Abstract
Ineffective treatment and poor patient management continue to plague the arena of clinical oncology. The crucial issues include inadequate treatment efficacy due to ineffective targeting of cancer deposits, systemic toxicities, suboptimal cancer detection and disease monitoring. This has led to the quest for clinically relevant, innovative multifaceted solutions such as development of targeted and traceable therapies. Mesenchymal stem cells (MSCs) have the intrinsic ability to “home” to growing tumors and are hypoimmunogenic. Therefore, these can be used as (a) “Trojan Horses” to deliver gene therapy directly into the tumors and (b) carriers of nanoparticles to allow cell tracking and simultaneous cancer detection. The camouflage of MSC carriers can potentially tackle the issues of safety, vector, and/or transgene immunogenicity as well as nanoparticle clearance and toxicity. The versatility of the nanotechnology platform could allow cellular tracking using single or multimodal imaging modalities. Toward that end, noninvasive magnetic resonance imaging (MRI) is fast becoming a clinical favorite, though there is scope for improvement in its accuracy and sensitivity. In that, use of superparamagnetic iron-oxide nanoparticles (SPION) as MRI contrast enhancers may be the best option for tracking therapeutic MSC. The prospects and consequences of synergistic approaches using MSC carriers, gene therapy, and SPION in developing cancer diagnostics and therapeutics are discussed. STEM CELLS 2010; 28:1686–1702.
Keywords: Stem cell tracking and imaging, Magnetic nanoparticles, Mesenchymal stem cells, Cancer, Nanotechnology, Gene therapy, SPION
CURRENT ISSUES IN CANCER IMAGING AND THERAPY
Approximately 25 million people live with cancer [1] and ∼13% of all deaths are attributed to this disease [2] worldwide. As specific molecular technologies improve, cancer is increasingly recognized as a highly heterogeneous disease. Despite improvements in anticancer therapies, the lack of tumor-specificity results in significant treatment-associated morbidity, ultimately limiting efficacy due to dosage limitations. Research priorities must now seek to refine the specificity and accuracy of cancer detection and treatment as well as develop strategies that target a wider repertoire of cancer cells. An important aim should be to achieve optimal patient management and improved quality of life through early detection of cancer and metastases, improved treatment delivery, and monitoring of outcomes through accurate and sensitive imaging techniques. Although magnetic resonance imaging (MRI) and computed tomography (CT) are currently integral to patient assessment and management, lesions <1 cm are still difficult to detect owing to the subjective nature of interpretation that may lead to inaccurate assessment [3,4].
Recent developments in real-time in vivo imaging technologies using image contrast enhancers offer tangible options to better guide treatment delivery and monitor outcome. Furthermore, improved treatment specificity may be achieved through gene therapy-based approaches. Using viral and nonviral vectors, genetic material can be specifically targeted to cancer cells, for example, to compensate for mutations in tumor suppressor genes, to potentiate anticancer immune responses, or to cause oncolysis [5]. However, obstacles to effective delivery of both contrast agents and gene vectors remain. Immune and reticuloendothelial sequestration or nonspecific vector uptake by nontarget organs dramatically reduces treatment efficacy. No single agent has offered a solution, but recent developments in cancer targeting using stem cell (SC) carriers and nanotechnology have led to innovative possibilities. We discuss the prospects of using SCs as gene therapy carriers and review strategies combining these with nanocarriers to facilitate monitoring and therapy.
SCs AS CARRIERS OF CANCER THERAPY
The ability of SCs to migrate to pathological sites including wounds, ischemia, and cancer (including micrometastases) [6–13] underpins their development as carriers of therapy, thus, providing an exciting paradigm for targeted cancer therapeutics. The importance of the microenvironment in tumorigenesis was first recognized in Paget's seminal (1889) “seed and soil” hypothesis [14]. Stroma provides the architectural framework for tumor development while facilitating molecular crosstalk via cytokines and growth factors to promote cellular turnover and angiogenesis. Thus, tumorigenesis closely resembles wound healing, leading to description of tumors as “wounds that do not heal” [15]. Further, extracellular matrix (ECM) remodeling is mediated by SC and tumor cells [16–18].
