIALS AND METHODS
Plant Growth and Pathology Assays
Arabidopsis (Arabidopsis thaliana) plants were grown on soil in a 16-h-light/8-h-dark regime or on Murashige and Skoog (MS) medium supplemented with 0.5% Suc and 0.3% phytagel or 0.8% agar, depending on the experiment. Bacterial and oomycete infections were performed as described (Li et al., 2001b). Briefly, bacterial pathogens were inoculated on the abaxial leaf surfaces of 4-week-old plants using a needless syringe. Leaf discs (with an area of 0.38 cm2) were collected on the day of infection (day 0) and 3 d later (day 3) from different plants. Hyaloperonospora arabidopsidis isolates were spray inoculated onto adaxial leaf surfaces of 2-week-old seedlings and stained using lactophenol trypan blue 7 d later using a protocol described by Koch and Slusarenko (1990).
Mutant Isolation and Genetic Crosses
T-DNA mutants were obtained from the Arabidopsis Biological Resource Center or Nottingham Arabidopsis Stock Centre and genotyped by PCR using primers flanking the insertions. mac5a-1 was isolated and genotyped using the primers 5′-CACTCCTTAGGGGAGGTATC-3′ and 5′-GGTGTTTAGGTGGCGACCTGG-3′. The other two alleles, mac5a-2 and mac5a-3, were identified first by phenotypic comparison with mac5a-1 and then confirmed by crossing to test for allelism. For this, homozygous mac5a-1 females were crossed with males homozygous for either the mac5a-2 or mac5a-3 allele, and the F1 progeny were analyzed for complementation. Heterozygosity at the mac5a-1 locus in the F1 plants was confirmed by genotyping. mac5b-1 was isolated and genotyped using the primers 5′-CAGCTTCAACACTAAGAAAC-3′ and 5′-TAGAGTGTGGATCGAAACGG-3′. mac5c-1 was isolated and genotyped using the primers 5′-GGTCAGTGTAAAAGAGGTGCC-3′ and 5′-GCTTGAACCATCACTCTCTTG-3′. Isolation and genotyping of snc1 (Li et al., 2001b; Zhang et al., 2003), mos4-1, Atcdc5-1, and prl1-2 (Palma et al., 2007), and mac3a mac3b (Monaghan et al., 2009) have been previously described, as has the isolation of npr1-1 (Cao et al., 1994), eds1-2 (Col-0; Bartsch et al., 2006), and ndr1 (Century et al., 1995). The snc1 mac5a-1, snc1 mac5b-1, and snc1 mac5c-1 double mutants were obtained by crossing a homozygous snc1 single mutant plant with homozygous mac5a-1, mac5b-1, or mac5c-1 plants. The F1 progeny were allowed to self, and the doubles were isolated in the F2 generation using a combination of phenotyping and PCR-based genotyping. A similar procedure was used to create the mac5a-1 mac5b-1, mac5a-1 mac5c-1, mac5b-1 mac5c-1, mac5a-1 mos4-1, mac5a-1 Atcdc5-1, mac5a-1 mac3a mac3b, and mac5a-1 prl1-2 double and triple mutants. All genotypes were confirmed by genotyping with mutation-specific primers.
