Abstract
Plant oils containing ω-7 fatty acids (FAs; palmitoleic 16:1Δ9 and cis-vaccenic 18:1Δ11) have potential as sustainable feedstocks for producing industrially important octene via metathesis chemistry. Engineering plants to produce seeds that accumulate high levels of any unusual FA has been an elusive goal. We achieved high levels of ω-7 FA accumulation by systematic metabolic engineering of Arabidopsis (Arabidopsis thaliana). A plastidial 16:0-ACP desaturase has been engineered to convert 16:0 to 16:1Δ9 with specificity >100-fold than that of naturally occurring paralogs, such as that from cat's claw vine (Doxantha unguis-cati). Expressing this engineered enzyme (Com25) in seeds increased ω-7 FA accumulation from <2% to 14%. Reducing competition for 16:0-ACP by down-regulating the β-ketoacyl-ACP synthase II 16:0 elongase further increased accumulation of ω-7 FA to 56%. The level of 16:0 exiting the plastid without desaturation also increased to 21%. Coexpression of a pair of fungal 16:0 desaturases in the cytosol reduced the 16:0 level to 11% and increased ω-7 FA to as much as 71%, equivalent to levels found in Doxantha seeds.
There is increasing interest in the use of plant oils as renewable sources of industrial chemical feedstocks (Dyer et al., 2008; Carlsson, 2009). Recent developments in olefin metathesis have demonstrated that long-chain monoene FA from vegetable oils can be efficiently split into the corresponding short-chain α-olefin and ω-alkenoic acids (Rybak et al., 2008; Meier, 2009). Thus, ethenolytic metathesis of ω-7 fatty acids (FAs), such as palmitoleic or cis-vaccenic acids, yields 1-octene and 9-decenoate. 1-Octene is a high-demand feedstock with a global consumption of over half a million tons per year that is primarily used as a comonomer in the expanding production of linear low density polyethylene. It is mainly synthesized from petroleum-derived ethylene via oligomerization to yield a complex mixture of α-olefins or from coal-derived syngas (Anonymous, 2007).
A plant oil containing high (>70%) content of ω-7 FA would represent a new and sustainable feedstock for 1-octene production. Several natural plant oil sources of ω-7 FA have been reported, e.g. milkweed (Asclepias syriaca) accumulates approximately 25% ω-7 FA (comprising approximately 10% 16:1Δ9 and approximately 15% 18:1Δ11) and cat’s claw vine (Doxantha unguis-cati) accumulates approximately 72% ω-7 FA (comprising approximately 55% 16:1Δ9 and approximately 17% 18:1Δ11), but the low yields and poor agronomic properties of these plants preclude their commercial use for ω-7 FA production. Isolation of the Δ9-16:0-ACP desaturases genes responsible for the production of ω-7 FA from milkweed and Doxantha (Cahoon et al., 1997, 1998) presented an opportunity for their heterologous expression and ω-7 FA production in transgenic crops. However, heterologous expression of the milkweed desaturase in Arabidopsis (Arabidopsis thaliana) failed to produce detectable ω-7 FA, and when the Doxantha desaturase was expressed in Brassica napus, it resulted in the accumulation of only approximately 9% ω-7 FA (Bondaruk et al., 2007). Thus, upon expression of either naturally occurring gene, ω-7 FA accumulation was far below that of the source plant, suggesting that additional metabolic modifications are required to optimize ω-7 FA accumulation in a host oilseed plant. Low levels of unusual FA accumulation upon the heterologous expression of variant desaturases is a generally observed phenomenon for monoene-producing acyl-ACP desaturases (Suh et al., 2002), and efforts in this laboratory to express the Δ4-16:0 desaturases from coriander (Coriandrum sativum) and ivy (Hedra helix) and the Δ6-16:0 desaturase from Thunbergia alata in Arabidopsis seed resulted in <3% of the target FA accumulation in the best lines. Similarly, the accumulation of unusual FAs resulting from the expression of FAD2 integral membrane desaturase variants is much lower than the levels found in seeds of the native host (Napier, 2007). Together, these observations constitute a significant barrier for biotechnological efforts to produce sustainable and renewable sources of oleochemical-derived feedstocks for products such as octene.
To overcome the problem of poor ω-7 FA accumulation, we performed a series of systematic metabolic engineering experiments to optimize the accumulation of ω-7 FA in transgenic plants. We chose to demonstrate proof of concept in the model oilseed plant Arabidopsis because its rapid generation time and facile transformation allowed us to rapidly quantify the effects of various combinations of transgenic approaches and mutant backgrounds. Based on the poor performance of naturally occurring Δ9-16:0-ACP desaturases, our first step was to identify a laboratory-derived variant desaturase with improved kinetics relative to its natural counterparts. We next systematically quantified the effects of various metabolic perturbations and then stacked the most promising traits into a single line. In doing so, we demonstrated that coexpression of the laboratory-evolved plastidial desaturase, along with native extraplastidial 16:0Δ9-desaturases in a background in which the β-ketoacyl-ACP synthase II (KASII) elongase was strongly suppressed, resulted in increased accumulation of ω-7 FA from <2% in the wild type to 71% in the best engineered line. This level of ω-7 FA is higher than that found in Asclepias and equivalent to that found in Doxantha seeds.
