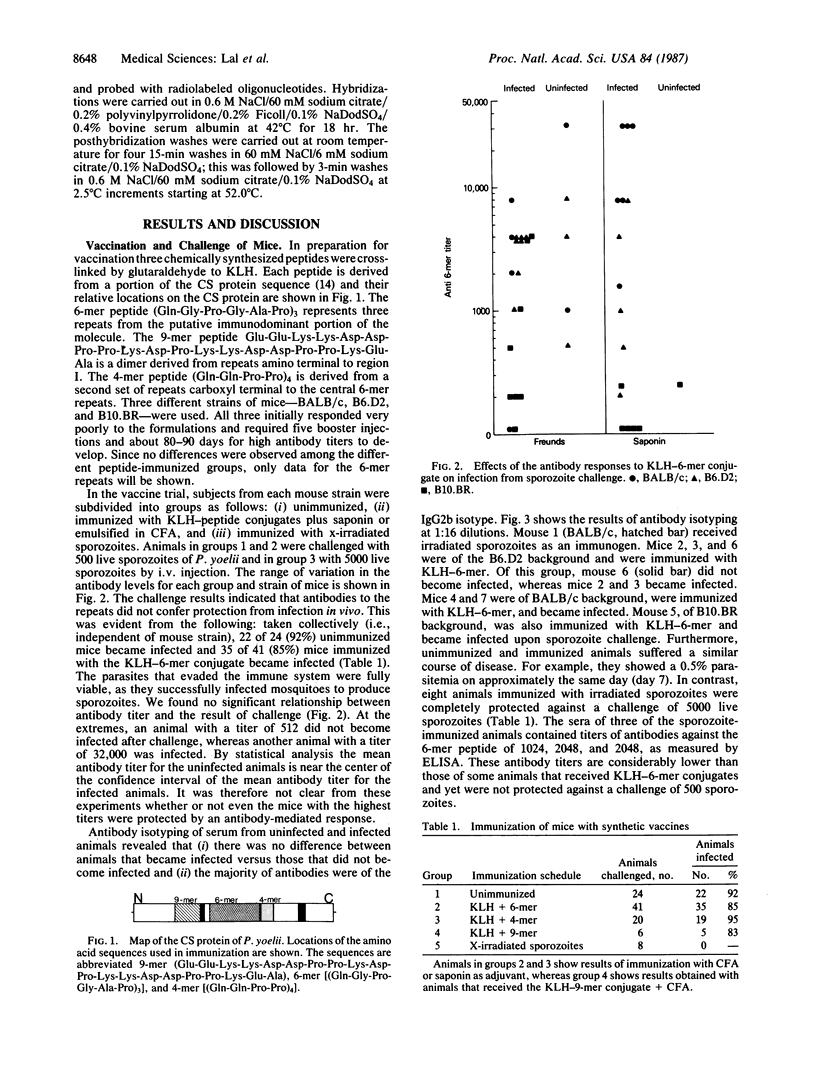
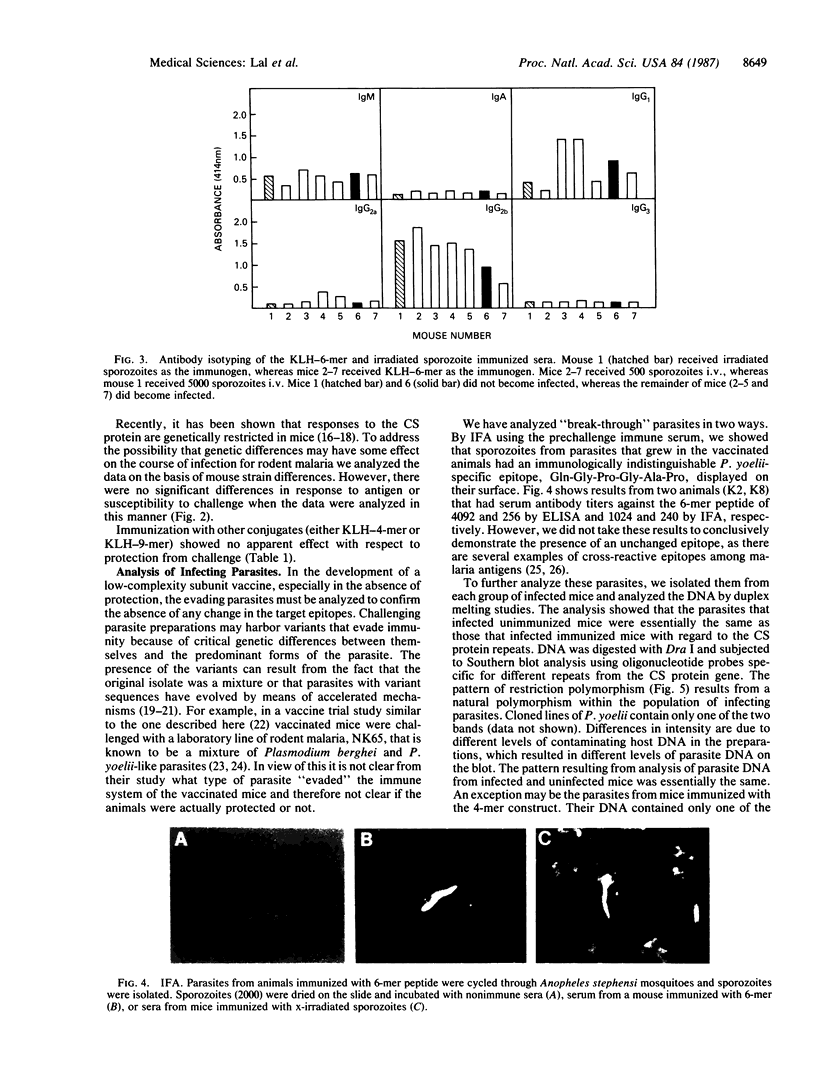
Abstract
To test the putative in vivo protective effects of antibodies to circumsporozoite (CS) protein repeats against malarial infection, different strains of mice were immunized against various repetitive regions of the Plasmodium yoelii CS protein in the form of synthetic peptides conjugated to keyhole limpet hemocyanin. Complete Freund's adjuvant or saponin was used as adjuvant. When vaccinated mice were challenged with 500 sporozoites almost all animals became infected. There were no significant protective effects in vaccinated versus unvaccinated mice. Furthermore, there was no correlation between the antibody titer to the CS repeats and infection. The parasites from infected animals were shown to encode a CS protein containing the same repeats as those used for immunization, indicating that the infections were not due to selection for variant parasites. These experiments demonstrate that antibodies to the CS repeats, as derived in vivo with peptides, despite being surface reactive, do not provide protection against sporozoite challenge in vivo. This conclusion is in contrast to previous conclusions based on studies showing protection by way of in vitro sporozoite neutralization procedures and passive transfer of monoclonal antibody.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986 Nov;8(6):529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Casaglia O., Dore E., Frontali C., Zenobi P., Walliker D. Re-examination of earlier work on repetitive DNA and mosquito infectivity in rodent malaria. Mol Biochem Parasitol. 1985 Jun;16(1):35–42. doi: 10.1016/0166-6851(85)90047-7. [DOI] [PubMed] [Google Scholar]
- Clyde D. F., Most H., McCarthy V. C., Vanderberg J. P. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973 Sep;266(3):169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- Conner B. J., Reyes A. A., Morin C., Itakura K., Teplitz R. L., Wallace R. B. Detection of sickle cell beta S-globin allele by hybridization with synthetic oligonucleotides. Proc Natl Acad Sci U S A. 1983 Jan;80(1):278–282. doi: 10.1073/pnas.80.