Abstract
The impact of gestational dam restraint stress on progeny immune and neuroendocrine temporal hormone responses to lipopolysaccharide (LPS) challenge was assessed. Maternal stress (5-min snout snare restraint stress during d 84 to 112 of gestation) increased (P < 0.05) the magnitude of tumor necrosis factor (TNF)-α, interleukin (IL)-6, epinephrine (E), norepinephrine (NE) and serum amyloid A (SAA) production following LPS infusion in the offspring. Moreover, these effects appear to be dependent on gender for TNF-α, E, EPI and cortisol production. However, maternal stress did not affect (P > 0.05) the normalization of proinflammatory cytokines or neuroendocrine hormones produced following LPS. Collectively, these results indicate that maternal stress impacts aspects of the proinflammatory cytokine and stress hormone response in their progeny following LPS dosing of the offspring. This response is potentially responsible in part for the resultant changes to SAA production. As several of the changes observed here are dependent on pig gender, these results are also the first evidence that inherent epigenetic factors coupled with maternal stress impacts the cumulative response to stress and LPS in young pigs.
Keywords: cytokine, gender, lipopolysaccharide, maternal stress, pig
1. Introduction
Routine swine industry management procedures such as restraint, transportation, and changes in housing conditions can be stressful for the gestating sow and cause activation of the hypothalamic-pituitary-adrenal (HPA) axis [1-3]. The resultant maternal glucocorticoid (GC) release can cross the placenta in pigs [4] and may exert secondary effects on fetal development. Stress of this nature is termed maternal or prenatal stress [2]. The influence of maternal GC on fetal HPA axis development may also affect function of this axis throughout the life of the progeny [3,5]. Specifically, maternally stressed offspring could have an HPA axis primed to be hyperactive to stressors and the subsequent altered production of stress-related hormones would adversely effect growth, health, reproduction and the general welfare of the animal. There is evidence that maternal reprogramming of the progeny's HPA axis can influence the in vitro response of their immune cells [6,7]. Whereas effects of maternal stress on the HPA axis of the offspring have been documented, little information is available concerning the effect of maternal stress on the activity of the sympathetic nervous system and concomitant release of adrenal catecholamines. There is also little evidence detailing the impact of maternal stress on the immune and stress responses to an in vivo immune challenge of the offspring. As previous results from this lab suggest temporal and gender effects on LPS-induced stress hormone and cytokine responses [8], the present report describes the effects of maternal stress on immune and stress responses of male and female progeny to in vivo LPS administration.
2.1 Materials and Methods
2.1. Animals and Experimental Design
All experimental procedures were in accordance with the Guide for the Care and Use of Agriculture Animals in Agricultural Research and Teaching and approved by the Institutional Animal Care and Use Committee of Texas A&M University, Kingsville. The effects of maternal stress on stress response and immune function in their offspring was evaluated. The sows were housed in gestation stalls, fed once daily, and allowed ad libitum access to water throughout gestation according to standard practices at the Texas Tech University Swine Farm. Sows were assigned to one of two treatment groups: non-stressed or stressed. The sows assigned to the stress treatment were subjected to restraint stress for 5 min each day from wk 12 to wk 16 (d 84 to 112) of gestation. Restraint of the sow was performed by using a nose sling comprised of a soft cotton material. Control sows continued through gestation without treatment. On d 112 of gestation, the sows were moved into farrowing crates. After farrowing (within 24 h) the pigs were processed according to standard practices at the Texas Tech University Swine Farm (needle teeth clipped, tail docked, ear notches for identification and any males were castrated). At weaning (20.0 ± 0.3 d of age) 2 barrows (B) and 2 gilts (G) from each of 10 control (NS) and 10 stressed litters (S; 40 pigs per experimental group, n=80 overall), were taken to the Livestock Issues Research Unit's nursery facility.
Pigs were weighed, placed in individual pens (4 ft × 2 ft), and allowed ad libitum access to food and water. The pigs were given 14 d to adjust to their surroundings and diet. All pigs were weighed and non-surgically fitted with an indwelling jugular catheter according to Carroll et al. [9] 1 d prior to LPS infusion. Pigs were then given 24 h to recover from the cannulation procedure before blood collection began. Prior to the first sample, an extension was attached to the catheter to allow for remote sampling without handling of the pigs. Blood samples were taken every 30 min from 1 h prior to and for 6 h after LPS (Escherichia coli 0111:B4; Sigma L-2630, Sigma Chemical, St. Louis, MO; 25 μg/kg body weight) infusion. Approximately 5 mL of blood were drawn at each time point into a serum tube, allowed to clot for 1 h at room temperature, centrifuged at 1400 × g for 20 min at 20°C, serum collected into micro-centrifuge tubes and then stored at -80°C for later analysis. Total white blood cell and white blood cell differential counts were performed on whole blood samples taken at -0.5, 5.5 and 24 h using a Cell-Dyn differential analyzer (Abbott Laboratories; Abbott Park, IL. USA).
2.2. Serum analysis
Serum concentration of cortisol was determined in duplicate using a commercially available Coat-a-Count assay kit (Diagnostic Products Corp.; Los Angeles, CA, USA). Serum concentrations of epinephrine and norepinephrine (pg/mL) were determined using an EIA kit (Tri-Cat-EIA; American Laboratory Products Company, Windham, New Hampshire) per manufacturer's directions. Concentration of serum cytokines (TNF-α, IL-1β, IFN-γ and IL-6) was determined according to the manufacturer's protocol using a porcine specific ELISA kit for proinflammatory cytokines (SearchLight Porcine Inflammatory Cytokine Array #84664; Pierce, Rockford, IL). The acute phase proteins serum amyloid A, C-reactive protein and haptoglobin were also analyzed with commercially available kits (Tridelta PHASE™ RANGE Serum Amyloid A, C-reactive protein and Haptoglobin assays; Tridelta Diagnostic Products Inc., Morris Plains, NJ, USA). All assays were performed in duplicate and intra- and inter-assay coefficient of variance (CV) values calculated. The intra- and inter-assay coefficients of variation (CV) were less than from 8 and 9%, respectively for cytokine analyses and 11 and 12%, respectively for the catecholamines. For acute phase proteins, the intra- and inter-assay CV were less than 7 and 6%, respectively.
2.3. Statistical analysis
Calculations for area under the curve (AUC) were determined using the trapezoid method of SigmaPlot (Systat Software, Inc., San Jose, CA). All data were subjected to analysis of variance specific for repeated measures using the mixed procedure of SAS (SAS Inst., Inc., Cary, NC). Sources of variation included, sow, maternal treatment, time, sex, and their interactions. Specific treatment comparisons were made using Fisher's Protected Least Significant Difference with comparisons of P < 0.05 considered significant. Pearson's correlation coefficients were determined amongst either the magnitude of responses (peak concentrations) or amongst the duration of the responses (AUC).