RESULTS
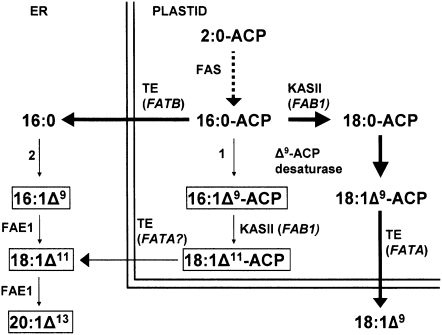
In wild-type Arabidopsis, FAs are synthesized de novo via the ACP pathway to a first branch point at the level of 16:0-ACP (Fig. 1). If acted on by fatty acid thioesterase B (FATB), the palmitoyl thioesterase, 16:0 free FA is released from the plastid to the cytoplasm, where it is esterified to CoA and subsequently transesterified onto phospholipids of the endomembrane system. Alternatively, KASII elongates the majority of 16:0-ACP to 18:0-ACP, whereupon it is desaturated by Δ9-stearoyl-ACP desaturase to produce oleoyl-ACP. FATA, the oleoyl-ACP thioesterase, releases the oleic acid that exits the plastid and, like palmitate, becomes activated to the CoA-thioester and is transferred to phospholipids. In the endoplasmic reticulum, oleate can be elongated to 20:1Δ11 via the action of fatty acid elongase 1 (FAE1) or becomes sequentially desaturated by the action of FAD2 and FAD3 to produce linoleic and linolenic acids, respectively.
Figure 1.
Schematic of FA synthesis and modification in the plastid and endoplasmic reticulum of Arabidopsis. Reactions mediated by 16:0 desaturases are indicated: 1, Δ9-16:0-ACP desaturase; 2, extraplastidial Δ916:0-desaturase. ω-7 FAs of interest in this study, i.e. 16:1Δ9 and 18:1Δ11, are boxed.
As Δ7-14:0-ACP desaturases are unknown,16:0-ACP is the earliest metabolite in the FA synthesis pathway that can be intercepted and committed to ω-7 FA production by desaturation to 16:1Δ9-ACP. To achieve this, we explored the feasibility of expressing a plastidial Δ9-16:0-ACP-desaturase under the control of a seed-specific promoter (Fig. 1, reaction 1).
Choice of Δ9-16:0-ACP Desaturase
Several plants, including Asclepias (Hopkins and Chisholm, 1961) and Doxantha (Chisholm and Hopkins, 1965), accumulate ω-7 FA in their seeds. Because the genes encoding these naturally occurring 16:0-ACP desaturases have been isolated (Cahoon et al., 1997, 1998), we first considered their use in efforts to engineer ω-7 FA accumulation. However, the measured in vitro activities of the desaturases from milkweed and Doxantha, like those of many variant desaturases, were considerably lower (milkweed, 4% and Doxantha, 11%; Cahoon et al., 1997, 1998) than those reported for archetypal stearoyl-ACP desaturases acting on 16:0-ACP (Whittle and Shanklin, 2001). Test expression of these enzymes in Arabidopsis seed showed the Doxantha desaturase resulted in approximately 10% ω-7 FA, to similar levels as those previously reported upon its expression in Brassica (Bondaruk et al., 2007), while no product was detected upon expression of the milkweed enzyme in Arabidopsis.
We next considered the use of variants of the castor desaturase, including the previously reported 5.2 (Whittle and Shanklin, 2001), along with a battery of previously undescribed variants, including Com25, that arose from enzyme evolution experiments designed to enhance the 16:0-desaturase activity of the castor Δ9-18:0-desaturase. Kinetic analysis was used to compare the various acyl-ACP desaturases; Com25 was chosen because it exhibited the highest kcat/Km (specificity factor) of 91 μm−1 min−1, i.e. equivalent to that of the castor wild-type desaturase for its natural 18:0-ACP substrate (Table I).
Table I. Comparison of the kinetic parameters of the castor desaturase and its variants with those of Doxantha.
| Enzyme | Substrate | kcata | Km | Specificity Factor kcat/Km |
| min−1b | μm | μm−1 min−1 | ||
| Com25 | 16:0-ACP | 11.10 (0.60) | 0.12 (0.03) | 91.00 |
| 5.2c | 16:0-ACP | 25.30 (1.10) | 0.55 (0.06) | 46.00 |
| Wild typec | 16:0-ACP | 2.80 (0.10) | 5.00 (0.50) | 0.56 |
| Wild typec | 18:0-ACP | 42.30 (1.60) | 0.46 (0.05) | 92.00 |
| Doxantha | 16:0-ACP | 0.31 (0.03) | 0.43 (0.12) | 0.72 |
kcat is reported per di-iron site.
Mean with se in parentheses (n = 10).
Data from Whittle and Shanklin (2001).
