Abstract
To allow successful germination and growth of a pollen tube, mature and dehydrated pollen grains (PGs) take up water and have to adjust their turgor pressure according to the water potential of the surrounding stigma surface. The turgor pressure of PGs of lily (Lilium longiflorum) was measured with a modified pressure probe for simultaneous recordings of turgor pressure and membrane potential to investigate the relation between water and electrogenic ion transport in osmoregulation. Upon hyperosmolar shock, the turgor pressure decreased, and the plasma membrane (PM) hyperpolarizes in parallel, whereas depolarization of the PM was observed with hypoosmolar treatment. An acidification and alkalinization of the external medium was monitored after hyper- and hypoosmotic treatments, respectively, and pH changes were blocked by vanadate, indicating a putative role of the PM H+ ATPase. Indeed, an increase in PM-associated 14-3-3 proteins and an increase in PM H+ ATPase activity were detected in PGs challenged by hyperosmolar medium. We therefore suggest that in PGs the PM H+ ATPase via modulation of its activity by 14-3-3 proteins is involved in the regulation of turgor pressure.
Plant cells, surrounded by a rigid cell wall, have an internal hydrostatic pressure, the turgor pressure. The turgor pressure depends on the concentration gradient of osmolytes across the plasma membrane (PM); therefore, changes in osmolytes lead to turgor-driven movements of entire plant organs, e.g. leaves of Samanea saman (Moran, 2007), Dionea trap leaves (Hodick and Sievers, 1989), or changes in the cell shape of guard cells (Roelfsema and Hedrich, 2005). Especially in guard cells turgor pressure values can reach up to 0.12 MPa (12 bar; Franks et al., 1998). Turgor pressure values can be estimated indirectly by observing plasmolysis or directly measured by impaling a pressure probe into the living plant cell still surrounded by an intact cell wall (Hüsken et al., 1978). The use of a pressure probe not only allows the monitoring of the turgor pressure over a time period but also determination of the hydraulic conductivity of the PM (LP), the elastic modulus of the cell wall (ε), and measurements of the permeability of substances across the PM (σ; Zimmermann, 1989; Steudle, 1993; Boyer, 1995; Tomos and Leigh, 1999). In guard cells changes in the activity of a number of ion transporters in the PM lead to a change in the concentration gradient of osmolytes and therefore, water following its potential gradient will either increase or decrease the turgor pressure, thus leading to opening and closing of the stomata, respectively (Roelfsema and Hedrich, 2005; Pandey et al., 2007). On the other hand, environmental changes, e.g. in the water potential of the soil will affect the turgor pressure of root cells that are responding to maintain their turgor pressure (Frensch and Hsiao, 1994). In hyperosmotically stressed Arabidopsis (Arabidopsis thaliana) root cells, a rapid, transient hyperpolarization of the PM was observed, whereas during recovery of turgor pressure, an uptake of ions (K+, Cl−Na+) was measured (Shabala and Lew, 2002). Furthermore, in suspension culture cells, hyper- and hypoosmotic shock caused corresponding changes in turgor pressure and also induced an acidification and alkalinization of the external medium, respectively, mainly carried by modulation of the PM H+ ATPase activity via 14-3-3 protein binding (Reuveni et al., 1987; Curti et al., 1993; Babakov et al., 2000; Felix et al., 2000; Kerkeb et al., 2002). This correlation between changes in turgor pressure and ion transport processes is widespread and can be observed in different plant organs and also in algae and fungi (Lew, 1996; Shabala et al., 2000; Lew et al., 2004; Bisson and Beilby, 2008). Therefore, one may assume that ion and water transport in plant cells are connected to each other not only by affecting physical parameters important for both transport processes (e.g. osmolyte/ion concentration and pressure) but also because of active regulation and signal cross talk between both processes as has been shown in yeast (Saccharomyces cerevisiae) cells (Muzzey et al., 2009).
Pollen grains (PGs), the male gametophytes of higher plants, are exposed to dramatic changes in environmental water potential. In the last stage of maturation, PGs are dehydrated and are then transferred to a stigma surface on which they will immediately take up water to rehydrate. Before germination occurs, the internal ion concentrations, pH, and also the turgor pressure have to be adjusted to allow the germination and growth of a pollen tube (PT) through the pistil tissue toward the ovules (Feijó et al., 1995). PGs do not foresee the water potential of the stigma on which they are going to land; interestingly, in vitro-cultivated PGs of the same species are capable of germinating in media containing various concentrations of osmolytes, e.g. lily (Lilium longiflorum) PGs can germinate in 5% to 12% Suc corresponding to 150 and 450 mosmol kg−1, respectively, with a more or less constant turgor pressure independent from the medium osmolality (Benkert et al., 1997). On the other hand, growing PTs are very sensitive to osmotic changes in the medium and stopped growth or increased the volume of the tip region (tip swelling; Pierson et al., 1994; Zonia and Munnik, 2004). The turgor pressure of lily PTs stays more or less constant (0.21 ± 0.06 MPa) and is independent from tube growth rates, tube length, and medium osmolality (Benkert et al., 1997). Artificial increases of turgor pressure by the pressure probe immediately stopped growth and further increase resulted in bursting of the tube tip with a burst pressure approximately twice the previous pressure, indicating a fine-regulated balance between turgor pressure and cell wall strength at the tube tip that is necessary for successful growth of PTs (Winship et al., 2010). However, PTs and grains are surrounded by an electrical field that is generated by localized influxes and effluxes of ions (Holdaway-Clarke and Hepler, 2003; Michard et al., 2009), thus changing the cytosolic osmolyte concentration. Additionally, metabolic activity during PT growth will also contribute to variations in osmolyte concentrations that will result in changes in the cytosolic water potential and, therefore, in water flux amount across the PM or even water flux direction. Therefore, one may assume an osmoregulation mechanism taking place in PGs that (1) somehow senses the osmotic conditions on the stigma surface, (2) allows an influx of water to account for the volume increase in germination and tube growth, and (3) adapts the turgor pressure to grow a PT without bursting. Several cellular components that are involved in osmoregulation as demonstrated for other plant cells were detected in pollen, too: ion channels (Obermeyer and Blatt, 1995; Fan et al., 1999; Mouline et al., 2002; Griessner and Obermeyer, 2003; Frietsch et al., 2007; Sze et al., 2004), a PM H+ ATPase (Obermeyer et al., 1996; Certal et al., 2008; Pertl et al., 2009), and 14-3-3 proteins (Pertl et al., 2005). In this study, invasive and noninvasive techniques were used to investigate whether PGs are capable to adjust and regulate their turgor pressure and which transport activities are affected by turgor changes and, therefore, are involved in osmoregulation of PGs.