3. Results
3.1. Proinflammatory Cytokines
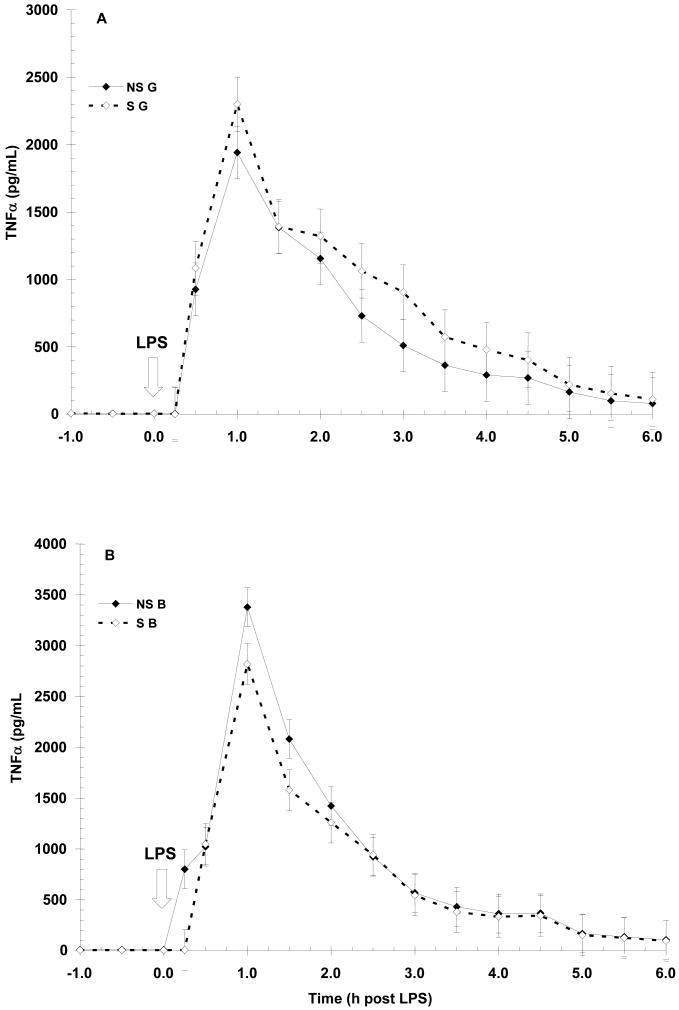
Prior to LPS (-1 to 0 h), NS animals had higher (P < 0.01) basal serum TNF-α concentration than S animals (7.4 ± 0.6 vs. 5.1 ± 0.6 pg/mL, respectively). As expected, LPS induced a time-dependent increase (P < 0.01) in TNF-α with initial increases apparent by 0.5 h post-LPS and peak concentration occurring at 1 h post-LPS (Fig. 1). Serum TNF-α then decreased for the remainder of the sampling period, reaching baseline concentration (i.e., pre-LPS) by 5 h post-LPS. Peak concentration was influenced by the main effect of sex (P < 0.06) with barrows (B) having a higher concentration of TNF-α than gilts (G; 736±37 vs. 640 ± 37 pg/mL, respectively). The overall temporal pattern was not affected by maternal treatment, however, a sex X maternal treatment interaction did exist (P < 0.05) with S B having a lower (P < 0.05) peak concentration than NS B (2819 ± 200 vs. 3378 ± 190 pg/mL, respectively). In contrast, peak concentration did not differ (P = 0.21) between NS G and S G. However, the temporal pattern of TNF-α production was similar in both maternal treatments (P = 0.99) and neither maternal treatment (P = 0.71) nor sex (P = 0.11) affected the duration of exposure to TNF-α as measured by area under the curve (AUC; 4404 ± 328 and 4577 ± 342 for NS and S, respectively; 4865 ± 333 and 4115 ± 338 for B and G, respectively). There was a tendency (P = 0.08) for a sex X maternal treatment interaction on the duration of exposure to TNF-α, as NS B tended to have a higher AUC than S B while S G tended to have a higher AUC than NS G (5197 ± 458 vs. 4534 ± 483 for NS B and S B, respectively; and 4619 ± 486 vs. 3610±471 for S G and NS G, respectively).
Figure 1.
Effect of maternal stress on the serum TNF-α response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM for gilts (NS G and S G, n = 19 and 18, respectively; Panel A) and for barrows (NS B and S B; n = 20 and 20, respectively; Panel B). Peak TNF-α concentration was influenced by the main effect of sex (P < 0.06) with barrows (B) having a higher (P < 0.05) concentration of TNF-α than gilts
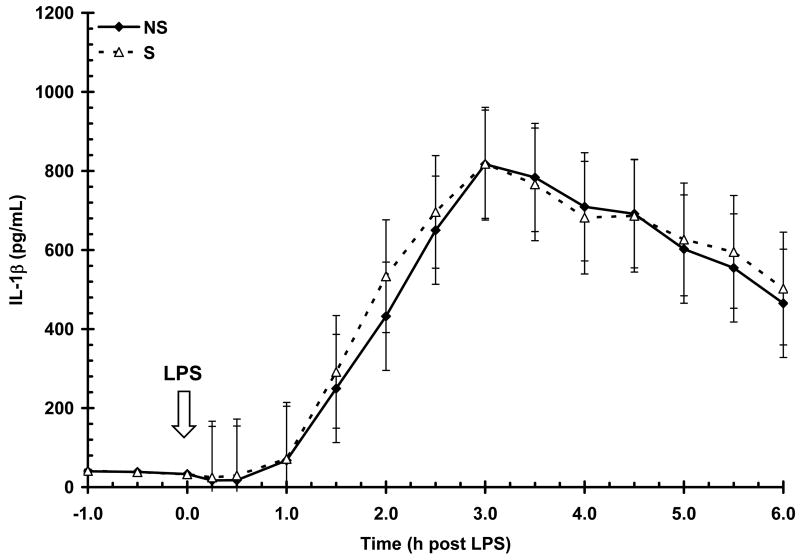
The concentration of serum IL-1β (37 ± 1 pg/mL) prior to LPS (-1 to 0 h) was not affected by sex (P = 0.78) or maternal treatment (P = 0.96). Lipopolysacharide induced a time-dependent increase (P < 0.01) in IL-1β concentration with an initial increase apparent by 1.5 h post-LPS and a peak concentration occurring at 3 h post-LPS (Fig. 2). Serum IL-1β then steadily decreased, but unlike TNF-α, remained elevated above baseline throughout the 6 h post-LPS sampling period. There was no influence of maternal treatment (P = 0.71) or sex (P = 0.34) on IL-1β production following LPS. Similarly, maternal treatment did not influence the temporal pattern (P = 1.0) or the duration of exposure to IL-1β (AUC = 2362 ± 623 and 2452 ± 650 for NS and S, respectively; P = 0.92). There was also no influence of sex on the temporal pattern (P = 0.99) or duration of IL-1β exposure (P = 0.67; AUC = 2218 ± 631 and 2596 ± 642 for B and G, respectively).
Figure 2.
Effect of maternal stress on the serum IL-1β response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM (NS, n = 39; S, n = 36). There was no influence of maternal treatment (P = 0.71) or sex (P = 0.34) on IL-1β production following LPS.