Expression of Com25 in Wild-Type Arabidopsis
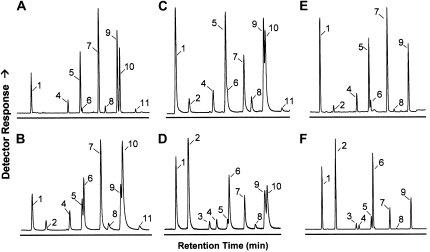
Expression of Com25 in transgenic seeds under the control of the seed-specific phaseolin promoter in wild-type Arabidopsis resulted in the accumulation of approximately 2% 16:1Δ9 and approximately 12% of its elongation product, 18:1Δ11, yielding a total of approximately 14% ω-7 FA. For a comparison of the gas chromatographic analysis of seed FA from wild-type Arabidopsis and the wild type expressing Com25, see Figure 2, A and B.
Figure 2.
Representative gas chromatographic separation of FAMEs upon expression of Com25 in various backgrounds of Arabidopsis. A and B, the wild type; C and D, fab1; E and F, fab1 fae1. A, C, and E, Untransformed; B, D, and F, transformed with Phas:Com25. FAME peaks are indicated: 16:0 (1), 16:1Δ9 (2), 16:2 (3), 18:0 (4), 18:1Δ9 (5), 18:1Δ11 (6),18:2 (7), 20:0 (8), 20:1Δ11 (9), 18:3+20:1Δ13 (10), and 22:1 (11).
Expression of Com25 in Arabidopsis Hosts Containing Increased Levels of 16:0
As described above, KASII competes with Com25 for substrate and elongates 16:0-ACP to 18:0-ACP. Therefore, we sought lines with lowered KASII activity that would contain increased levels of 16:0 substrate (see Fig. 2 for representative GC traces of seed FA methyl esters; James and Dooner, 1990). Despite many mutagenesis screens for altered FA profiles, only one KASII mutant, fab1, has been reported (James and Dooner, 1990) that exhibits increased 16:0 levels in leaves and seeds. The seed 16:0 levels increase to approximately 21% from approximately 10% in the wild type (Fig. 2C; Table II). Biochemical evidence has confirmed that the fab1 lesion is in KASII (Carlsson et al., 2002). Expression of Com25 in fab1 increased the accumulation of 16:1Δ9 and 18:1Δ11 to approximately 23% and approximately 16% respectively, yielding a total of approximately 39% ω-7 FA (Fig. 2D; Table II).
Table II. Comparison of the FA composition of various lines of transgenic Arabidopsis seeds with those of Doxantha.
| Genotype | FA Species |
Total ω-7a | Δ ω-7b | |||||||||
| 16:0 | 16:1Δ9 | 16:2 | 18:0 | 18:1Δ9 | 18:1Δ11 | 18:2 | 18:3 | 20:0 | 20:1 | |||
| Wild type | 10.4 (0.4)c | 0.1 (0.06) | 0 | 3.7 (0.4) | 14.5 (2.2) | 1.7 (0.2) | 26.6 (0.9) | 20.2 (1.1) | 2.1 (0.3) | 20.7 (0.6) | 1.8 (0.2) | |
| fab1/ | 20.8 (1.6) | 1.5 (0.5) | 0 | 3.7 (0.4) | 18.4 (1.3) | 2.8 (0.9) | 14.8 (2.7) | 19.1 (1.3) | 3.1 (0.8) | 15.8 (1.0) | 4.3 (1.3) | 2 |
| fab1, fae1/ | 26.8 (1.9) | 1.9 (0.3) | 0 | 3.6 (0.5) | 15.8 (1.5) | 6.8 (0.8) | 25.3 (2.5) | 19.7 (1.5) | 0.2 (0.1) | 0 | 8.7 (1.1) | 4 |
| Wild type, Com25 | 9.2 (1.5) | 1.6 (0.4) | 0 | 3.7 (0.3) | 5.7 (1.6) | 12.8 (1.2) | 26.6 (1.6) | 29.6 (2.2) | 2.1 (0.4) | 8.7 (2.1) | 14.4 (1.5) | 12 |
| fab1, Com25 | 18.6 (2.1) | 23.5 (3.7) | 1.6 (0.5) | 1.9 (0.3) | 2.9 (2.2) | 15.6 (3.3) | 9.6 (1.7) | 17.1 (1.8) | 1.0 (0.4) | 8.2 (3.4) | 39.1 (1.9) | 35 |
| fab1, fae1, Com25 | 22 (2.6) | 26.2 (2.9) | 2.0 (0.4) | 1.8 (0.4) | 3.7 (1.1) | 23.4 (2.3) | 8.6 (0.7) | 12.4 (1.8) | 0 | 0 | 49.6 (1.1) | 41 |
| fab1, fae1, Com25, Fab1-HPAS | 20.7 (2.1) | 30.3 (1.6) | 2.2 (0.2) | 0.9 (0.6) | 4.7 (1.4) | 24.5 (1.8) | 6.6 (0.9) | 10 (1.6) | 0 | 0 | 54.8 (1.5) | 5 |
| fab1, fae1, AnD9D,SnD9D | 12.7 (2.1) | 17.9 (1.8) | 0.8 (0.1) | 0.2 (0.1) | 17.7 (0.9) | 5.8 (0.7) | 24.1 (1.2) | 20.8 (0.9) | 0 | 0 | 23.7 (1.9) | 15 |
| fab1, fae1, Fab1-HPAS Com25, AnD9D,SnD9D | 11.2 (1.3) | 43.4 (3.3) | 0.7 (0.5) | 0.9 (0.2) | 4.6 (1.5) | 23.2 (1.1) | 8.4 (1.2) | 7.6 (1.6) | 0 | 0 | 66.6 (3.9) | 12 |
| Doxantha | 18.0 (0.5) | 54.6 (1.7) | 2.4 (0.5) | 2.0 (0.1) | 1.8 (0.2) | 17.3 (1.5) | 3.9 (0.7) | 0 | 0 | 0 | 71.9 (1.3) | |
Total ω-7 FAs, the mol % of 16:1Δ9 + 18:1Δ11 FA species; double bond positions validated by their coelution with authentic standards upon gas chromatographic separation and by mass spectrometry of their pyrrolidide derivatives.