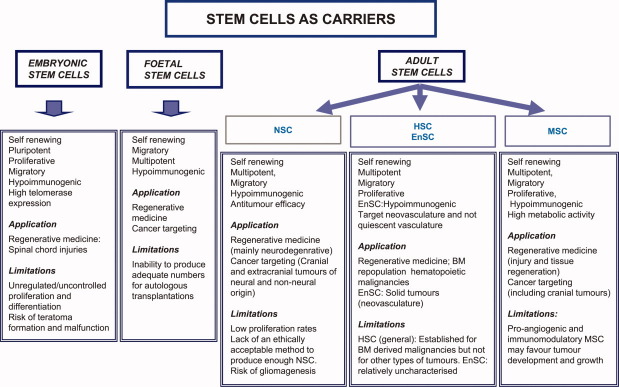
SCs from different sources have been explored for biomedical applications: embryonic SC; fetal multipotent SC; induced pluripotent SC; adult multipotent SC comprising neuronal SC (NSC), hematopoietic SC (HSC), and mesenchymal SC (MSC) (reviewed in [11]; Fig. 1 summarizes their properties, potential applications, and drawbacks). Overall, by virtue of their lineage plasticity and tumor tropism, adult SCs display the best attributes for targeting cancer. Both HSC and NSC have been explored with variable success, however, their application is limited either due to issues with production or inadequate characterization (Fig. 1; reviewed in [19–25]). MSCs are currently under intense investigation as potential clinical therapeutic carriers due to their high lineage plasticity [26] and minimal ethical concerns associated with their isolation and use [11]. This review will focus on the potential of MSC as cellular carriers in oncology.
Figure 1.
A schematic summarizing the properties, applications, and limitations of different stem cells for the treatment of biomedical conditions including cancer. Abbreviations: BM, bone marrow; EnSC, Endothelial Stem Cells; HSC, hematopoietic stem cell; MSC, mesenchymal stem cell; NSC, neuronal stem cell.
MSCs AND CANCER
MSCs are multipotent stromal cells with the ability to self-renew, differentiate into cells of diverse lineage [27], and migrate to sites of pathology [28]. First isolated as an adherent mononuclear cell fraction of bone marrow (BM) [29], MSCs are present virtually in all postnatal tissues [30]. The following MSCs properties make them ideal therapeutic cellular carriers (Table 1): ease of isolation and expansion in vitro; ease of ex vivo genetic modification; autologous transplantation in patients (overcome issues of host immune responses); and finally, hypoimmunogenicity (suitable for allogeneic transplantations). Indeed, approval of ∼107 clinical trials employing MSCs for regenerative medicine, stroke, and myocardial infarction (http://clinicaltrials.gov/ct2/results?term=Mesenchymal+stem+cells&show_flds=Y) [47] suggests the clinical feasibility of their use for cancer targeting.
Table 1.
Properties of MSCs relevant to applications in cancer imaging and therapy
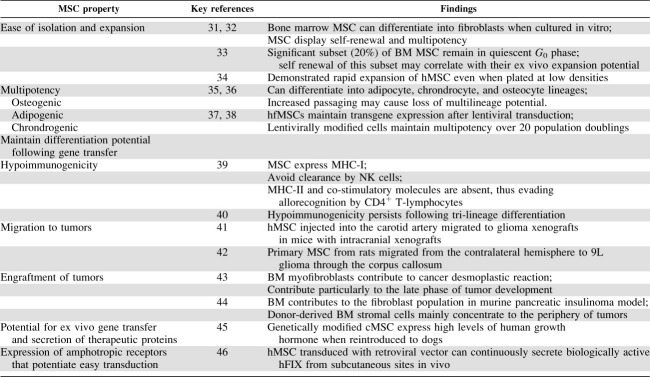 |
Abbreviations: BM, bone marrow; cMSC, canine MSC; hFIX, human factor; hfMSC, human fibroblastic MSC; hMSC, Human MSC 8; MSC, mesenchymal stem cells.