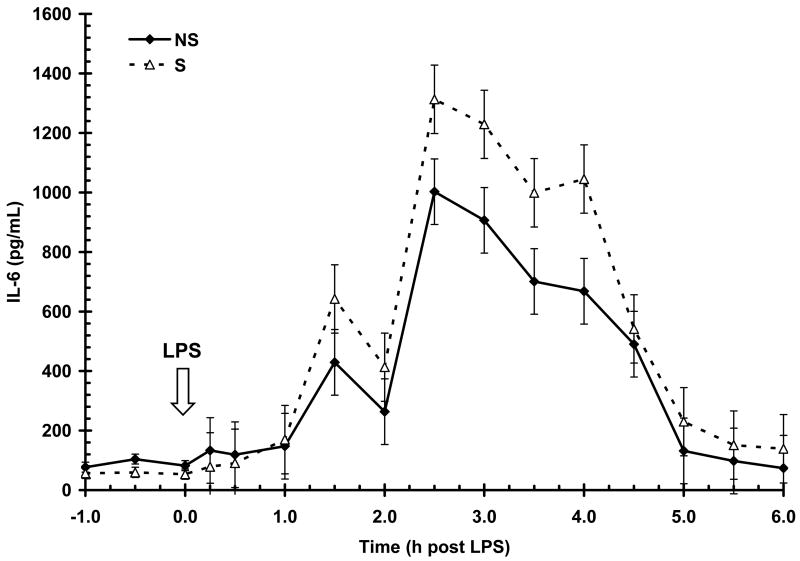
Basal serum IL-6 concentration prior to LPS (-1 to 0 h) was greater (P < 0.05) in NS animals than S (87 ± 9 vs. 56 ± 9 pg/mL, respectively). Lipopolysacharide induced a time-dependent increase (P < 0.01) in serum IL-6 with an initial increase apparent by 1.5 h post-LPS and peak concentration occurring at 2.5 h post-LPS (Fig. 3). Serum IL-6 then began to decrease and approached baseline concentration by the end of the 6 h post-LPS sampling period. Serum IL-6 post-LPS was influenced by maternal treatment (P < 0.01) with S pigs having a higher overall response than NS (506±30 vs. 374±29 pg/mL, respectively) and a higher (P < 0.05) magnitude of response (1312 ± 114 vs. 1002 ± 110 pg/mL, for S and NS respectively). The main effect of sex (P < 0.05) also influenced the IL-6 response to LPS, with G having a higher overall response than B in both NS and S pigs (485 ± 30 vs. 395±29 pg/mL, for G and B respectively). However, similar to IL-1β, the temporal pattern of IL-6 post-LPS was not influenced by maternal treatment (P = 0.60) or sex (P = 0.94). Duration of exposure to IL-6 was similar in both maternal treatments (2429 ± 457 and 2370 ± 476 for NS and S respectively; P = 0.20) and sexes (2571 ± 463 and 3128 ± 470 for B and G, respectively; P = 0.40).
Figure 3.
Effect of maternal stress on the serum IL-6 response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM (n = 39 NS and n = 36 S). Serum IL-6 post-LPS was influenced by maternal treatment (P < 0.01) with S pigs having a higher overall response than NS and a higher (P < 0.05) magnitude of response. The main effect of sex (P < 0.05) also influenced the IL-6 response to LPS, with G having a higher overall response than B in both NS and S pigs.
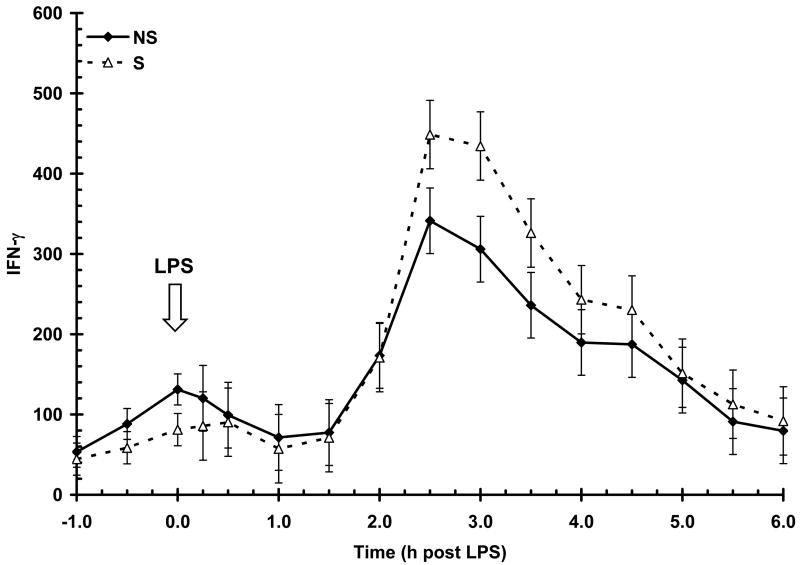
Serum IFN-γ concentration prior to LPS (-1 to 0 h) was similar (P = 0.06) between NS and S animals (90 ± 11 vs. 61 ± 11 pg/mL, respectively). Lipopolysacharide induced a time-dependent increase (P < 0.01) in serum IFN-γ, with an initial increase apparent at 2 h and peak concentration occurring at 2.5 h post-LPS (Fig. 4). The IFN-γ response was not affected by sex (P = 0.55) or maternal treatment (P = 0.11). The temporal pattern for the IFN-γ response was not influenced by maternal treatment (P = 0.63) nor sex (P = 0.94). The duration of exposure to IFN-γ was not influenced by maternal treatment (AUC = 910 ± 175 and 1089 ± 182 for NS and S, respectively; P = 0.48) nor sex (1018 ± 177 and 981 ± 180 for B and G, respectively; P = 0.88).
Figure 4.
Effect of maternal stress on the serum IFN-γ response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM (n = 39 NS and n = 36 S). The IFN-γ response was not affected by sex (P = 0.55) or maternal treatment (P = 0.11).
Correlation analysis indicated strong positive relationships between the duration of the cytokine responses (AUC), including IL-1β and IL-6 (r = 0.76, P < 0.01), IL-1β and IFN-γ (r = 0.66, P < 0.01). Similarly, there were positive relationships between the duration of the TNF-α and IL-6 and the TNF-α and IFN-γ responses following LPS (r = 0.52 and 0.43, P < 0.01, respectively). These relationships were not influenced by maternal treatment. However, positive relationships between the duration of the TNF-α and IL-1β responses was found only in NS pigs (r = 0.28, P < 0.05 vs. r = 0.18, P = 0.28 for NS vs. S pigs, respectively).