Δ ω-7, difference between the parental line and the line containing the introduced element as indicated by underlining in the genotype column.
Values represent mean (±se), n = 10 (or greater).
To simplify analysis, we combined the fab1 and fae1 mutations because FAE1 is responsible for the extraplastidial elongation of 18:1Δ11 to 20:1Δ13 (Fig. 1). The untransformed double mutant contains approximately 9% of ω-7 FAs, presumably reflecting increased desaturation of 16:0-ACP by the Δ9-18:0-ACP desaturase when presented with increased levels of 16:0-ACP substrate (Fig. 2E; Table II). Expression of Com25 in the fab1 fae1 background resulted in an increase of 16:1Δ9 and 18:1Δ11 to approximately 26% and approximately 23% respectively, yielding an increase of ω-7 FA to approximately 50% (Fig. 2F; Table II).
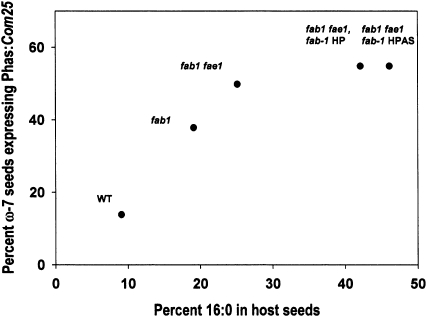
From the above results, increased accumulation of 16:1 in fab1 and fab1 fae1 mutant backgrounds correlates with increased 16:0, and so we sought additional lines in which 16:0 levels were elevated with respect to that of the fab1 fae1 double mutant. Two such lines were recently reported in which fab1 was suppressed, one by hairpin RNA interference (RNAi; Pidkowich et al., 2007) and the other by a novel method of suppression termed hairpin-antisense (HPAS) RNAi (Nguyen and Shanklin, 2009). These lines contain strongly elevated seed 16:0 accumulation levels at 42% and 46%, respectively (Fig. 3). Transformation with Com25 yielded a further increase of approximately 5% in ω-7 FA in both cases relative to the host background (Table II). Thus, when Com25 is expressed in host lines differing in 16:0 levels, the accumulation of ω-7 FA is proportional to 16:0 levels up to approximately 30% with little difference observed in hosts accumulating 42 or 46% 16:0 (Fig. 3).
Figure 3.
Relationship between 16:0 in host seeds versus ω-7 FA accumulation (as mol %).
Expression of Extraplastidial Δ9-16:0 Desaturases Increases ω-7 FA Accumulation
The approach to increasing ω-7 FA accumulation via the expression of a plastidial 16:0-ACP desaturase mimics the strategy employed by plants that naturally accumulate high levels of ω-7 FA. As noted above, the use of a host Arabidopsis with elevated 16:0 correlates with the formation of ω-7 FA. To test our hypothesis that the 16:0 had exited the plastid and was unavailable for Com25 to desaturate, we explored two approaches to reducing 16:0 accumulation in seed oil. One strategy was to increase levels of 16:0-ACP in the plastid by reducing the FATB thioesterase (Jones et al., 1995) activity responsible for the release of 16:0 from 16:0-ACP (Fig. 1). However, pilot experiments showed that suppression of FatB had only a minor effect on increasing ω-7 FA accumulation and so we pursued an alternative approach of desaturating the 16:0 after export from the plastid.