MSCs AND TUMOR TROPISM
MSCs show preferential migration toward sites of inflammation, injury, and cancer [6]. Typically, these are attracted to lesions where they engraft into the stroma and persist: in xenograft experiments, 40% of intratumoral fibroblasts in pancreatic lesions in mice were of BM origin [44]. Although, distributed throughout the tumor mass, both this and subsequent studies have shown a greater concentration of BM-derived cells toward the tumor periphery, indicative of the role of MSCs in the later stages of stromal induction as regulators of desmoplastic reactions [48]. Thus far, the tropism of MSCs for gliomas [42,49], pulmonary metastases [50–52], breast cancer metastases [53] ovarian carcinoma [54], and melanoma [55] has been demonstrated in several animal models.
Although not completely understood, MSCs “homing” to cancer may involve recruitment of resident fibroblasts and circulating MSCs into the tumor microenvironment through the release of growth factors and chemokines, where they proliferate and subsequently differentiate into tumor stroma forming fibrocytes, myofibroblasts, and neovascular pericytes [48]. Chemokine-receptor pairs including stromal-derived growth factor SDF-1/CXC chemokine Receptor-4 (CXCR4) [56], monocyte chemotactic protein-1/chemokine (C-C motif) receptor 2 [53], hepatocyte growth factor/c-met [57], and Vascular Endothelial Growth Factor (VEGF)/VEGF receptor [58] together with ECM proteins have been implicated [59] (reviewed in [60]). A clear understanding of these processes is crucial to improve MSCs “homing” to tumors in vivo. Characteristics unique to their migratory phenotype including the chemokine receptor status and triggering events such as cytokine release and matrix metalloproteinase (MMP) production at tumor site need to be identified to determine the optimal biological window for therapeutic MSCs targeting of tumors. For example, postresection production of cytokines that recruit MSCs to gliomas [61] could provide a window to target gliomas with therapeutic MSCs to remove residual disease. Further, to achieve optimal tumor targeting, specific identification of nonquiescent SC populations, which can migrate, target, and integrate into tumor tissue, is essential. This would require an assessment of relevant receptors on these cells and their responses to biological triggers using molecular imaging and appropriate ex vivo or in vitro three-dimensional models [62].
MSCs AND CANCER GENE THERAPY
Overall, the recognition that MSCs “home” toward tumors while evading immune clearance has led to extensive research into their use for cancer-specific gene delivery [11,48,50,55]. A primary consideration for such applications is to ensure their in situ efficacy and survival with the retention of their fundamental properties of migration, differentiation, and hypoimmunogenicity, after modification.
Cancer gene therapy delivered using MSC has been based on suicide-, apoptosis-, anti-angiogenesis-, immuno-stimulatory genes, or oncolytic viral vectors (reviewed in [63]) primarily, using the viral vectors. The use of MSCs as carriers for these vectors [5] can address the drawbacks associated with their direct use including: safety (e.g., insertional mutagenesis when integrating viral vectors [retroviruses] are used) [64]; inadequate tumor targeting; inefficient gene delivery resulting from vector and/or transgene immunogenicity; limited availability of virus-specific “receptors” on cancer cells or inefficient transduction of nondividing cells. Furthermore, ex vivo MSCs manipulation maximize transduction efficiency by allowing for the selection of cells carrying the desired gene before in vivo delivery.
Viral Vectors and MSC
Transduction of MSCs by integrating retroviral vectors is efficient, but their random genomic integration can lead to unwanted transformation, significantly increasing the risk of secondary malignancies. Despite continuing efforts toward the assessment and accurate mapping of safe insertion sites, currently, the risks may outweigh the advantages. Hence, nonintegrating vectors, such as adenoviruses (Ad), are appealing and are the most widely explored for cancer gene therapy. Ad can be grown to high titer (∼1012 virus particles per milliliter), yield high gene expression and importantly, transduce dividing and nondividing cells [64]. However, systemically administered Ad can be rapidly cleared by the immune system and hepatic Kupffer cells [64] and inactivated by Ad-neutralizing antibodies in humans [65]. This substantially compromises the efficiency of Ad gene delivery [66–69]. Because of their hypoimmunogenicity, MSCs may act as a “Trojan Horse” for the delivering Ad-mediated gene therapy directly into tumor lesions. This concept has generated significant interest and is the focus of the following section.