3.2. Stress hormones
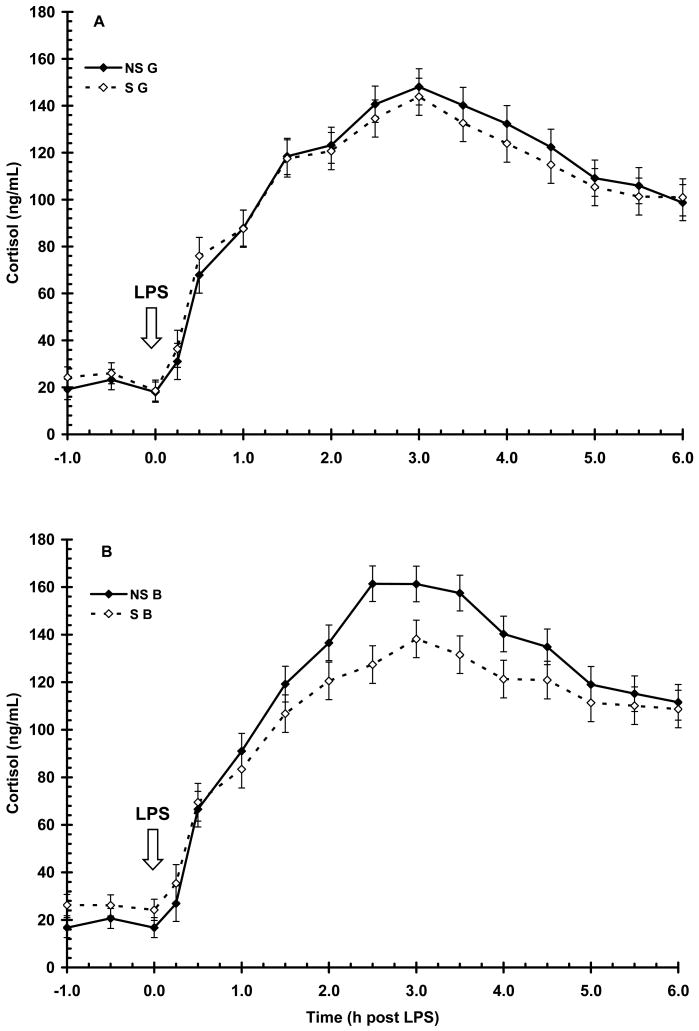
Basal serum cortisol concentration prior to LPS (-1 to 0 h) was greater (P < 0.05) in S pigs when compared to NS pigs (24 ± 1 vs. 19 ± 1 ng/mL, respectively). As expected, LPS induced a time-dependent increase (P < 0.01) in cortisol, with an initial increase apparent by 0.5 h and peak concentration occurring at 2.5 h post-LPS (Fig. 5). Serum cortisol then decreased for the remainder of the 6 h sampling period, but did not return to basal concentration (i.e., pre-LPS). There was a main effect of maternal treatment (P < 0.01) with NS pigs having a higher overall cortisol response to LPS than S pigs (107±1 vs. 100±1 ng/mL, respectively). A sex X maternal treatment interaction (P < 0.05) was evident with S B having a reduced (P < 0.01) magnitude of cortisol response following LPS than NS B (127 ± 7 vs. 161 ± 7 ng/mL, respectively), while S G and NS G had similar peak concentrations of cortisol following LPS. However, the temporal pattern of cortisol response following LPS was similar in both maternal treatments and sexes (P = 0.29 and 0.93, respectively). Furthermore, the duration of exposure to cortisol was not influenced by maternal treatment (AUC = 591 ± 24 and 547 ± 25 for NS and S pigs, respectively; P = 0.20) nor sex (AUC=577 ± 24 and 561 ± 24 for B and G, respectively; P = 0.63).
Figure 5.
Effect of maternal stress on the serum cortisol response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM for gilts (NS G and S G, n = 19 and 18, respectively; Panel A) and for barrows (NS B and S B; n = 20 and 20, respectively; Panel B). There was a main effect of maternal treatment (P < 0.01) with NS pigs having a higher overall cortisol response to LPS than S pigs.
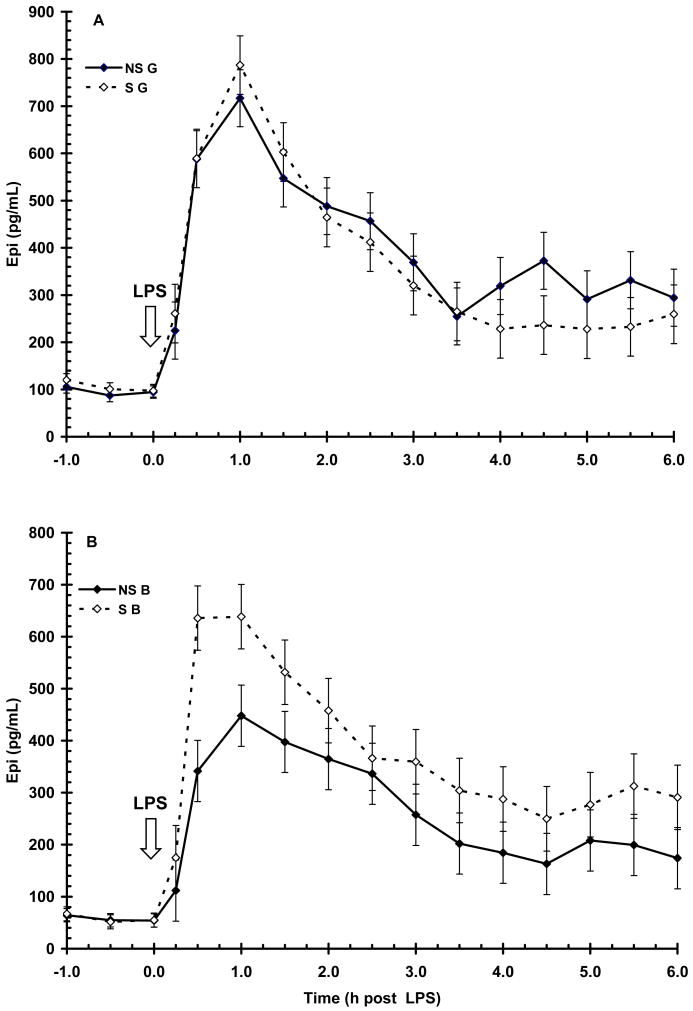
Prior to LPS (-1 to 0 h), serum concentration of E was higher (P < 0.01) in G than in B (100 ± 5 vs. 57 ± 5 pg/mL, respectively). Lipopolysacharide induced a time-dependent increase (P < 0.01) in E, with initial increase apparent by 0.25 h and peak concentration occurring at 0.5 to 1 h post-LPS (Fig. 6). Serum concentration of E decreased following peak and by the end of the sampling period concentrations were approaching baseline. There was a main effect of maternal treatment (P < 0.05) on serum E following LPS, with S pigs having a higher peak concentration of E at 1 h post-LPS than NS (712 ± 43 vs. 582 ± 42 pg/mL, respectively). Serum E concentration following LPS was also influenced by sex (P < 0.01) with G having a higher overall E response (372 ± 11 vs. 299 ± 11 pg/mL for G and B, respectively).
Figure 6.
Effect of maternal stress on the serum E response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM for gilts (NS G and S G, n = 19 and 18, respectively; Panel A) and for barrows (NS B and S B; n = 20 and 20, respectively; Panel B). There was a main effect of maternal treatment (P < 0.05) on serum E following LPS, with S pigs having a higher peak concentration of E at 1 h post-LPS than NS. Serum E concentration following LPS was also influenced by sex (P < 0.01) with G having a higher overall E response.
Similar to cortisol, serum E response to LPS was also influenced by a sex X maternal treatment interaction, with S B having a higher (P < 0.01) magnitude of response at 1 h post-LPS (638 ± 62 vs. 447 ± 58 pg/mL for S B vs. NS B, respectively). However, the magnitude of response in S G and NS G was similar (P > 0.05). As with cortisol, neither maternal treatment (P = 0.72) nor sex (P = 0.83) affected the temporal pattern of E post-LPS. The duration of exposure to E following LPS was not affected by maternal treatment (AUC = 1784 ± 180 and 1980 ± 187 for NS and S respectively; P = 0.45).