RESULTS
PG Turgor Pressure and Membrane Potential
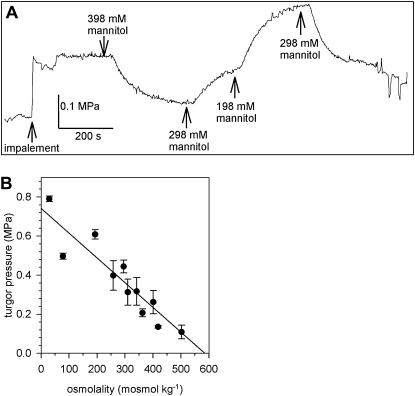
Immediately after impalement with the micropipette of the pressure probe, a stable turgor pressure of 0.317 ± 0.07 MPa (n = 17) was recorded in lily PGs bathed in standard medium (298 mm mannitol; Fig. 1). Exposing the PGs to various external mannitol concentrations that still allow germination and tube growth (Benkert et al., 1997), resulted in an adaptation of the turgor pressure to the new osmotic condition. The turgor pressure decreased to a lower value when the measuring chamber was perfused with a medium of higher osmolality (398 mm mannitol) or increased upon perfusion with a lower osmolar medium (198 mm mannitol; Fig. 1A). The changes in turgor pressure were always reversible in the range of media used for perfusion (approximately 50–520 mosmol kg−1) and lily PGs behave like a linear osmometer (Fig. 1B), indicating a reflection coefficient close to 1 for mannitol. At least no changes in the concentration of mannitol during the experiment due to an uptake by the PGs were detectable. A similar linear osmotic behavior was observed when lily PG protoplasts were exposed to various mannitol concentrations (Sommer et al., 2007). The osmotic potential of the cytosol could be estimated as 580 mosmol kg−1 from the regression line (Fig. 1B). During some measurements, the turgor pressure was manually changed by the pressure probe and its relaxation was recorded (Supplemental Fig. S1) to determine the hydraulic conductivity of the PM. The LP was calculated from the half time of the turgor pressure relaxations according to Steudle (1989) and Zimmermann (1989), giving 4.89 ± 5.20 10−8 m MPa−1s−1 (n = 14) that corresponds to an osmotic permeability coefficient (Pos) of approximately 7.4 μm s−1 that is well in the range of Pos values determined by swell assays of lily PG protoplasts (6.6 μm s−1; Sommer et al., 2007).
Figure 1.
Turgor pressure of PGs. A, A lily PG was impaled with the pressure probe in standard medium (298 mm mannitol). Perfusion of the chamber with hyper- (398 mm mannitol) and hypoosmolar (198 mm mannitol) media resulted in a reversible change of turgor pressure. At the end of the turgor pressure measurement, small pressure changes were induced and the corresponding volume of the PG measured to determine the elastic modulus ε. B, The measured turgor pressure plotted against the osmolality of the bath medium (n > 8 individual PGs) showing a linear osmotic behavior of lily PGs. Mean values ± sd.
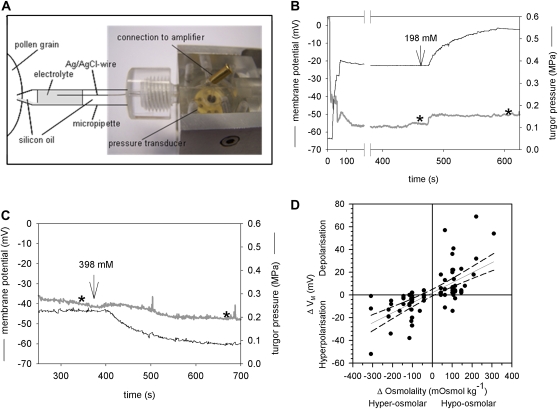
To monitor effects on electrogenic transport processes across the PM in parallel to turgor pressure changes, simultaneous measurements of the turgor pressure and the membrane potential were performed by impaling the PG with a micropipette that was partially filled with an electrolyte (1 m KCl) to ensure contact with an Ag/AgCl wire connected to a voltage amplifier (Fig. 2A). A glass capillary inside the micropipette allowed an electrical contact between the cytosol and the electrolyte-filled part of the pressure probe thus measuring the membrane potential of the impaled PG (Zhu, 1996). The PG turgor pressure values measured by the modified pressure probe were similar to those recorded with the original pressure probe. Perfusion with a hypoosmotic medium (198 mm mannitol) increased the turgor pressure and simultaneously depolarized the PM (Fig. 2B), whereas upon perfusion with a hyperosmotic medium (398 mm mannitol) the turgor pressure decreased as expected and the PM was hypolarized (Fig. 2C). For each measured PG, the change in medium osmolality (Δosmol) was plotted against the corresponding change in membrane potential (ΔVM), giving a linear relation with hyperosmotic media causing hyperpolarization and hypoosmotic media resulted in depolarization of the PM (Fig. 2D). These deflections of the membrane potential indicate the presence of an electrogenic transport process that is coupled to or responds to turgor pressure changes in lily PGs.