MSCs as Hypoimmunogenic Cellular Vehicles for Adenoviral Vectors
MSCs express major histocompatibility class (MHC)-I antigens, thereby avoiding clearance by natural killer cells, whereas the absence of MHC-II and costimulatory molecules permit immune evasion from CD4+ T-lymphocytes [39]. In vitro studies have demonstrated that MSCs do not cause the proliferation of allogeneic T-cells following interferon (IFN)-γ stimulation [70] and this hypoimmunogenicity persists even after tri-lineage differentiation [40]. Studies in several animal models (rodents, dogs, pigs) have shown that allogeneic-mismatched MSCs can engraft in vivo [71]. Importantly, recent studies have shown that MSC-Ad produce therapeutic transgenes even in the presence of physiological concentrations of sera that would otherwise neutralize adenovirus alone in vitro [52]. Thus, the dual benefits of MSCs homing and their potential for allogeneic transplantation without extensive immunosuppression can be exploited to increase Ad-gene delivery specifically to tumor sites.
Efficacy of MSCs Carrying Therapeutic Genes Against Cancer
High metabolic activity of MSC permits high-level transgene expression [72]. The use of MSC carriers to deliver Ad-vectors expressing therapeutic genes has been assessed in several preclinical models of cancer (Table 2). Specific delivery of cytokine transgenes to tumor sites has been attempted to mitigate the toxicities associated with systemic administration of the corresponding recombinant proteins. Unlike systemically administered IFN-β, systemically delivered MSC-expressing IFN-β suppressed tumor growth and prolonged survival in a lung melanoma model [50]. The antitumor effects were attributed to the local production of IFN-β within the tumor, thus, highlighting the importance of MSC engraftment for cancer-targeted delivery [34]. Similar benefits have been achieved using MSC-expressing interleukin-2 [42], fractalkine [74], and tumor necrosis factor-related apoptosis inducing ligand [52] against intracranial glioma, lung metastases, and lung carcinoma, respectively. The localized production of cytotoxic drug metabolites was also achieved using MSC-expressing cytosine-deaminase; local conversion of the prodrug, 5-fluorocytosine to 5-fluorouracil, resulted in inhibition of growth of colorectal cancer [76] and melanoma [77] xenografts.
Table 2.
Efficacy of adenovirus-transduced MSCs in preclinical tumor models
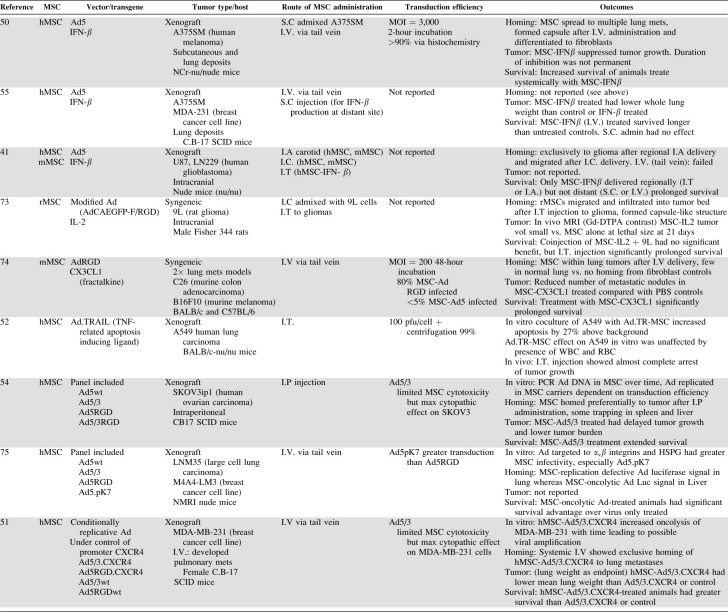 |
Ad5/3, Ad5 with chimeric fiber(Ad5+Ad3); Ad5, adenovirus serotype 5; Ad5.pK7, Ad5 with fiber containing polylysine (7 residues); Ad5RGD, Ad5 with integrin binding RGD motif in its fiber; Ad5wt, Ad5 wild type; AxFAEGFP-F/RGD, adenoviral vector carrying humanized variant of Aequoria victoria green fluorescent protein with RGD-mutated fiber under control of a CA promoter; CX3CL1, C-X3-C-motif ligand 1 (Fractalkine); CXCR4, cxc chemokine receptor 4 (Fusin); hMSC, human MSC; I.A., intraarterial; I.C., intracranial injection; IFN-β, Interferon beta; IL-2, interleukin-2; I.P., intraperitoneal injection; I.T., intratumoral injection; I.V., intravenous injection; mMSC, murine MSC; MOI, multiplicity of infection; MSC, mesenchymal stem cells; PBS, phosphate buffered saline; S.C., subcutaneous.