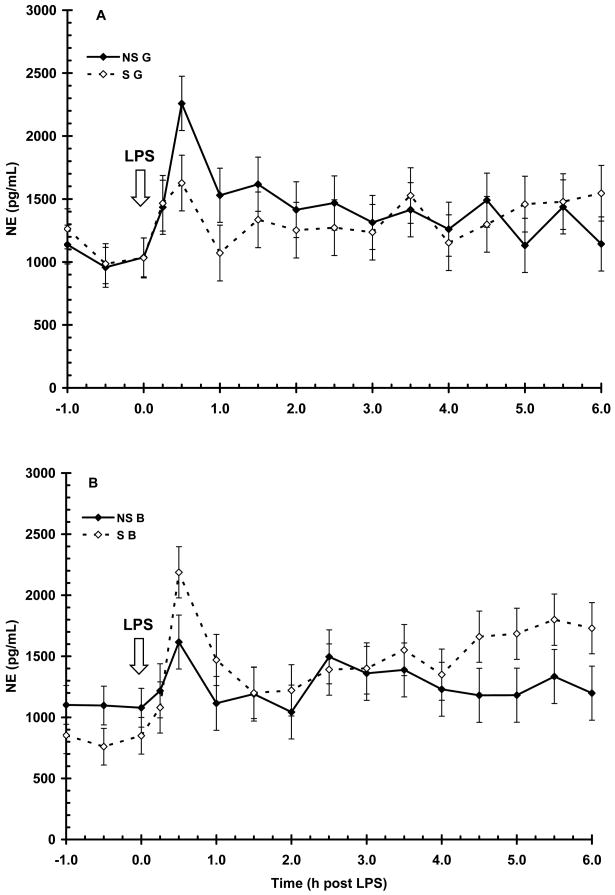
Serum NE concentration prior to LPS (-1 to 0 h) was (1024 ± 47 pg/mL) and was not influenced by maternal treatment (P = 0.23) or sex (P = 0.20). LPS induced a time-dependent increase (P < 0.01) in NE with an initial increase and peak concentration occurring by 0.5 h post-LPS (Fig. 7). Serum NE then rapidly decreased (P < 0.01) and approximated basal concentration for the remainder of the sampling period. There was no main effect of maternal treatment or sex on the NE response following LPS (P = 0.28 and 0.70, respectively). However, there was a sex X maternal treatment interaction (P < 0.05), with S B having a higher peak concentration of NE than NS B (2187 ± 220 vs. 1616 ± 209 pg/mL, respectively) while NS G had a higher peak concentration of NE then S G (2259 ± 215 vs. 1626 ± 221 pg/mL, respectively). Similar to the other stress hormones there was no main effect of maternal treatment (AUC = 6834±588 and 6913 ± 613 for NS and S, respectively; P = 0.92) nor sex (6791 ± 595 and 6955 ± 605 for B and G, respectively; P = 0.84) on the duration of exposure to NE following LPS.
Figure 7.
Effect of maternal stress on the serum NE response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM for gilts (NS G and S G, n = 19 and 18, respectively; Panel A) and for barrows (NS B and S B; n = 20 and 20, respectively; Panel B). There was no main effect of maternal treatment or sex on the NE response following LPS (P = 0.28 and 0.70, respectively).
Correlation analysis indicated a positive relationship between the magnitude of the E and NE response following LPS (r = 0.39, P < 0.01) that was not affected by maternal treatment. In contrast, there was a negative relationship between the magnitude of the NE and cortisol responses following LPS that was present in S pigs (r = -0.33, P < 0.05) but not in NS pigs (r = -0.14, P = 0.39). A relationship between the magnitude of the E and cortisol responses following LPS was not observed (r = -0.11, P = 0.36) regardless of maternal treatment. Correlation analysis also indicated a positive relationship between the duration of the E and NE responses following LPS (r = 0.52, P < 0.01) that was not influenced by maternal treatment. There were no relationships found between the duration of the cortisol and E responses (r = 0.02, P = 0.80) or cortisol and NE responses (r = -0.13, P = 0.25) regardless of maternal treatment. Positive relationships were also observed between the magnitude of the IL-1β and E responses (r = 0.56, P < 0.01) and the magnitude of the IL-6 and E responses (r = 0.52, P < 0.01) following LPS, that were not influenced by maternal treatment. There was also a positive relationship between the magnitude of the IL-6 and NE responses (r = 0.27, P < 0.05). A positive relationship was also found between the magnitude of the IL-1β and NE responses following LPS in NS pigs (r = 0.41, P < 0.01) that was not present in S pigs (r = 0.13, P = 0.44). In contrast, there was a positive relationship between the magnitude of the IFN-γ and E responses that was present only in S pigs (r = 0.51, P < 0.01). Correlation analysis also indicated positive relationships between the duration of the E response with the duration of exposure to IL-1β, IL-6 and IFN-γ following LPS (r = 0.61, 0.52 and 0.48; respectively; P < 0.01), regardless of maternal treatment. However, there was no relationship (P > 0.05) between the duration of exposure to E and the duration of TNF-α following LPS. The duration of NE response following LPS was also found to be positively related to the duration of all the proinflammatory cytokines measured in this study (r = 0.34, 0.45, 0.49 and 0.48 for TNF-α, IL-1β, IL-6 and IFN-γ, respectively; P < 0.05). However, these relationships were only present in NS pigs, and there were no relationships between the duration of NE and the proinflammatory cytokines in S pigs. There were no relationships found between the duration of the proinflammatory cytokine response and the cortisol response following LPS regardless of maternal treatment.
3.3. Leukocyte Counts
Mean WBC count prior to LPS infusion (0 h) was 15.06 ± 0.49 × 103 cells/μl, and was similar (P = 0.57) in both maternal treatments. As expected, lymphocytes and neutrophils comprised > 88% of the total WBC present in peripheral circulation, with monocytes, eosinophils, and basophils making up the remaining 11% (8.6, 1.3 and 1.6 %, respectively; Table 1). Lipopolysacharide induced an initial redistribution of leukocytes out of circulation into tissues, followed by proliferation-induced increases in leukocyte numbers. The initial redistribution of leukocytes in response to LPS was reflected by an approximate 65% decrease in circulating WBC by 5.5 h post-LPS (15.06 ± 0.5 vs. 5.28 ± 0.5 × 103 cells/μl at 0 vs. 5.5 h respectively) and was not affected by maternal treatment (P > 0.05). The redistribution was composed primarily of lymphocytes which decreased by 87% (6.62±0.15 vs. 0.84 ± 0.15 × 103 cells/μl at 0 vs. 5.5 h, respectively) and to a quantitatively lesser extent by neutrophils, monocytes, basophils, and eosinophils, which decreased by 87, 90, 89 and 91% respectively.
Table 1.
Effect of LPS challenge on WBC counts.