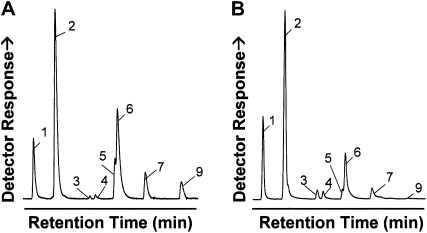
Upon exiting the plastid, free FAs are esterified to CoA by acyl-CoA synthases en route to their accumulation as triacylglycerols (Shockey et al., 2003). These cytoplasmic fatty acyl-CoAs and phospholipid-linked FA constitute pools of substrates that could potentially be intercepted by extraplastidial desaturases. In preliminary experiments, the expression of extraplastidial fungal desaturases from Stagonospora nodorum (SnΔ9D) and Aspergillus nidulans (AnΔ9D), either singly or in combination, was evaluated with respect to reducing 16:0 levels in Arabidopsis. These desaturases are homologs of ole1, the well-characterized acyl-CoA Δ9-desaturase from Saccharomyces cerevisiae. We observed that coexpression of the two fungal desaturases under the control of the phaseolin promoter yielded promising results in reducing 16:0 levels in wild-type Arabidopsis. We therefore tested the expression of these desaturases along with Com25 in a KASII HPAS-RNAi suppression line. Expression of the extraplastidial fungal desaturases resulted in the conversion of approximately half of the residual 16:0 to 16:1Δ9, resulting in a decrease of seed 16:0 from approximately 21% to approximately 11% (approximately the level seen in wild-type seeds) with a corresponding increase in 16:1Δ9 from approximately 30% to approximately 43% (Table II). The fae1 mutant is almost entirely devoid of 16:1Δ9 elongation activity so that levels of 18:1Δ11 are unaffected in the host fab1 fae1 Com25 line when transformed with SnΔ9D and AnΔ9D (approximately 25% and 23%, respectively). Coexpression of plastidial and extraplastidial desaturases yielded a mean accumulation of approximately 67% ω-7 FA, with individual lines showing >71% ω-7 FA (Fig. 4). Seeds of the highest ω-7 FA (Fig. 4). accumulating lines had equivalent oil content to those of the wild type, and germination, growth, and development appeared unaffected by the accumulation of ω-7 FA.
Figure 4.
Representative chromatograms of FAMES from the best fab1 fae1, Phas:Com25, Fab1-HPAS, AnD9D, and SnD9D transformant line (A) and Doxantha seed (B). Peak designations are as described in Figure 2.
DISCUSSION
It has been estimated that there may be upwards of 1,000 FA structures in nature (Millar et al., 2000). Many of these FA are synthesized by an array of variants of archetypal desaturases (Shanklin and Cahoon, 1998). The isolation of many of these desaturases and desaturase-like enzymes led to the expectation that their heterologous expression would lead to the production of the corresponding unusual FA at high levels. After more than a decade of effort, the production of commercial levels of unusual FA based on the expression of variant desaturase enzymes has yet to be reported (Napier, 2007). A contributing factor may be that unusual FA producing acyl-ACP desaturase enzymes have in vitro turnover numbers one or two orders of magnitude below those of the archetypal desaturases from which they evolved. This may reflect the fact that natural evolution proceeds via random mutagenesis, in which achieving the combinations of specific changes at specific amino acid locations necessary to effect changes in functionality is commonly accompanied by a large number of changes distributed throughout the gene, a subset of which degrade the enzyme’s kinetic properties. For instance, the Doxantha desaturase, the more active of the two naturally occurring desaturases investigated herein (Table I), has a kcat for 16:0 that is 135-fold lower than the approximately 1 s−1 of the castor wild-type desaturase with 18:0 substrate and 35-fold lower than the castor desaturase variant Com25 with 16:0 substrate. Others have suggested the poor in vitro performance of variant acyl-ACP desaturases may reflect a requirement for specific ferredoxins or ACPs (Suh et al., 1999; Schultz et al., 2000). However, despite work in our and other labs, to date there are no reports that coexpression of such factors has enhanced unusual FA accumulation.
Com25 exhibits a specificity factor for 16:0 equivalent to that of the castor wild-type desaturase for its natural 18:0 substrate. Thus, a key factor in the successful production of high levels of ω-7 FA in this study is the use of Com25 with its improved Km for 16:0-ACP allowing it to compete more effectively with FatB (the 16:0-ACP thioesterase) and KASII (the 16:0-ACP elongase) for substrate (see Fig. 1). That ω-7 FA increases proportionately with 16:0 levels up to approximately 30% suggests that the substrate is limiting in this range above which Com25, ferredoxin, reductant, or molecular oxygen may become limiting.
The strategy of expressing a 16:0-acyl-ACP desaturase to accumulate ω-7 FA was designed to mimic the strategy employed by naturally occurring ω-7 FA accumulating plants. The suppression of the KASII elongase activity resulted in increased release of 16:0 from ACP by FATB (Salas and Ohlrogge, 2002). FAs exiting the plastid traverse the cytosolic acyl-CoA pool en route to storage as triacylglycerols, presenting an additional opportunity to convert 16:0 to 16:1 in the cytoplasm. Coexpression of fungal 16:0-CoA desaturases facilitated the conversion of approximately 50% of the residual 16:0 into 16:1Δ9 product.
Here, we demonstrate proof of concept in Arabidopsis seeds for a strategy to accumulate desired ω-7 monoene products to industrially relevant levels. Different strategies will likely be necessary to optimize the accumulation of oxygenated FAs, such as ricinoleic or vernolic acid (Lee et al., 1998), for which downstream enzymes, such as acyltransferases, with improved specificity for the unusual FAs appear to be important for improving the accumulation of the unusual FAs (Burgal et al., 2008). In another example involving the production of laurate in canola, the coexpression of a coconut (Cocos nucifera) lysophosphatidic acid acyltransferase with the California bay medium-chain thioesterase enhanced laurate accumulation from 50% to 60% in the best example of unusual FA production to date (Knutzon et al., 1999). It is possible that the levels of ω-7FA reported herein could be further optimized by expression of appropriate acyltransferases or by fine-tuning the expression of thioesterases.