Figure 2.
Simultaneous measurement of turgor pressure and membrane potential in single PGs. A, Modified pressure probe with a chlorided silver wire reaching into the electrolyte solution. Except for the indicated part of the micropipette, the pressure probe as well as the other parts of the micropipette was filled with silicon oil. B, Typical recording of the turgor pressure (black) and membrane potential VM (gray). A change from iso- to hypoosmolar medium (198 mm mannitol) increased the turgor pressure and depolarized the PM whereas a change to hyperosmolar medium (398 mm mannitol) led to a hyperpolarization of the PM (C). Stars indicate the time at which the data were used to create Figure 2D. D, Summary of all PGs in which turgor pressure and VM were measured simultaneously. Hyperpolarization of the PM upon hyperosmolar treatments are given as negative ΔVM and Δosmolality values, respectively. A regression line with the 95% confidence intervals (dashed line) was fitted to the data. [See online article for color version of this figure.]
External pH Changes upon Osmotic Stress
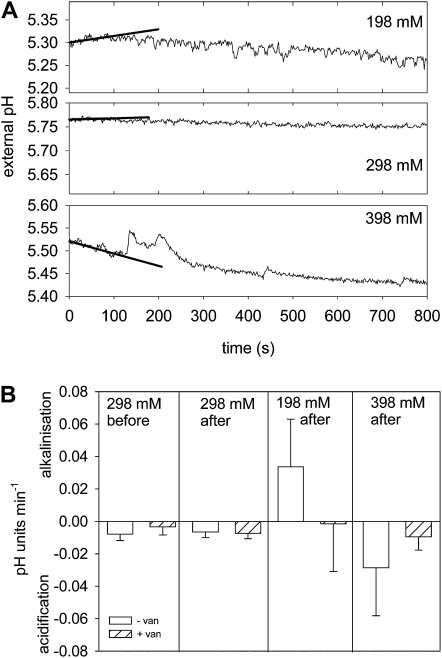
PGs incubated in in vitro germination medium extrude H+ and acidify the external medium (Southworth, 1983; Rodriguez-Rosales et al., 1989). The acidification of the external medium is inhibited by vanadate and promoted by fusicoccin, two well-characterized modulators of the PM H+ ATPase in plants. Therefore, monitoring a vanadate-sensitive acidification of the external medium provides a noninvasive method to record the PM H+ ATPase activity during various osmotic treatments. Under standard osmotic conditions (298 mm mannitol) an acidification of the medium was recorded for 1 h after suspending the lily PGs in the incubation chamber (Supplemental Fig. S2). Addition of 500 μm Na+ vanadate clearly inhibited acidification (Fig. 3B; Supplemental Fig. S2), whereas the addition of 1 μm fusiccocin stimulated the H+ extrusion (Supplemental Fig. S2). Immediately after the exposure to hypo- or hyperosmotic solutions, transient changes in the acidification rate could be observed, leading to a slight alkalinization or to a faster acidification during the first 100 s after osmotic stress (Fig. 3A). Addition of an isoosmotic solution did not affect the previously recorded acidification rate (Fig. 3A). The observed alkalinization as well as the acidification after the osmotic stress were both sensitive to vanadate. In the presence of 500 μm Na+ vanadate, almost no changes in the external pH were detectable after osmotic shock (Fig. 3B). Thus, the activity of the PM H+ ATPase might be responsible for the pH as well as for the membrane potential changes induced by osmotic challenges. Therefore, one might assume that during exposure to hyperosmotic conditions, the PM H+ ATPase activity is increased, resulting in a hyperpolarization of the PM and a higher acidification rate of the external medium; on the contrary, a hypoosmotic stress reduces the PM H+ ATPase activity, leading to a depolarization of the PM and a transient alkalinization of the medium.
Figure 3.
Vanadate-sensitive H+ transport changes upon hypo- and hyperosmotic shock. A, PGs were incubated in standard medium (298 mm mannitol), and the pH of the external medium was monitored. Upon sudden osmotic changes (198, 298, and 398 mm mannitol) at t = 0 min, temporal changes in the pH values can be noticed, namely an alkalinization after hypoosmotic shock, no changes after isoosmotic additions, and an increased acidification rate after hyperosmotic challenge. The first 100 s were fitted with a linear regression line giving a pH change rate of 0.0081, 0.0010, and −0.0180 pH units min−1 for 198, 298, and 398 mm mannitol treatments, respectively. B, The low acidification rate before the osmotic challenge was sensitive to vanadate and acidification rates did not change after addition of isoosmolar medium. The alkalinization in the first 100 s after hypoosmotic and the increased acidification rate after hyperosmotic challenge were inhibited by the presence of 500 μm vanadate. Mean values ± sd (n ≥ 3).