MSCs have also been used to carry and support the replication of oncolytic viruses, which infect tumor cells when released into the tumor mass. This strategy relies on the optimal balance between minimizing cytotoxicity to the cellular carriers and maximizing cytopathic effects on cancer cells. Thus, systemically delivered MSC-bearing oncolytic viruses have been successful against lung metastases [51] and orthotopic breast and lung tumors [75] displaying cytopathic effects against cancer cells with minimal toxicity to the MSC themselves. Similarly, extended host survival and delayed tumor growth was seen following intraperitoneal delivery of MSC-bearing oncolytic Ad against ovarian cancer [54]. In comparison, the same doses of virus injected systemically showed only liver accumulation [75], validating the MSC cell-carrier approach to more efficiently target cancer. However, the low transduction efficiency of MSC with Ad vectors due to the low expression of Ad-receptor Coxsackie-adenovirus receptor (CAR) is a limiting factor [78]. This could not be improved by increasing the multiplicity of infection or time of exposure to the Ad [79]. However, alteration of the Ad tropism with genetic modification of Ad-fiber-knob to contain poly-L-lysine or addition of the Ad35 fiber improved MSC transduction by16- to 460-fold [80]. Cumulatively, these studies indicate the superiority of Ad-modified MSC over the use of Ad alone, although, their therapeutic success is conditional on the efficiency at which MSC are transduced and their subsequent engraftment and persistence within the tumors.
Limitations of MSC Carriers for Cancer Gene Therapy
Despite tremendous interest in MSC, the unpredictability of their in vivo biological properties such as migration and potential for contribution to the neoplastic phenotype poses a serious obstacle. Some studies have raised concerns that proangiogenic and immunomodulatory properties of MSC may potentiate growth and metastatic capacity of epithelial cancer cells particularly when MSCs are mixed with the cancer cells prior to implantation [81]. Others have shown no apparent effect of exogenous MSC on tumor progression with proven MSC migration but lack of proliferation and differentiation [82,83]. Thus, exhaustive investigative studies are essential prior to any clinical application [63], for example, an assessment of the time required to generate pathology free cells, genetic modification, expansion and phenotypic and genotypic characterization, and then certification for human use needs to be established. The investment in time and resources to produce clinical grade MSC showing acceptable levels of genetic modification and expansion to a therapeutic dose for use in patients is considerable. A timely completion of such characterizations is particularly challenging when dealing with primary cells prior to transplantation. Furthermore, MSC from different sources show different properties [84,85]. For example, MSC from BM proliferate less efficiently than those from umbilical chord or adipose tissue. Some of these constraints can be addressed by development of immortalized, clonal MSC lines that after exhaustive characterization, especially, with respect to their tumorigenicity (e.g., based on type of immortalization gene, its insertion site), may be optimal for clinical use [63].
Additionally, variations in persistence, survival, and interactions of MSC in the tumor microenvironment can affect the duration and level of gene expression at the tumor site [63]. Chemotherapy or the immunogenicity of the transgene and/or vector may impact on MSC survival in situ. Overall, adequately long systemic survival of these carriers is mandated to ensure therapeutic efficacy against cancer. This could in part be supported by the production of immunosuppressive chemokines or cytokines, such as VEGF, Interleukin 10 (IL10), or immunosuppression of T effector-, antigen-presenting- or regulatory T-cells. A better understanding of MSC biology can be exploited to prolong their systemic survival, for example, recognition that CD47 marker expression can prevent SC phagocytosis by macrophages [86,87]. Ultimately, all new approaches must be assessed using human data to evaluate safety and efficacy. Toward that end, a rapid translation of the findings will be greatly facilitated by monitoring SC survival and behavior in vivo through use of longitudinal, noninvasive imaging technology [4,82,88–91].