CONCLUSION
The optimization of any unusual FA will likely require the stacking of multiple traits (Napier, 2007). Thus, our approach of (1) identifying and expressing a natural or engineered desaturase with favorable kinetic parameters, (2) quantifying incremental improvements resulting from additional transgene coexpression, and (3) modulation of endogenous activities followed by (4) trait stacking is a general strategy that will likely result in the successful optimization of a variety of unusual FAs.
Specifically, to optimize the accumulation of ω-7 FA we (1) introduced an engineered enzyme optimized for its specificity factor (kcat/Km) capable of competing with endogenous enzymes for substrate in vivo, (2) optimized the level of substrate by manipulating the levels of the competing enzyme KASII, and (3) increased product yield by expressing additional and compartment-specific 16:0-CoA desaturases.
In summary, we exemplified a strategy for metabolically engineering the sustainable production of an ω-7 FA feedstock for the production of a high-demand product, octane, in higher plants.
MATERIALS AND METHODS
Arabidopsis Growth and Transformation
Arabidopsis (Arabidopsis thaliana) plants were grown in soil under continuous exposure to 300 μE of light (1 μE = 1 mol of light) in E7/2 controlled environment growth chambers (Conviron). The plants were transformed according to Clough and Bent’s method (Clough and Bent, 1998) using Agrobacterium tumefaciens strain GV3101. We identified individual T1 seeds carrying the transgenes by the fluorescence emitted (Stuitje et al., 2003) upon illumination with green light from an X5 LED flashlight (Inova) in conjunction with a 25A red camera filter (Pidkowich et al., 2007). A WILD M3Z dissection microscope equipped with an Olympus U-LH100HG illumination system was used to discriminate between seeds carrying Zs-Green and Ds-Red markers with the use of filters fluorescein isothiocyanate 535 and fluorescein isothiocyanate 515, respectively. Seed-specific expression was achieved by placing constructs under the control of the phaseolin seed storage protein promoter or the LTP170 promoter (Slightom et al., 1983; van der Geest and Hall, 1997).
Source of Com25
Com25 is a variant of the Ricinus communis Δ9-18:0-ACP desaturase that arose from a program of combinatorial saturation mutagenesis/selection designed to identify variants with improved activity toward acyl chains of <18C in length (Whittle and Shanklin, 2001). Com25 differs from the parental castor desaturase at the following 5 amino acid positions: M114S, T117R, L118C, P179L, and G188T (numbered according to the mature castor desaturase Protein Databank entry 1AFR).
Plasmid Constructs
Phas:Com25
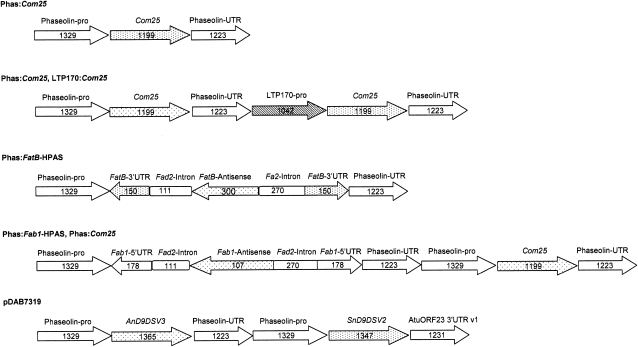
The entire open reading frame of the castor variant Com25, engineered to contain its authentic transit peptide and flanked by 5′ PacI and 3′ XhoI restriction sites, was cloned into the corresponding sites of plasmid pDs-Red-Phas (Pidkowich et al., 2007; with Ds-Red marker) to create Phas:Com25 (Fig. 5).
Figure 5.
Schematic arrangement of DNA elements in constructs described in this article. Numbers indicate size in base pairs.
Phas:Fab1-HPAS
Construction of the Phas:Fab1-HPAS construct was described previously (Nguyen and Shanklin, 2009).
Phas:FatB-HPAS
This construct was created in two steps: first, the construction of Phas:FatB-HP, and afterward the insertion of an antisense portion of the FatB gene to replace part of the Fad2 intron separating the sense and antisense portions of the FatB gene comprising the hairpin. To achieve this, 150 bp of the Arabidopsis FatB 3′ untranslated region was amplified from genomic DNA in both sense (using primers FatB-hps-5′PstI 5′-GGGCTGCAGAAACAAGTTTCGGCCACCAACCC-3′ and FatB-hps-3′XhoI 5′-CCCCTCGAGACATCAGAATTCGTAATGAT-3′) and antisense (using primers FatB-hpa-5′NheI 5′-GGGGCTAGCAAGTTTCGGCCACCAACCC-3′ and FatB-hpa-3′PacI 5′-CCCTTAATTAAACATCAGAATTCGTAATGAT-3′) orientations. These fragments were restricted with PstI/XhoI and NheI/PacI and used to replace the 5′ untranslated region sense and antisense portions of Fab1 in pGEM-T-Easy-HTM3 (Pidkowich et al., 2007) at their equivalent sites to create the intermediate plasmid pGEM-T-Easy-HTM4. To create a 300-bp antisense portion of the FatB coding region, a fragment was amplified with primers FatB-Exon-5′Sp-Bam (5′-CCACTAGTGGATCCACCTCTGCTACGTCGTCATT-3′) and FatB-Exon-3′Bg-Sal (5′-GGAGATCTGTCGACGTAGTTATAGCAGCAAGAAG-3′), and the fragment, restricted with BamHI and SalI, was used to replace part of the Fad2 intron after restriction with BglII and SpeI to create pGEM-T-Easy-HTM5.