PM-Associated 14-3-3s and PM H+ ATPase Activity
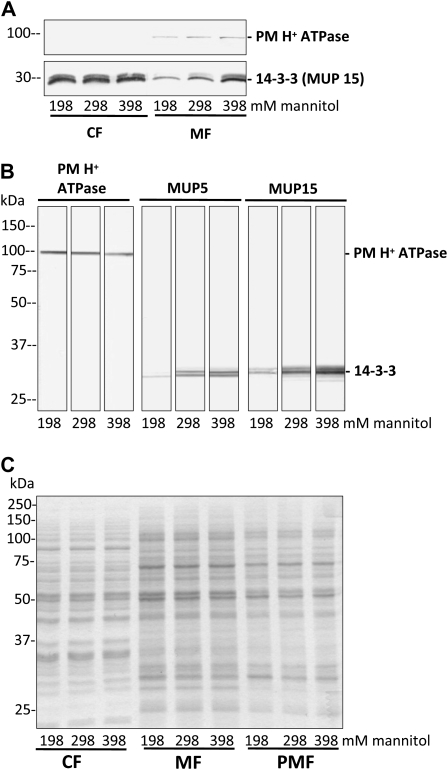
To test whether the up- or down-regulation of the PM H+ ATPase activity is mediated by 14-3-3 proteins, the abundance of PM-associated 14-3-3 proteins after different osmotic treatments was detected. Intact PGs were suspended in standard germination medium M (298 mm mannitol) for 15 min and were then incubated in iso-, hypo-, and hyperosmolar medium M for another 15 min before PM vesicles were isolated by Suc step gradient centrifugation. The amount of PM H+ ATPases was unaffected by the osmotic treatment, showing similar band intensities after immunodetection in the crude membrane (membrane fraction [MF]) as well as in the PM-enriched fraction (PMF; Fig. 4, A and B, left). The amount of 14-3-3 proteins in the soluble protein, cytosolic fraction (CF), was also not affected upon osmotic treatment, whereas membrane-associated 14-3-3 proteins clearly show a decrease, no effect, and an increase in the crude MF after hypo-, iso-, and hyperosmolar treatments, respectively (Fig. 4A). In isolated PM vesicles, 14-3-3 proteins were detected with two different antibodies (MUP5 and MUP15; Pertl et al., 2005) and showed changes in signal intensities upon osmotic treatments (Fig. 4B). Compared with controls (isoosmolar treatment) hypo- and hyperosmotic shock resulted in less or more PM-associated 14-3-3 proteins (Fig. 4B) that was confirmed in three independent PMF preparations after osmotic shock treatment. The Coomassie-stained protein gel (Fig. 4C) serves as a loading control to demonstrate the equal amounts of protein loaded on each lane. In addition, similar results were observed when 14-3-3 proteins in the PMF were detected with antibodies against Arabidopsis 14-3-3s (at-82, Santa Cruz Biotechnology) and Hordeum vulgare 14-3-3s (Hv14-3-3A, Hv14-3-3B, Hv14-3-3C, Agrisera; and gifts from Bert de Boer; data not shown).
Figure 4.
PM H+ ATPase and membrane-associated 14-3-3 proteins after osmotic treatment. PGs were incubated in standard medium (298 mm mannitol) for 15 min and exposed to different media osmolalities (198, 298, 398 mm mannitol) for another 15 min. The cytosolic (CF), membrane (MF), and PMF for each treatment were isolated. Proteins were separated by gel electrophoresis, blotted onto polyvinylidene difluoride membranes, and detected by specific antibodies as indicated. A, No changes in signal intensities of the PM H+ ATPase in the MF and of 14-3-3 proteins in the CF are observed, whereas 14-3-3 proteins in the MF showed a signal change dependent on the osmotic pretreatment. Ten micrograms of protein per lane. B, Compared with isoosmolar treatment (298 mm), higher 14-3-3 signals after hyperosmotic shock (398 mm) and lower 14-3-3 signals after hypoosmotic shock (198 mm) can be observed in the PMF with two different antibodies against 14-3-3 proteins. The amount of PM H+ ATPase was not affected by osmotic treatments. Twenty micrograms of protein per lane. C, Coomassie-stained gel of fractions used in A and B for immunodetection to verify the loading of 10 μg protein per lane (loading control).
Additionally, an increase in vanadate-sensitive ATP hydrolysis was detectable after hyperosmolar treatment, indicating an activation of the PM H+ ATPase activity in PM-enriched vesicles (Table I). Compared with the ATPase activity under isoosmotic conditions the vanadate-sensitive ATPase activity decreased by 30% after hypoosmotic shock whereas upon hyperosmotic treatment the ATPase activity increased by 26%, probably due to the interaction with 14-3-3 proteins.
Table I. PM H+ ATPase activity.
The vanadate-sensitive ATP hydrolysis was measured in PMFs prepared from PGs incubated in hypo- (198 mm), iso- (298 mm), and hyperosmolar (398 mm mannitol) germination medium. The specific activity is given in nmol mg−1 min−1. Mean values ± sd (n = 3).
| Mannitol Concentration | Specific Activity | Osmotic Treatment |
| mm | nmol Pi mg−1 min−1 | |
| 198 | 89.50 ± 19.92 | Hypo |
| 298 | 130.20 ± 7.35 | Iso |
| 398 | 164.29 ± 30.30 | Hyper |
DISCUSSION
Generally, PTs growing in in vitro culture are very sensitive to external perturbations and stop growth immediately or even burst at their tips. Small changes in external parameters like pH, K+, and Ca2+ concentrations or osmolality lead to temporal cessation of growth or change in growth rate oscillations that are often accompanied by morphological changes of the tube tip region (Pierson et al., 1994; Malhó and Trewavas, 1996; Messerli and Robinson, 2003; Zonia and Munnik, 2004; Zerzour et al., 2009). This flexibility to react to small environmental changes may help the PT to grow toward the ovary as fast as possible despite varying conditions along the style. In particular, PTs have to adapt their cell wall strength to osmotic changes that would otherwise lead to tube bursting (Zerzour et al., 2009; Winship et al., 2010). The mechanisms and pathways, how PTs sense or regulate their turgor pressure, osmolyte concentrations, and water fluxes, are still not well characterized. PGs also need to adapt to osmotic conditions of their environment, especially after landing on a receptive stigma when they first take up water and have to adjust their internal ion and osmolyte concentrations to build up a turgor pressure that drives the germination of the tube but still is balanced with the cell wall strength to prevent bursting of the germinating grain (Winship et al., 2010). In contrast to growing PTs that reflect osmotic changes irreversibly in their tube morphology, most manipulations, osmotic changes in particular (Fig. 1), are reversible in PGs, making them a suitable experimental system for first investigations of the principles of osmoregulation in pollen.