MSCs AND IMAGING
To translate MSCs benefits to the clinic, their accurate detection and localization in real time using clinically relevant imaging techniques is essential. An ideal imaging modality should be noninvasive, sensitive, and provide objective information on cell survival, function, and location. In context of cancer, MRI, CT, positron emission tomography (PET), and single photon emission computed tomography (SPECT) are the most explored (specific features of different imaging modalities are reviewed in [3,91–95]. Overall, while nuclear imaging by PET or SPECT leads to greater sensitivity (>5 × 103 cells; [96]), these are primarily limited by lack of anatomical context [97]. MRI provides accurate anatomical detail but does not yield information about cell viability and show poor sensitivity (>105 cells; [98]). Although, none of these modalities is ideal, MRI is the most preferred for cellular tracking (comprehensively reviewed in [95,99–102]). Through detection of proton relaxations in the presence of magnetic field (1.5 Tesla [T]–3 T for clinical imaging), it provides tomographic images with excellent soft tissue contrast and can locate the cells of interest in context of the surrounding milieu (edema or inflammation) [103–105] without the use of harmful ionizing radiations (as with CT, PET, SPECT). In addition, MRI offers a greater tracking window in comparison to PET and SPECT that are limited by the decay of short-lived radioactive isotopes.
During MRI, the intrinsic tissue contrast is affected by local microenvironment including magnetic inhomogeneities of the contrast agents, usually measured as changes in two relaxation time constants T1 (brightening) and T2 (darkening) times [106]. In this context, nanotechnology-based contrast agents have rapidly come to the forefront to improve SC detection in situ. In the following sections, after a brief introduction of the nanotechnology platform as it applies to cancer targeting its potential synergistic applications involving the MSCs carriers are discussed.
Nanotechnology and Cancer: Potential for Synergies with Cellular Carriers for Targeting Cancer
Initiated by the discovery that particles of ∼50–100 nm “passively” accumulate in cancer deposits, nanotechnology has fast emerged as a tool for imaging and/or delivery of therapies in oncology. This passive uptake occurs via an “enhanced permeability and retention effect” where inherently leaky tumor vasculature coupled with poor intratumoral lymphatic drainage allows extravasation and entrapment of the nanoparticles [107,108]. Further, nanoparticle surfaces can be modified with cancer-specific antibodies or peptides for the “active” targeting of tumor cells [109]. Thus, nanotechnology platforms offer flexibility and versatility. Not only can nanoparticles deliver targeted therapeutic payloads (drugs or genes), their intrinsic components can simultaneously serve as enhancers for imaging [110]. For optimal targeting and efficacy in vivo, these particles should be biocompatible (based on their size, shape, surface coatings, and chemical or immunotoxicity [111,112]), easily targeted (through surface interactions with cancer targeting antibodies, peptides or ligands), and easily tracked by virtue of their composition to allow clinical imaging [110].
Of the ever expanding catalogue of nanoparticles including polymers, dendrimers, liposomes, carbon nanotubes, nanoshells, and magnetic nanoparticles [110], several have gained Food and Drugs (FDA) approval for cancer therapeutics (Doxil, DaunoXome) and imaging (Resovist) [107,110,113]. However, their first clinical application may be as imaging agents [114–116] and in that the best developed are superparamagnetic iron-oxide nanoparticles (SPION).