| Time (h post LPS) | ||||
|---|---|---|---|---|
| Item (×103/μL) | 0 | 5 | 24 | SEM |
| WBC | 15.06a | 5.28b | 18.56c | 0.50 |
| Monocytes | 1.30a | 0.13b | 1.05c | 0.05 |
| Lymphocytes | 6.62a | 0.84b | 6.80c | 0.15 |
| Neutrophils | 6.69a | 4.28b | 9.76c | 0.43 |
| Basophils | 0.19a | 0.02b | 0.18a | 0.01 |
| Eosinophils | 0.24a | 0.02b | 0.73c | 0.02 |
Values within a row with different superscripts differ, P<0.01
Correlation analysis indicated negative relationships between the duration of exposure to TNF-α, IL-1β, IL-6 and IFN-γ with total WBC counts at 5.5. h (r = -0.24, -0.32, -0.41 and -0.42, respectively; P < 0.05) along with IL-1β, IL-6 and IFN-γ with lymphocytes (r = -0.23, -0.20, and -0.25, respectively; P < 0.05). Negative relationships between neutrophil number and serum TNF-α, IL-1β, IL-6 and IFN-γ at 5.5 h post-LPS (r = -0.26, -0.31, -0.40 and -0.42, respectively; P < 0.05) were detected. There was also a negative relationship between the duration of exposure to IL-6 and monocyte count at 5.5 h post-LPS (r = -0.23, P < 0.05). Duration of exposure to the catecholamines (E and NE) was negatively associated with total WBC (-0.30 and -0.29, respectively; P < 0.05), lymphocytes (r = -0.30 and -0.24, respectively; P < 0.05) and neutrophils (r = -0.27 and -0.28, respectively; P < 0.05). In addition a negative relationship was detected between the duration of the E response and monocytes at 5.5 h post-LPS (r = -0.22, P < 0.05). There were no relationships between leukocyte counts at 5.5 h post-LPS and the duration of exposure to cortisol following LPS. Proliferation of leuckocytes was apparent at 24 h post-LPS as indicated by a 23% increase in WBC relative to numbers at 0 h (Table 1). Although there were increases in all WBC types, the quantitatively greatest increase occurred in neutrophils (6.69 ± 0.43 vs. 9.67 ± 0.43 × 103 cells/μl at 0 vs. 24 h, respectively). Correlation analysis identified positive relationships between the duration of IL-1β, IL-6 and IFN-γ responses with neutrophil count at 24 h (r = 0.34, 0.28 and 0.26, respectively; P < 0.05). Positive relationships were found between the duration of the E response and total WBC and neutrophils at 24 h post LPS (r = 0.25 and 0.41, respectively; P < 0.05). There were no relationships (P > 0.05) found between the duration of the cortisol or NE responses following LPS and the proliferation of leukocytes at 24 h post-LPS.
3.4. Acute Phase Proteins
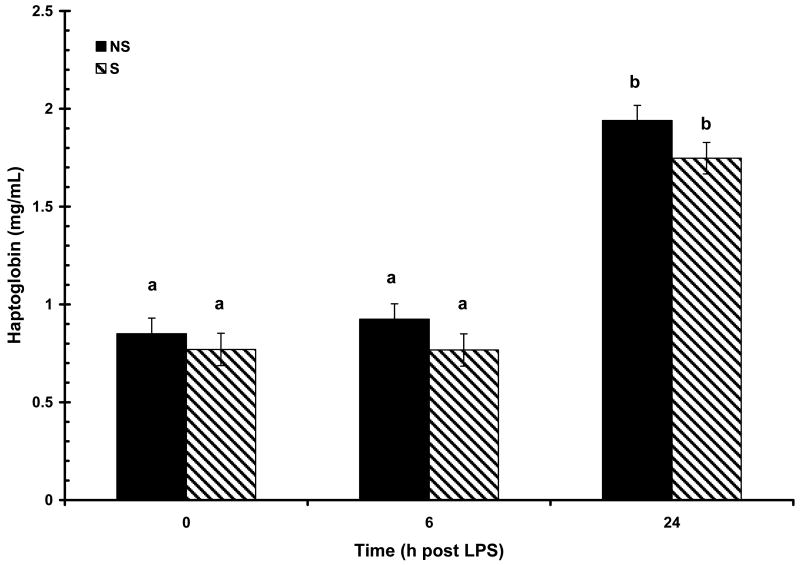
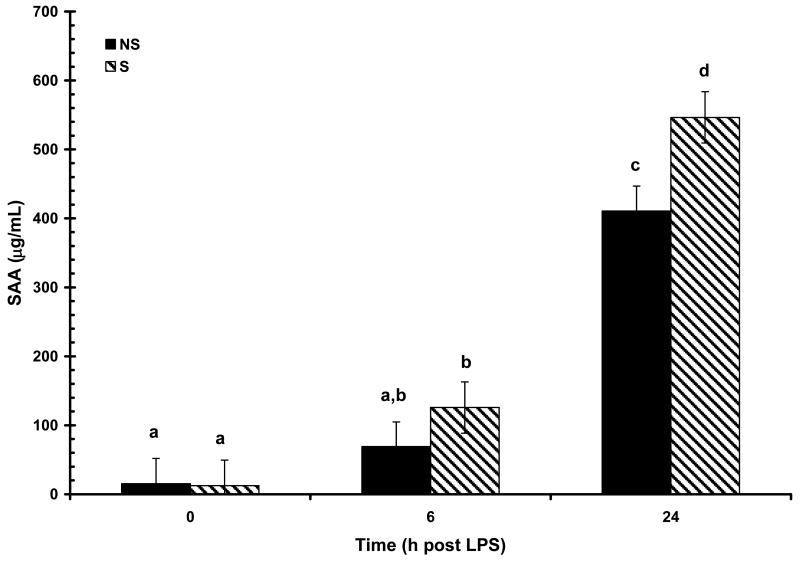
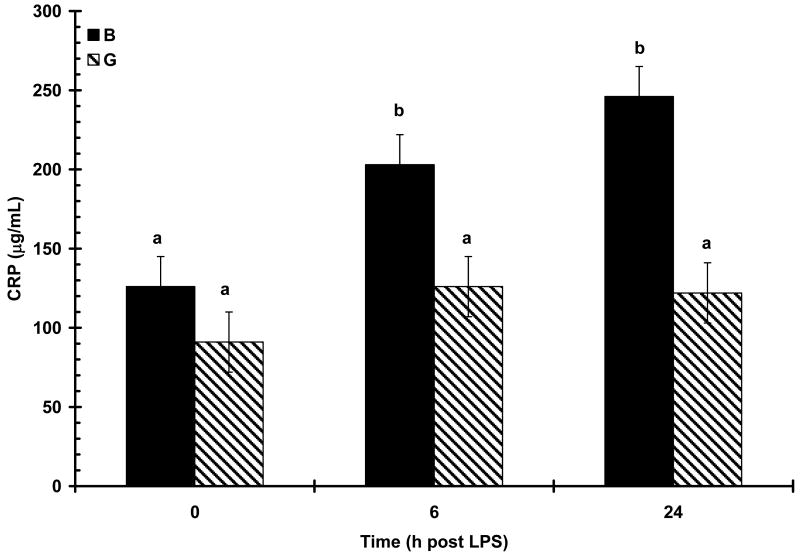
The acute phase proteins CRP, SAA and haptoglobin, were all detectable at low concentrations prior to LPS challenge at 0 h (108 ± 13, 13 ± 26 μg/mL, and 0.81 ± 0.05 mg/mL, respectively) and were not affected by maternal treatment or sex (P > 0.05). Concentrations of all APP increased (P < 0.01) in response to LPS challenge. Increased (P < 0.01) serum haptoglobin concentration was apparent at 24 h post-LPS (Fig. 8). There was a main effect of treatment (P < 0.05) on haptoglobin response following LPS as a result of NS pigs having consistently higher numerical concentration of haptoglobin at all time points compared to S pigs (0.85 ± 0.07 vs. 0.77 ± 0.08, 0.92 ± 0.07 vs. 0.76 ± .08, and 1.94 ± 0.07 vs. 1.74 ± 0.08 mg/mL, for 0, 6, and 24 h; respectively). However, maternal treatment did not affect (P = 0.77) the temporal pattern of haptoglobin response following LPS and there was no effect of sex (P = 0.99). Maternal treatment also influenced the SAA response to LPS (P < 0.05). An initial increase in SAA was apparent at 6 h post-LPS in S pigs and at 24 h in NS pigs (Fig. 9). At 24 h post-LPS S pigs had a higher (P < 0.01) concentration of SAA than NS pigs (410 ± 36 vs. 546 ± 37 μg/mL, respectively). However, there was no influence of maternal treatment on the temporal pattern of SAA following LPS (P = 0.17). As with haptoglobin, there was no effect of sex on the SAA response following LPS (P = 0.82). In contrast to haptoglobin and SAA, the CRP response to LPS was not influenced by maternal treatment (P = 0.66), but was effected by the main effect of sex (P < 0.01). There was also a tendency for a maternal treatment X time interaction (P = 0.08; Fig. 10). Basal concentration (0 h) of CRP did not differ between the sexes (P = 0.20; 126 ± 19 and 91 ± 19 μg/mL for B and G respectively). However, by 6 h post-LPS there was an increase (P < 0.01) in serum concentration of CRP in barrows that remained at 24 h post-LPS, while there was no increase (P > 0.05) in serum CRP in gilts following LPS.