The assembled HPAS fragment was excised with the use of PacI and XhoI and cloned into the equivalent sites of pZs-Green-Phas:Com25 (plasmid pDs-Red-Phas:Com25, described above, in which the fluorescence marker pCVMV:Ds-Red was replaced by a green fluorescent protein marker pCVMV:Zs-Green [Clontech]) to create plasmid Phas:FatB-HPAS (Fig. 5).
Phas:AnΔ9D and Phas:SnΔ9D
Two fungal acyl-CoA Δ9 desaturases were combined in plasmid pDAB7318 with both genes being driven by the seed-specific Phas promoter from Phaseolus vulgaris. The first gene in the construct was an acyl-CoA Δ9-desaturase from Aspergillus nidulans that was redesigned and synthesized for optimal expression in plants and fused to the 3′ untranslated region and 3′ MAR from the P. vulgaris phaseolin gene. The second desaturase gene in this construct was an acyl-CoA Δ9-desaturase from Stagonospora nodorum that was also redesigned and synthesized for plant expression and fused to the A. tumefaciens ORF23 3′ untranslated region (Fig. 5). This desaturase was identified by homology searches of the S. nodorum genome sequence released by the Stagonospora nodorum Sequencing Project (Broad Institute of Harvard and MIT; http://www.broad.mit.edu). It was shown to have a preference for desaturation of palmitate by complementation of the ole1 mutant of Saccharomyces cerevisiae (Stukey et al., 1990).
Phas:Fab1-HPAS, Phas:Com25
To simplify gene stacking experiments, we constructed plasmid Phas:Fab1/HPAS and Phas:Com25 to combine Com25 expression with KASII suppression. To achieve this, the EcoRV-EcoRV fragment containing the phaseolin promoter driving Com25 along with the phaseolin terminator was isolated from Phas:Com25 and cloned into the intermediate vector pBL to create pBL-Phas:Com25-PhasTer. This Com25 expression cassette was excised using flanking EcoRI-EcoRI restriction sites and cloned into the corresponding site within Phas:Fab1-HPAS to create Phas:Fab1-HPAS-Phas-Com25 (Fig. 5).
FA Analysis
To analyze the FAs of single seeds, we prepared fatty acid methyl esters (FAMEs) by incubating the seeds with 0.2 m trimethylsulfonium hydroxide in methanol (Butte et al., 1982). To similarly analyze bulk seeds, FAMEs were prepared by incubation in 0.5 mL BCl3 for 1 h at 80°C, extracting them with 1 mL of hexane, and then drying under N2. FAMEs were analyzed either with an HP6890 gas chromatograph-flame ionization detector (Agilent Technologies) or an HP5890 gas chromatograph-mass spectrometer (Hewlett-Packard) fitted with 60-m × 250-μm SP-2340 capillary columns (Supelco). The oven temperature was raised during the analyses from 100°C to 240°C at a rate of 15°C min−1 with a flow rate of 1.1 mL min−1. Mass spectrometry was performed with an HP5973 mass selective detector (Hewlett-Packard). We determined the double-bond positions of monounsaturated FAMEs by dimethyl disulfide derivatization (Yamamoto et al., 1991).
Acknowledgments
We thank Mark Thompson for identification of the Arabidopsis LTP170 promoter and Paul Roessler for helpful discussions.
References
- Anonymous (2007) Process Evaluation/Research Planning Report, On-Purpose Octene-1. Nexant ChemSystems, White Plains, NY [Google Scholar]
- Bondaruk M, Johnson S, Degafu A, Boora P, Bilodeau P, Morris J, Wiehler W, Foroud N, Weselake R, Shah S. (2007) Expression of a cDNA encoding palmitoyl-acyl carrier protein desaturase from cat’s claw (Doxantha unguis-cati L.) in Arabidopsis thaliana and Brassica napus leads to accumulation of unusual unsaturated fatty acids and increased stearic acid content in the seed oil. Plant Breed 126: 186–194 [Google Scholar]
- Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J. (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6: 819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte W, Eilers J, Hirsch K. (1982) Trialkysulfonium-hydroxides and trialkylselonium-hydroxides for the pyrolytic alkylation of acidic compounds. Anal Lett 15: 841–850 [Google Scholar]
- Cahoon EB, Coughlan SJ, Shanklin J. (1997) Characterization of a structurally and functionally diverged acyl-acyl carrier protein desaturase from milkweed seed. Plant Mol Biol 33: 1105–1110 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Shah S, Shanklin J, Browse J. (1998) A determinant of substrate specificity predicted from the acyl-acyl carrier protein desaturase of developing cat’s claw seed. Plant Physiol 117: 593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson AS. (2009) Plant oils as feedstock alternatives to petroleum - A short survey of potential oil crop platforms. Biochimie 91: 665–670 [DOI] [PubMed] [Google Scholar]
- Carlsson AS, LaBrie ST, Kinney AJ, von Wettstein-Knowles P, Browse J. (2002) A KAS2 cDNA complements the phenotypes of the Arabidopsis fab1 mutant that differs in a single residue bordering the substrate binding pocket. Plant J 29: 761–770 [DOI] [PubMed] [Google Scholar]