SPION comprise a crystalline iron-oxide core coated with biocompatible materials such as, dextran, starch, or polyol derivatives, that confer stability in vivo and can be conjugated with cancer-targeting ligands or gene-vectors for active targeting. These display magnetism only under the influence of an external magnetic field [117], which also avoids self-aggregation. Importantly, SPION are biocompatible and are eliminated through the body's normal iron metabolism. SPION have been studied for cancer therapy (hyperthermia), magnetic field-assisted targeting, and as contrast enhancers for MRI and targeted molecular imaging [118,119]. The promise shown in such studies have initiated clinical evaluation of SPION for the detection and management of liver metastases with enhanced sensitivity of up to 95% [120] to nodal metastases in both head and neck [121] and genitourinary cancers [122,123]. Although these studies have shown SPION usage to be safe [124], some issues associated with their use need to be addressed.
Issues with Use of Iron-Oxide-Based Nanoparticles.
Major limitations of SPION beyond MRI of the Reticuloendothelial system (RES), include their uptake by phagocytic cells leading to their rapid clearance from the blood [125], in vivo toxicity resulting from the coating materials, and surface chemistry together with unwanted cellular or tissue distribution [118,126]. SPION can also cross the blood-brain barrier and accumulate in the liver (80%–90%), spleen (5%–8%), and BM (1%–2%) [127]. Their ability to agglomerate in the presence of a magnetic field can cause embolization [126,128]. Excessive iron-oxide could also lead to an imbalance in its homeostasis and may lead to toxicity [129]. Therefore, toxicity of any new formulations of SPIONs has to be established and would require extensive characterization terms in terms of SPION composition, coatings, size, and dosing regimens in vivo. Thus, the use of the nanoparticles under the “camouflage” of MSCs may resolve some of these issues. Particularly for cellular tracking, the delivery of nanoparticle-labeled MSCs directly into tumor deposits will not only allow the tracking of the labeled cells but also the targeted tumor deposits.
Magnetic Nanoparticles and Tracking of SCs In Vivo
Several paramagnetic and magnetic nanoparticles have been evaluated for labeling SCs to enhance their tracking by MRI. Paramagnetic gadolinium (Gd)- and Mn-based nanoparticles lead to image brightening (T1-based) while those based on SPIONS (50–200 nm), ultraSPION (∼35 nm), and micron-sized (MPION) lead to image darkening (T2 and T2*-based) [114,130]. Of these, only SPION are approved for clinical imaging and are the general focus of this review.
SPION and MRI of SCs.
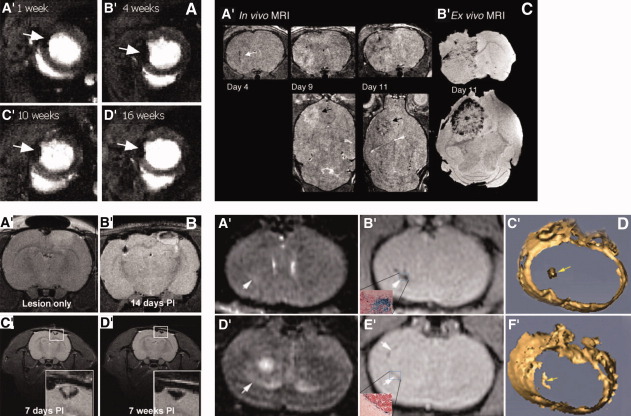
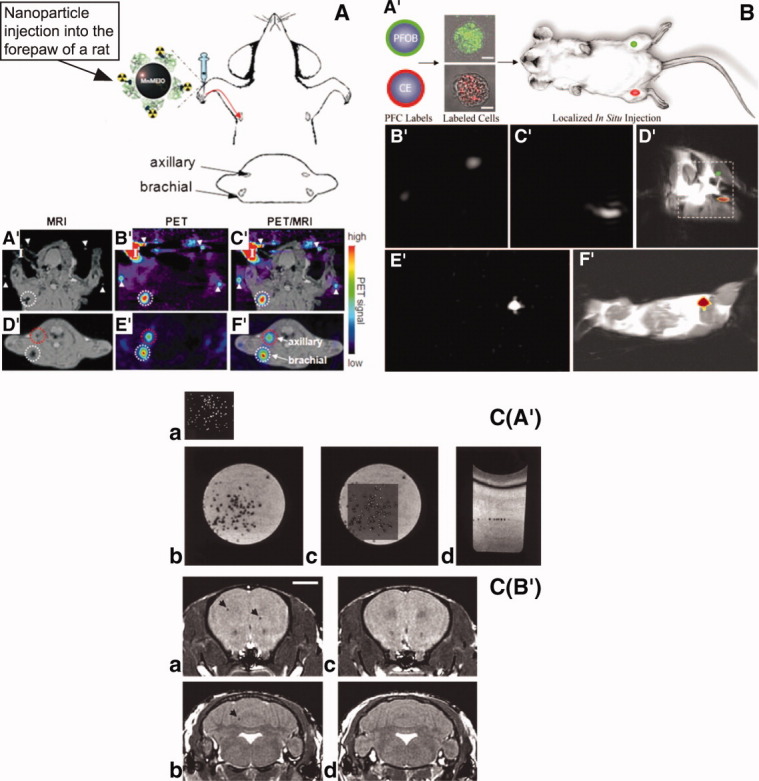