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame J. B., McCutchan T. F. Cloning and characterization of a ribosomal RNA gene from Plasmodium berghei. Mol Biochem Parasitol. 1983 Jul;8(3):263–279. doi: 10.1016/0166-6851(83)90048-8. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Egan J. E., Weber J. L., Ballou W. R., Hollingdale M. R., Majarian W. R., Gordon D. M., Maloy W. L., Hoffman S. L., Wirtz R. A., Schneider I. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- Fine E., Aikawa M., Cochrane A. H., Nussenzweig R. S. Immuno-electron microscopic observations on Plasmodium knowlesi sporozoites: localization of protective antigen and its precursors. Am J Trop Med Hyg. 1984 Mar;33(2):220–226. doi: 10.4269/ajtmh.1984.33.220. [DOI] [PubMed] [Google Scholar]
- Galinski M. R., Arnot D. E., Cochrane A. H., Barnwell J. W., Nussenzweig R. S., Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987 Jan 30;48(2):311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz R. W., Cochrane A. H., Nussenzweig V., Nussenzweig R. S. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ. 1979;57 (Suppl 1):165–173. [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Nardin E. H., Tharavanij S., Schwartz A. L., Nussenzweig R. S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984 Feb;132(2):909–913. [PubMed] [Google Scholar]
- Hollingdale M. R., Zavala F., Nussenzweig R. S., Nussenzweig V. Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J Immunol. 1982 Apr;128(4):1929–1930. [PubMed] [Google Scholar]
- Ikuta S., Takagi K., Wallace R. B., Itakura K. Dissociation kinetics of 19 base paired oligonucleotide-DNA duplexes containing different single mismatched base pairs. Nucleic Acids Res. 1987 Jan 26;15(2):797–811. doi: 10.1093/nar/15.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A. A., de la Cruz V. F., Welsh J. A., Charoenvit Y., Maloy W. L., McCutchan T. F. Structure of the gene encoding the circumsporozoite protein of Plasmodium yoelii. A rodent model for examining antimalarial sporozoite vaccines. J Biol Chem. 1987 Mar 5;262(7):2937–2940. [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig R. S., McGregor I. A., Bryan J. H. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science. 1979 Nov 2;206(4418):597–599. doi: 10.1126/science.386511. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967 Oct 14;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Development of a sporozoite malaria vaccine. Am J Trop Med Hyg. 1986 Jul;35(4):678–688. doi: 10.4269/ajtmh.1986.35.678. [DOI] [PubMed] [Google Scholar]
- Peters W., Chance M. L., Lissner R., Momen H., Warhurst D. C. The chemotherapy of rodent malaria, XXX. The enigmas of the 'NS lines' of P. berghei. Ann Trop Med Parasitol. 1978 Feb;72(1):23–36. doi: 10.1080/00034983.1978.11719276. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Freeman R. R., Holder A. A. Vaccination with a purified blood-stage malaria antigen in mice: correlation of protection with T cell mediated immunity. Clin Exp Immunol. 1985 Oct;62(1):19–23. [PMC free article] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann K. H., Carson P. E., Beaudoin R. L., Cassells J. S., Sell K. W. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1974;68(3):258–259. doi: 10.1016/0035-9203(74)90129-1. [DOI] [PubMed] [Google Scholar]
- Togna A. R., Del Giudice G., Verdini A. S., Bonelli F., Pessi A., Engers H. D., Corradin G. Synthetic Plasmodium falciparum circumsporozoite peptides elicit heterogenous L3T4+ T cell proliferative responses in H-2b mice. J Immunol. 1986 Nov 1;137(9):2956–2960. [PubMed] [Google Scholar]
- Vanderberg J., Nussenzweig R., Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. V. In vitro effects of immune serum on sporozoites. Mil Med. 1969 Sep;134(10):1183–1190. [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Sequence variation in putative functional domains of the circumsporozoite protein of Plasmodium falciparum. Implications for vaccine development. J Biol Chem. 1987 Sep 5;262(25):11935–11939. [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., Welsh J. A., McCutchan T. F. Evolution of the immunodominant domain of the circumsporozoite protein gene from Plasmodium vivax. Implications for vaccines. J Biol Chem. 1987 May 15;262(14):6464–6467. [PubMed] [Google Scholar]