Molecular Cloning and Expression Analysis
A genomic fragment spanning the full-length MAC5A gene including its native promoter was amplified from Col-0 DNA using the primers 5′-CGGGGTACCCGGTTCCAATGTCACCGGCAG-3′ (KpnI) and 5′-AAAACTGCAGCTGAGACGAACCAGTAGCTGT-3′ (PstI), cloned into pGreen0229 in frame with a C-terminal HA or GFP tag (Hellens et al., 2000), and confirmed by sequencing. The open reading frame of MAC5B was amplified from Col-0 cDNA using the primers 5′-CACCATGGCGCATAGAATACTGAG-3′ and 5′-TTGAGACGAACCAGTAGTAAC-3′. This Gateway-adapted PCR fragment was cloned into pENTR using the Gateway pENTR/D-Topo Kit (Invitrogen). Entry vectors were confirmed by sequencing using the M13F and M13R primers. Recombination into a destination binary vector containing a constitutive 35S promoter was carried out with Gateway LR Clonase (Invitrogen). Transgenic seedlings were selected on soil with the herbicide Basta and confirmed by PCR. For mRNA expression analysis, RNA was extracted from 20-d-old seedlings grown on MS medium using the Totally RNA Kit (Ambion). RT was performed using SuperScript II reverse transcriptase (Invitrogen). The primers used to monitor MAC5A expression were either 5′-TGCAAGATATGTACACGACC-3′ and 5′-AGTGCCCATCTCACCAGCTT-3′ (fragment size, 525 bp) or 5′-ACGCGTCGACGATGGCTCACAGAATACTGAG-3′ and 5′-ATAGTTTAGCGGCCGCCGTCCTGAATAGCGGGAAAC-3′ (full-length transcript, 1,528 bp). The primers used for MAC5B expression were 5′-GCAAATCTGCTCTTCAAGGT-3′ and 5′-GCCGGGTACAGATCTTACAC-3′. The primers used to monitor the expression of PCNA2 were 5′-GATGGTAGCGACACTGTTAC-3′ and 5′-CCGATATCACCTGCTGTTGA-3′. The primers used to amplify PR-1, PR-2, and ACTIN1 have been described previously (Zhang et al., 2003). Primers used to detect alternative transcript variants for U1-70K, AtSRp34/SR1, and AtSRp30 are described by Savaldi-Goldstein et al. (2003). TUBULIN was amplified using the primers 5′-ACGTATCGATGTCTATTTCAACG-3′ and 5′-ATATCGTAGAGAGCCTCATTGTCC-3′.
Nuclear Protein Extraction and Immunoprecipitation
Approximately 15 g of leaf tissue from complementing mac5a-1 plants expressing PMAC5A-MAC5A-HA was used to isolate the nuclear protein fraction with a procedure described by Monaghan et al. (2009). Immunoprecipitation was carried out using anti-HA microbeads (Miltenyi Biotec) as described (Monaghan et al., 2009). The eluted fraction was loaded on a 12% SDS-PAGE gel followed by western-blot analysis using anti-HA (Roche) or anti-AtCDC5 (Palma et al., 2007) antibodies.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NP_563788, NP_180518.1, NP_196323.1, NP_193422.1, Q94BR4, ACO38702, NP_566599, NP_172448, CAA58031, and NP_180517.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Multiple sequence alignment of MAC5A homologs in Arabidopsis, human, and budding yeast.
Supplemental Figure S2. Differential expression of MAC5A, MAC5B, and MAC5C in Arabidopsis development.
Supplemental Figure S3. The mac5a-1, mac5a-2, and mac5-3 mutants are allelic.
Supplemental Figure S4. Expression analysis of PCNA2 and MAC5B in the mac5b-1 mutant.
Supplemental Figure S5. Morphology of mac5a-1 mac5c-1 and mac5b-1 mac5c-1 double mutants.
Supplemental Figure S6. mac5b-1 and mac5c-1 do not suppress snc1 autoimmunity.
Supplemental Figure S7. Overexpression of MAC5B restores snc1 morphology in snc1 mac5a-1.
Supplemental Figure S8. Growth of P.s.t. DC3000 in mac5c-1.
Supplemental Figure S9. mac5a-1 is resistant against H.a. isolates Emwa1 and Cala2.
Supplemental Figure S10. Growth of P.s.t. DC3000 in higher order MAC mutants.
Supplemental Table S1. Summary of phenotypes reported for MAC mutants in Arabidopsis.
Supplementary Material
Acknowledgments
We thank Mark Tessaro (University of British Columbia) for his assistance isolating T-DNA insertion lines provided by the Arabidopsis Biological Resource Center (The Ohio State University). Prof. Brian Ellis (University of British Columbia), Prof. Lacey Samuels (University of British Columbia), and Prof. Jean Greenberg (University of Chicago) provided helpful comments on an earlier version of the manuscript.