Figure 8.
Effect of maternal stress on the serum haptoglobin response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM (NS, n = 39; S n = 36). Columns with differing superscripts differ (P < 0.05).
Figure 9.
Effect of maternal stress on the serum amyloid-A (SAA) response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM (NS, n = 39; S n = 36). Columns with differing superscripts differ (P < 0.05).
Figure 10.
Effect of maternal stress on the serum c-reactive protein (CRP) response of pigs to an i.v. challenge with 25 μg/kg LPS. Pigs were obtained from sows subjected to either a daily 5-min restraint stress (stressed; S) from d 84 to 112 of gestation or managed per current industry standards (non-stressed; NS). Values represent the mean ± SEM (barrows, B, n = 40 and gilts, G, n = 37). Columns with differing superscripts differ (P < 0.05).
Correlation analysis indicated positive relationships between the duration of the TNF-α, IL-6 and IFN-γ responses and concentration of SAA at 24 h post-LPS (r = 0.54, 0.55 and 0.56, respectively; P < 0.05) that were not influenced by maternal treatment. A positive relationship was also present between IL-1β and 24 h SAA (r = 0.43; P < 0.01) in NS pigs, but this relationship was not significant in S pigs (P > 0.05). As all three APP measured in this study are type I APP the lack of relationships between IL-1β with CRP and haptoglobin was unexpected. Correlation analysis also indicated positive relationships between the duration of the NE response with 24 h SAA (r = 0.58, P < 0.05) and between NE and 24 h CRP (r = 0.31, P < 0.05) in NS pigs, while these relationships were non-significant in S pigs. Interestingly, there were negative relationships found between the duration of E response in S pigs with 24 h haptoglobin (r = -0.38, P < 0.05). There were no relationships found between the duration of cortisol response with 24 h APP production regardless of maternal treatment.
4. Discussion
Previous reports on the effects of maternal stress in pigs have found that maternal stress is capable of influencing the cellular and humoral branches of the immune system in the offspring [5]. While the temporal pattern and the duration of exposure to the proinflammatory cytokines remained unaffected, maternal treatment did influence the magnitude of the TNF-α and IL-6 response to LPS. The TNF-α response also suggests that the effects of maternal stress can be gender specific.
Collectively, the data indicate that maternal stress is capable of altering both basal and post-LPS concentrations of inflammatory cytokines and stress hormones in offspring. The temporal pattern of TNF-α and IL-1β response to LPS found in this study are similar to previous reports on LPS response in pigs [10-12]. Similarly, the temporal pattern of IL-6, INF-γ, cortisol and catecholamines in this study are similar to those reported elsewhere [12-15]. These results are also consistent with previous reports on the effects of maternal stress on the offspring's stress response in both rats and pigs [2, 6-19]. The increased basal concentration of cortisol in S pigs found in this study is similar to studies in rats that reported higher basal ACTH and corticosterone concentrations in stressed animals compared to non-stressed [16, 19, 20]. However, the decreased concentration of cortisol in S barrows is in contrast to reports by Henry et al. [17], and Takahashi et al. [16], that reported increased corticosterone response to stressors in maternally stressed rats, and Haussmann et al. [2], who reported increased cortisol response to mixing stress in maternally stressed pigs. These contradicting reports may reflect differences in the type of stressor (immune challenge vs. acute physical stressors) as stressor-specific patterns of stress hormones have been documented [reviewed in 21].
The gender effects elucidated in this study are consistent with our previous results [8]. The lack of maternal treatment influence on the S gilt cortisol response to LPS is supported by a previous report that showed the same lack of maternal stress influence on cortisol response to LPS in female offspring [22]. This result is further supported by correlation analysis that indicated a negative relationship between the magnitude of the NE and cortisol response in S pigs. Stressed barrows had a higher magnitude of NE which was then followed by a lower magnitude of cortisol response. While S G had a lower magnitude of NE response and maintained a normal cortisol response. To our knowledge, there are no reports describing the effects of maternal stress on the E response to stressors. The increased concentration of E in S B however is supported by correlation analysis showing a positive relationship between NE and E production. Increased NE in S B could result in increased E in S B, since peak production of NE occurs before peak E. We have shown that the temporal pattern of stress hormone response to LPS was not influenced by maternal treatment. This is supported by data from Takahashi et al. [16] who found that although maternally stressed rats had increased levels of ACTH and corticosterone in response to stress, there was not an effect of maternal treatment on the temporal pattern in response to stress.
The lack of a discernable correlation between the proinflammatory cytokines and cortisol observed here contrasts the premise that the proinflammatory cytokines feed back at the level of the hypothalamus to promote the production of cortisol [reviewed in 23, 24]. However, the positive relationship between the catecholamines and IL-6 production is supported by previous literature that catecholamines can induce the production of IL-6 in systemic circulation [reviewed in 25]. Furthermore, the positive relationships between catecholamine production and the proinflammatory cytokine production is consistent with in vitro and in vivo evidence indicating that the catecholamines can modulate (enhance or inhibit) the response of immune cells to antigenic challenge [26, 27].
Consistent with previous observations and with reports from a number of other species, LPS induced an initial redistribution of leukocytes out of circulation into tissues, followed by proliferation-induced increases in leukocyte numbers [28-30]. These results agree with previous reports on the ability of LPS or live Echerichia coli to induce a rapid redistribution of lymphocytes and monocytes out of peripheral circulation [28-30]. The negative relationships between catecholamine production and leukocyte counts at 5.5 h post-LPS suggests that the catecholamines are involved in inducing the redistribution of leukocytes. This is supported by reports that acute stress or administration of catecholamines is capable of inducing redistribution of leukocytes [26, 27] and immune cell proliferation through activation of Th cells [31, 32]. This is further supported here by correlation analysis suggesting positive relationships between the duration of IL-1β, IL-6 and IFN-γ responses with neutrophil count at 24 h and consistent with their role in an immune response [33, 34]. The results presented here are also consistent with the ability of proinflammatory cytokines to induce APP production [34, 35]. Specifically, TNF-α and IL-1β induce the production of type I APP, and IL-6 to synergistically enhance this effect [36, 37]. However, the lack of correlation between cortisol and 24 h APP production was unexpected as cortisol is known to synergistically enhance the production of APP during an immune response [reviewed in 33, 34]. The data presented here suggest that catecholamine production is capable of influencing APP production. To our knowledge these are the first reports on the presence of these relationships.