The turgor pressure immediately responded to osmotic changes, and lily PGs react like a linear osmometer using mannitol as an osmolyte (Fig. 1). Simultaneously with the turgor pressure the membrane potential difference (VM) also changed, resulting in a hyper- and depolarization of the PM after hyper- and hypoosmotic shock, respectively (Fig. 2D). Similar changes in VM upon osmotic treatments have been reported from other plant cells: In broad bean (Vicia faba) mesocarp cells VM was sensitive to tugor pressure changes (Li and Delrot, 1987) and Arabidopsis root cells showed a transient hyperpolarization after addition of 100 mm mannitol (Shabala and Lew, 2002). A correlation between turgor pressure and changes in VM or in electrogenic transport across the PM was also reported from algal and fungal cells (Bisson and Kirst, 1995; Lew et al., 2004; Lew and Levina, 2007; Bisson and Beilby, 2008). In Arabidopsis and carrot (Daucus carota) cell culture cells (Reuveni et al., 1987; Curti et al., 1993) as well as in leaf mesophyll tissue of beans (Phaseolus vulgaris; Shabala et al., 2000), an increase in (vanadate-sensitive) proton extrusion was detected after turgor pressure decreases by external addition of mannitol, indicating an involvement of the PM H+ ATPase in the turgor pressure-induced changes in VM. By measuring the pH changes (acidification or alkalinization) of the germination medium during in vitro culturing of pollen, the activity of the PM H+ ATPase can be recorded in a noninvasive way (Southworth, 1983; Tupy and Rihova, 1984; Rodriguez-Rosales et al., 1989). In lily pollen cultures a vanadate-sensitive and fusicoccin-stimulated acidification is detectable (Supplemental Fig. S2) and upon hyper- and hypoosmotic treatment an acidification and alkalinization, respectively, was observed (Fig. 3). Both changes in external pH could be inhibited by vanadate, indicating that the changes in VM are caused by changes in the electrogenic H+ transport of the PM H+ ATPase that are also reflected in the increase or decrease of the ATP hydrolysis activity of the PM H+ ATPase. In general, the activity of PM H+ ATPase is modulated by the binding of 14-3-3 proteins to the C-terminal autoinhibitory domain that has been reversibly phosphorylated (see Duby and Boutry, 2009 and refs. therein). 14-3-3 proteins have been detected in almost all cellular compartments of lily PGs and tubes (Pertl et al., 2005) and an increase in PM-associated 14-3-3 proteins is observed after treatment of PGs with hyperosmotic medium. A similar correlation between hyperosmolar treatment, activated H+ transport, or H+ efflux and abundance of 14-3-3 proteins at the PM was observed in suspension-cultured cells of tomato (Solanum lycopersicum; Kerkeb et al., 2002) and sugar beet (Beta vulgaris; Babakov et al., 2000). In contrast to Kerkeb et al. (2002) who observed an increase only in H+ transport but not in ATP hydrolysis activity upon hyperosmotic shock with NaCl, the H+ transport and the ATP hydrolysis of the PM ATPase in lily PGs are both stimulated by hyperosmotic treatment with mannitol.
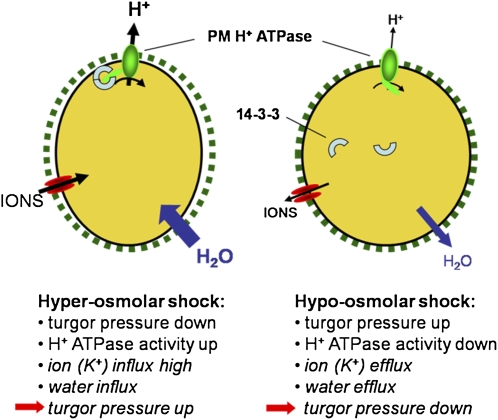
CONCLUSION
These data support the hypothesis that short-term osmoregulation in plants cells including PGs, involves a modulation of the PM H+ ATPase activity by interactions with 14-3-3 proteins (Fig. 5). Upon hyperosmolar shock, the turgor pressure decreases and a hyperpolarization of the PM can be measured due to an activation of the PM H+ ATPase via 14-3-3 interaction. Consequently, a membrane potential more negative than the reversal potential of K+ allows a higher K+ influx that in turn promotes water uptake to readjust the turgor pressure in the long term. On the other hand, a hypoosmotic shock increases the turgor pressure and down-regulates the PM H+ ATPase activity via dissociation of 14-3-3 proteins. The resulting depolarization of the PM enables K+ efflux followed by water efflux and the turgor pressure finally decreases again to the previous value. The short-term effects on turgor pressure and H+ ATPase activity have been measured in this study whereas the hypothesized changes in K+ and water transport and the subsequent readjustment of the turgor pressure still need to be demonstrated. Additionally, due to the fact that the interaction between the C-terminal autoinhibitory domain of the PM H+ ATPase and 14-3-3 proteins requires a change in the phophorylation status of probably both proteins several kinase- and phosphatase-including signal transduction pathways may be involved, e.g. a MAP kinase kinase is activated upon hydration of tobacco (Nicotiana tabacum) pollen (Voronin et al., 2004). Although the knowledge on osmoregulation in plant cells is still fragmentary, PGs may serve as a suitable model system for investigating plant osmoregulation and search for an osmosensor.