References
- Addepalli B, Hunt AG. (2008) Ribonuclease activity is a common property of Arabidopsis CCCH-containing zinc-finger proteins. FEBS Lett 582: 2577–2582 [DOI] [PubMed] [Google Scholar]
- Ajuh P, Kuster B, Panov K, Zomerdijk JC, Mann M, Lamond AI. (2000) Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry. EMBO J 19: 6569–6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE. (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18: 1038–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessonov S, Anokhina M, Will CL, Urlaub H, Lührmann R. (2008) Isolation of an active step I spliceosome and composition of its RNP core. Nature 452: 846–850 [DOI] [PubMed] [Google Scholar]
- Briggs GC, Osmont KS, Shindo C, Sibout R, Hardtke CS. (2006) Unequal genetic redundancies in Arabidopsis: a neglected phenomenon? Trends Plant Sci 11: 492–498 [DOI] [PubMed] [Google Scholar]
- Burd CG, Dreyfuss G. (1994) Conserved structures and diversity of functions of RNA-binding proteins. Science 265: 615–621 [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century KS, Holub EB, Staskawicz BJ. (1995) NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc Natl Acad Sci USA 92: 6597–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SP, Kao DI, Tsai WY, Cheng SC. (2003) The Prp19p-associated complex in spliceosome activation. Science 302: 279–282 [DOI] [PubMed] [Google Scholar]
- Cheng YT, Germain H, Wiermer M, Bi D, Xu F, García AV, Wirthmueller L, Després C, Parker JE, Zhang Y, et al. (2009) Nuclear pore complex component MOS7/Nup88 is required for innate immunity and nuclear accumulation of defense regulators in Arabidopsis. Plant Cell 21: 2503–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Hartmuth K, Boehringer D, Behzadnia N, Will CL, Kastner B, Stark H, Urlaub H, Lührmann R. (2006) Protein composition and electron microscopy structure of affinity-purified human spliceosomal B complexes isolated under physiological conditions. Mol Cell Biol 26: 5528–5543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KJ, Xu R, Zhang J, Li QQ, Yun KY, Falcone DL, Hunt AG. (2006) Calmodulin interacts with and regulates the RNA-binding activity of an Arabidopsis polyadenylation factor subunit. Plant Physiol 140: 1507–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZQ, Guo M, Jeong BR, Tian F, Elthon TE, Cerny RL, Staiger D, Alfano JR. (2007) A type III effector ADP-ribosylates RNA-binding proteins and quells plant immunity. Nature 447: 284–288 [DOI] [PubMed] [Google Scholar]
- Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, Marzioch M, Rau C, Jensen LJ, Bastuck S, Dümpelfeld B, et al. (2006) Proteome survey reveals modularity of the yeast cell machinery. Nature 440: 631–636 [DOI] [PubMed] [Google Scholar]
- Goritschnig S, Weihmann T, Zhang Y, Fobert P, McCourt P, Li X. (2008) A novel role for protein farnesylation in plant innate immunity. Plant Physiol 148: 348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritschnig S, Zhang Y, Li X. (2007) The ubiquitin pathway is required for innate immunity in Arabidopsis. Plant J 49: 540–551 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Herold N, Will CL, Wolf E, Kastner B, Urlaub H, Lührmann R. (2009) Conservation of the protein composition and electron microscopy structure of Drosophila melanogaster and human spliceosomal complexes. Mol Cell Biol 29: 281–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr AJ, Molnàr A, Jones A, Baulcombe DC. (2006) Defective RNA processing enhances RNA silencing and influences flowering of Arabidopsis. Proc Natl Acad Sci USA 103: 14994–15001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen TH, Oubridge C, Teo CH, Pritchard C, Nagai K. (1991) Identification of molecular contacts between the U1 A small nuclear ribonucleoprotein and U1 RNA. EMBO J 10: 3447–3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Koch E, Slusarenko A. (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jia D, Chen X. (2001a) HUA1, a regulator of stamen and carpel identities in Arabidopsis, codes for a nuclear RNA binding protein. Plant Cell 13: 2269–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Clarke JD, Zhang Y, Dong X. (2001b) Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol Plant Microbe Interact 14: 1131–1139 [DOI] [PubMed] [Google Scholar]
- Lim GH, Zhang X, Chung MS, Lee DJ, Woo YM, Cheong HS, Kim CS. (2010) A putative novel transcription factor, AtSKIP, is involved in abscisic acid signaling and confers salt and osmotic tolerance in Arabidopsis. New Phytol 185: 103–113 [DOI] [PubMed] [Google Scholar]