These data indicate that maternal stress changes typical proinflammatory cytokine and stress hormone response profiles of the offspring resulting in altered responses to LPS infusion, and that these changes can be influenced by gender. Specifically, immune and stress responses of barrows from stressed sows appear to be impacted more when compared to their gilt counterparts and barrows from non-stressed sows. These data highlight the importance of managing the stress of pregnant sows to promote immune and stress responses in her offspring that are conducive for optimal production. These results also suggest a potential epigenetic stress response mechanism whereby subsequent generations are primed for handling particularly persistent environmental stressors that impact across multiple generations. The gender-specific nature of these epigenetic modifications also highlights the future need to describe their physiological relevance across generations to the respective genders. As such, these data also have important implications for human mothers and the health of their offspring as the pig is a viable model for human studies.
Footnotes
Mention of trade names or proprietary products does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products that may also be suitable.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lay DC, Jr, Randel RD, Friend TH, Jenkins OC, Neuendorff DA, Bushong DM, Lanier EK, Bjorge MK. Effects of prenatal stress on suckling calves. J Anim Sci. 1997;75:3143–3151. doi: 10.2527/1997.75123143x. [DOI] [PubMed] [Google Scholar]
- 2.Haussmann MF, Carroll JA, Weesner GD, Daniels MJ, Matteri RL, Lay DC., Jr Administration of ACTH to restrained, pregnant sows alters their pigs' hypothalamic-pituitary-adrenal (HPA) axis. J Anim Sci. 2000;78:2399–2411. doi: 10.2527/2000.7892399x. [DOI] [PubMed] [Google Scholar]
- 3.Otten W, Kanitz E, Tuxhscherer M, Nurnberg G. Effects of prenatal restraint stress on hypothalamic-pituitary-adrenocortical and sympatho-adrenomedullary axis in neonatal pigs. J Anim Sci. 2001;73:279–287. [Google Scholar]
- 4.Klemcke HG. Placental metabolism of cortisol at mid- and late gestation of swine. Biol Reprod. 1995;3:1293–1301. doi: 10.1095/biolreprod53.6.1293. [DOI] [PubMed] [Google Scholar]
- 5.Tuchscherer M, Kanitz E, Otten W, Tuchscherer A. Effects of prenatal stress on cellular and humoral immune responses in neonatal pigs. Vet Immunol Immunopathol. 2002;86:195–203. doi: 10.1016/s0165-2427(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 6.von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol. 2002;109:923–28. doi: 10.1067/mai.2002.124776. [DOI] [PubMed] [Google Scholar]
- 7.Burdick NC, Banta JP, Neuendorff DA, White JC, Vann RC, Laurenz JC, Welsh TH, Jr, Randel RD. Interrelationships among growth, endocrine, immune and temperament parameters in neonatal Brahman calves. J Anim Sci. 2009;87:3202–3210. doi: 10.2527/jas.2009-1931. [DOI] [PubMed] [Google Scholar]
- 8.Williams PN, Collier CT, Carroll JA, Welsh TH, Jr, Laurenz JC. Temporal pattern and effect of sex on lipopolysaccharide-induced stress hormone and cytokine response in pigs. Domest Anim Endocrinol. 2009;37:139–147. doi: 10.1016/j.domaniend.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Carroll JA, Daniel JA, Keisler DH, Matteri RL. Non-surgical catheterization of the jugular vein in young pigs. Lab Anim. 1999;33:1–6. doi: 10.1258/002367799780578345. [DOI] [PubMed] [Google Scholar]
- 10.Carroll JA, Gaines AM, Spencer JD, Allee GL, Kattesh HG, Roberts MP, Zannelli ME. Effect of menhaden fish oil supplementation and lipopolysaccharide exposure on nursery pigs I. Effects on the immune axis when fed diets containing spray-dried plasma. Domest Anim Endocrinol. 2003;24:341–351. doi: 10.1016/s0739-7240(03)00017-1. [DOI] [PubMed] [Google Scholar]
- 11.Carroll JA, Gaines AM, Allee GL. Immunity in Swine: An Overview of Nutritional Modulation of the Acute Phase Response. Proceedings of the Prince Agri Products Inc. Twenty-fifth Annual Feed Ingredient Conference; Kansas City, Missouri. Aug 31, 2005. [Google Scholar]
- 12.Frank JW, Carroll JA, Allee GL, Zannelli ME. The effects of thermal environment and spray-dried plasma on the acute-phase response of pigs challenged with lipopolysaccharide. J Anim Sci. 2003;81:1166–176. doi: 10.2527/2003.8151166x. [DOI] [PubMed] [Google Scholar]
- 13.Carroll JA, Carter DB, Korte SW, Prather RS. Evaluation of the acute phase response in cloned pigs following a lipopolysaccharide challenge. Domest Anim Endocrinol. 2005;29:564–572. doi: 10.1016/j.domaniend.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Webel DM, Fink BN, Baker DH, Johnson RW. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J Anim Sci. 1997;75:1514–1520. doi: 10.2527/1997.7561514x. [DOI] [PubMed] [Google Scholar]
- 15.Wright KJ, Balaji R, Hill CM, Dritz SS, Knoppel EL, Minton JE. Integrated adrenal, somatotropic, and immune responses of growing pigs to treatment with lipopolysaccharide. J Anim Sci. 2000;78:1892–1899. doi: 10.2527/2000.7871892x. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi LK, Kalin NH. Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res. 1991;558:75–8. doi: 10.1016/0006-8993(91)90715-8. [DOI] [PubMed] [Google Scholar]
- 17.Henry C, Kabbaj M, Simon J, LeMoal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neruroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 18.McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- 19.Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R. Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav. 1998;64:439–44. doi: 10.1016/s0031-9384(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 20.Fameli M, Kitraki E, Stylianopoulou F. Effects of hyperactivity of the maternal hypothalamic-pituitary-adrenal (HPA) axis during pregnancy on the development of the HPA axis and brain monoamines of the offspring. Int J Dev Neurosci. 1994;12:651–9. doi: 10.1016/0736-5748(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 21.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress disorders. Endocrine Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 22.De Groot J, Kranendonk G, Fillerup M, Hopster H, Boersma W, Hodgson D, Van Reenen K, Taverne M. Response to LPS in female offspring from sows treated with cortisol during pregnancy. Physiol Behav. 2007;90:612–8. doi: 10.1016/j.physbeh.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 24.Haddad JJ, Saade NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 25.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 26.Dhabhar FS. Stress-induced augmentation of immune function – The role of stress hormones, leukocyte trafficking, and cytokines. Brain Behavior Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 27.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – An integrative interface between two supersystems: The brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 28.Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behavior Immun. 2007;21:807–815. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gehad AE, Lillehoj HS, Hendricks GL, III, Mashaly MM. Initiation of humoral immunity. II. The effects of T-independent and T-dependent antigens on the distribution of lymphocyte populations. Dev Comp Immunol. 2002;26:761–771. doi: 10.1016/s0145-305x(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 30.Trout JM, Mashaly MM, Siegel HS. Changes in blood and spleen lymphocyte populations following antigen challenge in immature chickens. Br Poult Sci. 1996;37:819–827. doi: 10.1080/00071669608417911. [DOI] [PubMed] [Google Scholar]
- 31.Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. TEM. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 32.Mossmann TR, Sad S. The expanding universe of T-Cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 33.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 34.Pettersen HH, Neilsen JP, Heegaard PMH. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- 35.Suffredini AF, Fantuzzi F, Badolato R, Oppenheim JJ, O'Grady NP. New Insights into the biology of the acute phase response. J Clin Immunol. 1999;19:203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]