Figure 5.
Schematic summary of osmoregulation in lily PGs. The first two events after osmotic shock were measured in this study. Subsequent, hypothetical events finally leading to a readjustment of turgor pressure are given in italics. For further explanation see “Conclusion.” [See online article for color version of this figure.]
MATERIALS AND METHODS
Plant Material
Lily (Lilium longiflorum) plants were grown in a greenhouse under environmental light and temperature conditions. PGs were collected from fully developed flowers and either used immediately or frozen as single anthers (turgor pressure measurements and pH measurements) or in aliquots of 25 flowers (biochemical experiments) in liquid N2 and stored at −80°C. In parallel to or before the experiments, aliquots of the frozen PG batches were tested for their germination ability. In all experiments shown, no differences in germination frequency or tube morphology were observed between the frozen and fresh pollen.
Combined Turgor Pressure and Membrane Potential Measurements
The turgor pressure of ungerminated PGs was measured with the pressure probe (Zimmermann et al., 1969) as described in Benkert et al. (1997). Ungerminated PGs were suspended in standard bath medium [Med M, in mm: 298 mannitol, 1 KCl, 0.1 Ca(OH)2, 1.6 H3BO3, 25 MES adjusted to pH 5.6 with Tris (Tris{hydroxymethyl}amino-methane), approximately 320 mosmol kg−1] and transferred to a perfusion chamber. A PG was impaled with the oil-filled micropipette of the pressure probe while gently holding the PG at the bottom of the chamber with a second pipette. After successful impalement the holding pipette was carefully withdrawn and the impaled PG was lifted from the chamber bottom. The chamber was constantly perfused with bath solutions of different osmolalities adjusted with mannitol (190–500 mosmol kg−1). The turgor pressure was recorded with a chart recorder (Norma Goerz Instruments) or digitized at a sampling frequency of 100 Hz and filtered at 10 Hz cutoff frequency using the Digidata 1200 A/D converter and the Axoscope software (Molecular Devices). During some turgor pressure measurements pressure pulses were applied to calculate the hydraulic conductivity (Lp) from the subsequent pressure relaxation curves or the volumetric elastic modulus (ε) from small pressure increments (Steudle, 1993; Boyer, 1995).
For simultaneous recordings of turgor pressure and membrane potential a new pressure probe was constructed according to Zhu (1996; Fig. 2A). Micropipettes were pulled from glass capillaries containing a glass filament (GC 120F-10, Science Products) with a horizontal pipette puller (Sachs-Flaming PC-84, Sutter Instruments). The tip of the micropipettes was filled with silicon oil (AS-4, Wacker Chemie) and back filled with electrolyte (90 mm K+ acetate, pH 7.3, 10 mm KCl; see Fig. 2A). When mounting the filled micropipette into the pressure probe holder, an Ag/AgCl wire reaching into the electrolyte solution was connected to a voltage amplifier (Y-Science). The electrolyte solution was drawn to the micropipette tip due to the capillary forces between the glass filament and the pipette wall. The electric connection was tested after the micropipette and the reference electrode (1 m KCl) was submersed into the standard bath medium. PGs were impaled as described above and the membrane potential difference (VM) and the turgor pressure were recorded with a two-channel chart recorder.
External pH Measurements
Mature PGs of one anther were resuspended in an unbuffered medium (Med M) containing (in mm): 298 mannitol, 1 KCl, 0.1 CaCl2, 1.6 H3BO3, and the pH was adjusted to 5.6 with tiny amounts of MES or Tris, respectively, if necessary. The PGs were washed three to five times with Med M by short centrifugations to remove the pollenkitt, ions, or other compounds bound to the cell wall. Finally, 1 mL of the pollen suspension was added to 1 mL Med M in the multiport measurement chamber (WPI) housing the reference as well as the pH electrode (Kwik-Tip, WPI). The electrodes were connected to an electrometer (610C, Keithley Instruments) and the data were sampled at 333.3 Hz by an A/D converter (DigiData 1200, Molecular Devices), filtered at 10 Hz cutoff frequency and recorded with the Axoscope 7 software. The quality of the pH electrode was tested before each experiment by measuring solutions with known pH values, giving slopes of 50 to 56 mV per pH unit. The pH value was calculated from the recorded voltage using the calibration curves. The acidification (negative values) or alkalinization rates (positive values) are given as ΔpH min−1. During the measurements the PG suspension was constantly stirred at slow speed and osmolality was changed by adding appropriate volumes of hyper- or hypoosmotic mannitol stock solutions (with all other components as Med M) to give 198 and 398 mm mannitol, respectively, as final concentrations.
PM Isolation, Gel Electrophoresis, and Immunodetection
PGs from 75 flowers were incubated for 15 min in Med M, split to three aliquots with each aliquot incubated for another 15 min in isoosmolar Med M (298 mm mannitol), in hypoosmolar Med M (198 mm mannitol), and in hyperosmolar Med M (398 mm mannitol). Fractions of soluble cytosolic (CF), crude membranes (MF), and PMF proteins were prepared from each pollen aliquot by differential centrifugation followed by discontinuous Suc density gradient centrifugation as described by Pertl et al. (2005, 2009).