- Liu M, Yuan L, Liu NY, Shi DQ, Liu J, Yang WC. (2009) GAMETOPHYTIC FACTOR 1, involved in pre-mRNA splicing, is essential for megagametogenesis and embryogenesis in Arabidopsis. J Integr Plant Biol 51: 261–271 [DOI] [PubMed] [Google Scholar]
- Lorković ZJ, Barta A. (2002) Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res 30: 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasik E, Takken FL. (2009) STANDing strong, resistance proteins instigators of plant defence. Curr Opin Plant Biol 12: 427–436 [DOI] [PubMed] [Google Scholar]
- McDonald WH, Ohi R, Smelkova N, Frendewey D, Gould KL. (1999) Myb-related fission yeast cdc5p is a component of a 40S snRNP-containing complex and is essential for pre-mRNA splicing. Mol Cell Biol 19: 5352–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll C, von Lyncker L, Zimmermann S, Kägi C, Baumann N, Twell D, Grossniklaus U, Gross-Hardt R. (2008) CLO/GFA1 and ATO are novel regulators of gametic cell fate in plants. Plant J 56: 913–921 [DOI] [PubMed] [Google Scholar]
- Monaghan J, Xu F, Gao M, Zhao Q, Palma K, Long C, Chen S, Zhang Y, Li X. (2009) Two Prp19-like U-box proteins in the MOS4-associated complex play redundant roles in plant innate immunity. PLoS Pathog 5: e1000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaville P, Dai Y, Cheung CY, Giller K, Becker S, Michalak M, Webb SE, Miller AL, Krebs J. (2006) Nuclear translocation of the calcium-binding protein ALG-2 induced by the RNA-binding protein RBM22. Biochim Biophys Acta 1763: 1335–1343 [DOI] [PubMed] [Google Scholar]
- Németh K, Salchert K, Putnoky P, Bhalerao R, Koncz-Kálmán Z, Stankovic-Stangeland B, Bakó L, Mathur J, Okrész L, Stabel S, et al. (1998) Pleiotropic control of glucose and hormone responses by PRL1, a nuclear WD protein, in Arabidopsis. Genes Dev 12: 3059–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi MD, Link AJ, Ren L, Jennings JL, McDonald WH, Gould KL. (2002) Proteomics analysis reveals stable multiprotein complexes in both fission and budding yeasts containing Myb-related Cdc5p/Cef1p, novel pre-mRNA splicing factors, and snRNAs. Mol Cell Biol 22: 2011–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Yu HJ, Ngo QA, Rajani S, Mayalagu S, Johnson CS, Capron A, Xie LF, Ye D, Sundaresan V. (2005) Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132: 603–614 [DOI] [PubMed] [Google Scholar]
- Palma K, Zhang Y, Li X. (2005) An importin alpha homolog, MOS6, plays an important role in plant innate immunity. Curr Biol 15: 1129–1135 [DOI] [PubMed] [Google Scholar]
- Palma K, Zhao Q, Cheng YT, Bi D, Monaghan J, Cheng W, Zhang Y, Li X. (2007) Regulation of plant innate immunity by three proteins in a complex conserved across the plant and animal kingdoms. Genes Dev 21: 1484–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Aviv D, Davydov O, Fluhr R. (2003) Alternative splicing modulation by a LAMMER kinase impinges on developmental and transcriptome expression. Plant Cell 15: 926–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarn WY, Hsu CH, Huang KT, Chen HR, Kao HY, Lee KR, Cheng SC. (1994) Functional association of essential splicing factor(s) with PRP19 in a protein complex. EMBO J 13: 2421–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufighi K, Brady SM, Austin R, Ly E, Provart NJ. (2005) The Botany Array Resource: e-northerns, expression angling, and promoter analyses. Plant J 43: 153–163 [DOI] [PubMed] [Google Scholar]
- Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D, et al. (2004) Identification of genes required for embryo development in Arabidopsis. Plant Physiol 135: 1206–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Guo Y, Wu C, Yang G, Li Y, Zheng C. (2008) Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genomics 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. (2007) An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi N, Takeda S, Matsumoto N, Okada K. (2009) VAJ/GFA1/CLO is involved in the directional control of floral organ growth. Plant Cell Physiol 50: 515–527 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cheng YT, Bi D, Palma K, Li X. (2005) MOS2, a protein containing G-patch and KOW motifs, is essential for innate immunity in Arabidopsis thaliana. Curr Biol 15: 1936–1942 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goritschnig S, Dong X, Li X. (2003) A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15: 2636–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X. (2005) A putative nucleoporin 96 is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1,constitutive 1. Plant Cell 17: 1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. (2009) Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12: 414–420 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.