- Chisholm MJ, Hopkins CY. (1965) Fatty acids of Doxantha seed oil. J Am Oil Chem Soc 42: 49–50 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dyer JM, Stymne S, Green AG, Carlsson AS. (2008) High-value oils from plants. Plant J 54: 640–655 [DOI] [PubMed] [Google Scholar]
- Hopkins CY, Chisholm MJ. (1961) Development of oil in the seed of Asclepias syriaca L. Can J Biochem Physiol 39: 829–835 [DOI] [PubMed] [Google Scholar]
- James DW, Dooner HK. (1990) Isolation of EMS-induced mutants in Arabidopsis altered in seed fatty acid composition. Theor Appl Genet 80: 241–245 [DOI] [PubMed] [Google Scholar]
- Jones A, Davies HM, Voelker TA. (1995) Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell 7: 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutzon DS, Hayes TR, Wyrick A, Xiong H, Davies M, Voelker TA. (1999) Lysophosphatidic acid acyltransferase from coconut endosperm mediates the insertion of laurate at the sn-2 position of triacylglycerols in lauric rapeseed oil and can increase total laurate levels. Plant Physiol 120: 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Lenman M, Banaś A, Bafor M, Singh S, Schweizer M, Nilsson R, Liljenberg C, Dahlqvist A, Gummeson PO, et al. (1998) Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation. Science 280: 915–918 [DOI] [PubMed] [Google Scholar]
- Meier MAR. (2009) Metathesis with oleochemicals: new approaches for the utilization of plant oils as renewable resources in plant science. Macromol Chem Phys 210: 1073–1079 [Google Scholar]
- Millar AA, Smith MA, Kunst L. (2000) All fatty acids are not equal: discrimination in plant membrane lipids. Trends Plant Sci 5: 95–101 [DOI] [PubMed] [Google Scholar]
- Napier JA. (2007) The production of unusual fatty acids in transgenic plants. Annu Rev Plant Biol 58: 295–319 [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Shanklin J. (2009) Altering Arabidopsis oilseed composition by a combined antisense-hairpin RNAi gene suppression approach. J Am Oil Chem Soc 86: 41–49 [Google Scholar]
- Pidkowich MS, Nguyen HT, Heilmann I, Ischebeck T, Shanklin J. (2007) Modulating seed beta-ketoacyl-acyl carrier protein synthase II level converts the composition of a temperate seed oil to that of a palm-like tropical oil. Proc Natl Acad Sci USA 104: 4742–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak A, Fokou PA, Meier MAR. (2008) Metathesis as a versatile tool in oleochemsitry. Eur J Lipid Sci Technol 110: 797–804 [Google Scholar]
- Salas JJ, Ohlrogge JB. (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403: 25–34 [DOI] [PubMed] [Google Scholar]
- Schultz DJ, Suh MC, Ohlrogge JB. (2000) Stearoyl-acyl carrier protein and unusual acyl-acyl carrier protein desaturase activities are differentially influenced by ferredoxin. Plant Physiol 124: 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J, Cahoon EB. (1998) Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol 49: 611–641 [DOI] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse J. (2003) Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme a synthetases. Plant Physiol 132: 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom JL, Sun SM, Hall TC. (1983) Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci USA 80: 1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje AR, Verbree EC, van der Linden KH, Mietkiewska EM, Nap JP, Kneppers TJ. (2003) Seed-expressed fluorescent proteins as versatile tools for easy (co)transformation and high-throughput functional genomics in Arabidopsis. Plant Biotechnol J 1: 301–309 [DOI] [PubMed] [Google Scholar]
- Stukey JE, McDonough VM, Martin CE. (1990) The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J Biol Chem 265: 20144–20149 [PubMed] [Google Scholar]
- Suh MC, Schultz DJ, Ohlrogge JB. (1999) Isoforms of acyl carrier protein involved in seed-specific fatty acid synthesis. Plant J 17: 679–688 [DOI] [PubMed] [Google Scholar]
- Suh MC, Schultz DJ, Ohlrogge JB. (2002) What limits production of unusual monoenoic fatty acids in transgenic plants? Planta 215: 584–595 [DOI] [PubMed] [Google Scholar]
- van der Geest AH, Hall TC. (1997) The beta-phaseolin 5′ matrix attachment region acts as an enhancer facilitator. Plant Mol Biol 33: 553–557 [DOI] [PubMed] [Google Scholar]
- Whittle E, Shanklin J. (2001) Engineering delta 9-16:0-acyl carrier protein (ACP) desaturase specificity based on combinatorial saturation mutagenesis and logical redesign of the castor delta 9-18:0-ACP desaturase. J Biol Chem 276: 21500–21505 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Shibahara A, Nakayama T, Kajimoto G. (1991) Determination of double-bond positions in methylene-interrupted dienoic fatty acids by GC-MS as their dimethyl disulfide adducts. Chem Phys Lipids 60: 39–50 [Google Scholar]