Proteins were separated by SDS-PAGE by denaturing protein fractions (10–20 μg protein) in sample buffer (300 mm dithiothreitol, 30% [w/v] SDS, 15% [w/v] glycerol, 180 mm Tris/HCl pH 6.8, bromphenol blue) at room temperature for 15 min and loading onto a discontinuous gel system (Laemmli, 1970) with 4% stacking gel and 10% separation gel (Protean 3 system, Bio-Rad). Proteins were stained with Coomassie Brilliant Blue R-250. The protein concentration was determined using a Lowry DC assay (Bio-Rad).
For immunodetection separated proteins were transferred onto polyvinylidene difluoride membranes (Roth) by electroblotting with 20 V for 1 h (semi dry electrophoretic transfer cell, Bio-Rad) and immunodetection of the PM H+ ATPase and 14-3-3 proteins was performed as described previously (Pertl et al., 2005) with the following antibody concentrations and combinations: MUP5 and MUP15 diluted 1:250 and detected by goat anti-mouse IgG-alkaline phosphatase conjugated (1:5,000 or 1:10,000, Sigma) for 14-3-3 detection. For immunodetection of the PM H+ ATPase the primary antibody was diluted 1:2,500 and secondary antibody goat anti-mouse IgG-alkaline phosphatase was diluted 1:5,000 (Sigma).
PM H+ ATPase Activity
Hydrolysis of ATP by the vanadate-sensitive PM H+ ATPase was monitored by determination of released inorganic phosphate (Ames, 1962) as described in Pertl et al. (2005).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Determination of the hydraulic conductivity (LP).
Supplemental Figure S2. Acidification of the external medium by an active PM H+ ATPase.
Supplementary Material
Acknowledgments
We thank Bert de Boer (University of Amsterdam) for his generous gift of antibodies against Hordeum vulgare 14-3-3 proteins.
References
- Ames BN. (1962) Assays of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8: 115–118 [Google Scholar]
- Babakov AV, Chelysheva VV, Klychnikov OI, Zorinyanz SE, Trofimova MS, De Boer AH. (2000) Involvement of 14-3-3 proteins in the osmotic regulation of H+-ATPase in plant plasma membranes. Planta 211: 446–448 [DOI] [PubMed] [Google Scholar]
- Benkert R, Obermeyer G, Bentrup FW. (1997) The turgor pressure of growing lily pollen tubes. Protoplasma 198: 1–8 [Google Scholar]
- Bisson MA, Beilby MJ. (2008) Transport systems of Ventricaria ventricosa: asymmetry of the hyper- and hypotonic regulation mechanisms. J Membr Biol 225: 13–25 [DOI] [PubMed] [Google Scholar]
- Bisson MA, Kirst GO. (1995) Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften 82: 461–471 [Google Scholar]
- Boyer JS. (1995) Measuring the Water Status of Plants and Soils. Academic Press, San Diego, pp 103–142 [Google Scholar]
- Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguéz-Léon J, Wu HM, Cheung AY, et al. (2008) Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell 20: 614–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti G, Massardi F, Lado P. (1993) Synergistic activation of plasma membrane H+ ATPase in Arabidopsis thaliana cells by turgor decrease and by fusicoccin. Physiol Plant 87: 592–600 [Google Scholar]
- Duby G, Boutry M. (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch 457: 645–655 [DOI] [PubMed] [Google Scholar]
- Fan LM, Wu WH, Yang HY. (1999) Identification and characterization of the inward K+ channel in the plasma membrane of Brassica pollen protoplasts. Plant Cell Physiol 40: 859–865 [DOI] [PubMed] [Google Scholar]
- Feijó JA, Malhó R, Obermeyer G. (1995) Ion dynamics and its possible role during in vitro pollen germination and tube growth. Protoplasma 187: 155–167 [Google Scholar]
- Felix G, Regenass M, Boller T. (2000) Sensing of osmotic pressure changes in tomato cells. Plant Physiol 124: 1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD. (1998) A study of stomatal mechanics using the cell pressure probe. Plant Cell Environ 21: 94–100 [Google Scholar]
- Frensch J, Hsiao TC. (1994) Transient responses of cell turgor and growth of maize roots as affected by changes in water potential. Plant Physiol 104: 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF. (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessner M, Obermeyer G. (2003) Characterization of whole-cell K+ currents across the plasma membrane of pollen grain and tube protoplasts of Lilium longiflorum. J Membr Biol 193: 99–108 [DOI] [PubMed] [Google Scholar]
- Hodick D, Sievers A. (1989) On the mechanism of trap closure of Venus flytrap (Dionea muscipula Ellis). Planta 179: 32–42 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke T, Hepler PK. (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159: 539–563 [DOI] [PubMed] [Google Scholar]
- Hüsken D, Steudle E, Zimmermann U. (1978) Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol 61: 158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkeb L, Venema K, Donaire JP, Rodríguez-Rosales MP. (2002) Enhanced H+/ATP coupling ratio of H+-ATPase and increased 14-3-3 protein content in plasma membrane of tomato cells upon osmotic shock. Physiol Plant 116: 37–41 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lew RR. (1996) Pressure regulation of the electrical properties of growing Arabidopsis thaliana L. root hairs. Plant Physiol 112: 1089–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RR, Levina NN. (2007) Turgor regulation in the osmosensitive cut mutant of Neurospora crassa. Microbiology 153: 1530–1537 [DOI] [PubMed] [Google Scholar]
- Lew RR, Levina NN, Walker SK, Garrill A. (2004) Turgor regulation in hyphal organisms. Fungal Genet Biol 41: 1007–1015 [DOI] [PubMed] [Google Scholar]
- Li ZS, Delrot S. (1987) Osmotic dependence of the transmembrane potential difference of broadbean mesocarp cells. Plant Physiol 84: 895–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhó R, Trewavas AJ. (1996) Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8: 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli MA, Robinson KR. (2003) Ionic and osmotic disruptions of the lily pollen tube oscillator: testing proposed models. Planta 217: 147–157 [DOI] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijó JA. (2009) The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int J Dev Biol 53: 1609–1622 [DOI] [PubMed] [Google Scholar]
- Moran N. (2007) Osmoregulation of leaf motor cells. FEBS Lett 581: 2337–2347 [DOI] [PubMed] [Google Scholar]
- Mouline K, Véry AA, Gaymard F, Boucherez J, Pilot G, Devic M, Bouchez D, Thibaud JB, Sentenac H. (2002) Pollen tube development and competitive ability are impaired by disruption of a Shaker K(+) channel in Arabidopsis. Genes Dev 16: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzey D, Gómez-Uribe CA, Mettetal JT, van Oudenaarden A. (2009) A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138: 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer G, Blatt MR. (1995) Electrical properties of intact pollen grains of Lilium longiflorum: characteristics of the non-germinating pollen grain. J Exp Bot 46: 803–813 [Google Scholar]
- Obermeyer G, Kriechbaumer R, Strasser D, Maschessnig A, Bentrup FW. (1996) Boric acid stimulates the plasma membrane H+ ATPase of ungerminated lily pollen grains. Physiol Plant 98: 281–290 [Google Scholar]
- Pandey S, Zhang W, Assmann SM. (2007) Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett 581: 2325–2336 [DOI] [PubMed] [Google Scholar]
- Pertl H, Gehwolf R, Obermeyer G. (2005) The distribution of membrane-bound 14-3-3 proteins in organelle-enriched fractions of germinating lily pollen. Plant Biol (Stuttg) 7: 140–147 [DOI] [PubMed] [Google Scholar]
- Pertl H, Schulze WX, Obermeyer G. (2009) The pollen organelle membrane proteome reveals highly spatial-temporal dynamics during germination and tube growth of lily pollen. J Proteome Res 8: 5142–5152 [DOI] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK. (1994) Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. Plant Cell 6: 1815–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni M, Colombo R, Lerner HR, Pradet A, Poljakoff-Mayber A. (1987) Osmotically induced proton extrusion from carrot cells in suspension culture. Plant Physiol 85: 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rosales MP, Roldán M, Belver A, Donaire JP. (1989) Correlation between in vitro germination capacity and proton extrusion in olive pollen. Plant Physiol Biochem 27: 723–728 [Google Scholar]
- Roelfsema MRG, Hedrich R. (2005) In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol 167: 665–691 [DOI] [PubMed] [Google Scholar]
- Shabala S, Babourina O, Newman I. (2000) Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J Exp Bot 51: 1243–1253 [PubMed] [Google Scholar]
- Shabala SN, Lew RR. (2002) Turgor regulation in osmotically stressed Arabidopsis epidermal root cells: direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Mahlknecht G, Obermeyer G. (2007) Measuring the osmotic water permeability of the plant protoplast plasma membrane: implication of the nonosmotic volume. J Membr Biol 215: 111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southworth D. (1983) pH changes during pollen germination in Lilium longiflorum. Mulcahy DL, Ottaviano E, eds, Pollen: Biology and Implications for Plant Breeding Elsevier, New York, pp 61–65 [Google Scholar]
- Steudle E. (1989) Water flow in plants and its coupling to other processes: an overview. Methods Enzymol 174: 183–225 [Google Scholar]
- Steudle E. (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue, and organ level. Smith JAC, Griffiths H, eds, Water Deficits: Plant Responses from Cell to Community βios Scientific Publishers Ltd, Oxford, pp 5–36 [Google Scholar]
- Sze H, Padmanaban S, Cellier F, Honys D, Cheng NH, Bock KW, Conéjéro G, Li X, Twell D, Ward JM, et al. (2004) Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol 136: 2532–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomos AD, Leigh RA. (1999) The pressure probe: a versatile tool in plant cell physiology. Annu Rev Plant Physiol Plant Mol Biol 50: 447–472 [DOI] [PubMed] [Google Scholar]
- Tupy J, Rihova L. (1984) Changes and growth effect of pH in pollen tube culture. J Plant Physiol 115: 1–10 [DOI] [PubMed] [Google Scholar]
- Voronin V, Aionesei T, Limmongkon A, Barinova I, Touraev A, Laurière C, Coronado MJ, Testillano PS, Risueño MC, Heberle-Bors E, et al. (2004) The MAP kinase kinase NtMEK2 is involved in tobacco pollen germination. FEBS Lett 560: 86–90 [DOI] [PubMed] [Google Scholar]
- Winship LJ, Obermeyer G, Geitmann A, Hepler PK. (2010) Under pressure, cell walls set the pace. Trends Plant Sci 15: 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerzour R, Kroeger J, Geitmann A. (2009) Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev Biol 334: 437–446 [DOI] [PubMed] [Google Scholar]
- Zhu GL. (1996) A new turgor/membrane potential probe simultaneously measures turgor and electrical membrane potential. Bot Acta 109: 51–56 [Google Scholar]
- Zimmermann U. (1989) Water relations of plant cells: pressure probe technique. Methods Enzymol 174: 338–366 [Google Scholar]
- Zimmermann U, Raede H, Steudle E. (1969) Kontinuierliche druckmessung in pflanzenzellen. Naturwissenschaften 56: 634 [Google Scholar]
- Zonia L, Munnik T. (2004) Osmotically induced cell swelling versus cell shrinking elicits specific changes in phospholipid signals in tobacco pollen tubes. Plant Physiol 134: 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.