SPION display greater magnetic susceptibility in comparison to conventional Gd and engender significant signal loss to delineate areas of interest. Thus, SPION-labeled cells display a “blooming” artifact' that extends beyond the size of particles making the cells more visible for detection. Clinically, about 1–30 pg Fe per cell is adequate for detection of labeled cells by MRI without alterations in the proliferation, migration, differentiation, reactive oxygen species formation, and apoptosis rates [118,131]. Hence, with the increasing use of MSCs for therapy of tissue injury [132,133], MRI tracking protocols have gained prominence generating crucial information about their migration and survival. MRI signals from intramyocardial implanted SPION-labeled MSC could be detected for up to 16 weeks (Fig. 2A) [132–134] and specific migration of intravenously given SPION-labeled MSCs to the infarct area and not to the healthy surrounding viable myocardium was shown [136]. Further, through MRI of SPION-labeled porcine MSC, improved survival in the infarct zone than in healthy myocardium was shown [137]. Similarly, in models of brain injury and stroke, injury-specific migration of SPION-labeled MSCs (injected contralateral to the area of injury or infused intravenously) could be tracked by MRI (Fig. 2B) [8,28,138,139]. Thus, MRI-based demonstration of retention of injury-specific migration and improved survival of labeled MSCs at the site of injury suggests the feasibility of this approach in clinical oncology.
Figure 2.
MRI of superparamagnetic iron-oxide nanoparticles (SPION)-labeled stem cells showing their persistence, migration, and tumor homing in vivo. (A): Demonstrates long-term mesenchymal stem cell (MSC) traceability using SPION. Rat MSCs labeled with iron particles injected into the infracted heart could be detected as hypointense regions from 1 week and detected for up to 16 weeks. Volume of the signal void reduced to lesser extent in severely infarcted hearts in comparison with milder infarcts. ©AlphaMedPress, April 20th, 2006; Reprinted from [134], with permission from Wiley-Liss, Inc. a subsidiary of John Wiley & Sons, Inc. (B): Migration of MSCs to site of pathology: Endoderm (SPION)-labeled rat MSCs migrate toward the lesion of brain (A′) could be detected by MR up to 7 weeks after implantation (C′ and D′) in the contralateral hemisphere MSCs (B′). Reprinted from [7], with permission from Macmillan Publishers Ltd., © 2007 Nature Publishing Group. (C): Tumor homing and engraftment by Sca-1 positive bone marrow (BM) cells (target the tumor vasculature) by MRI: Serial MRI in tumor bearing mice that received magnetically labeled Sca-1+ BM cells. (A′): Three-dimensional (3D) RARE images show dark regions developing within and around tumors due to incorporation of labeled cells into the vasculature and parenchyma of tumor, images are acquired on day 4, 9, and 11. By day 11, a dark rim appear on the tumor periphery. (B′): Corresponding ex vivo gradient images of the same mouse on day 11. MR evidence of labeled cell incorporation demonstrates that neovascularization occurs primarily at the tumor periphery in the later stages of tumor development. Reproduced from [135], with permission from (This research was originally published in Blood) © 2007 The American Society of Hematology. (D): Tumor homing by SPION-labeled MSCs: The pattern of MSCs distribution, their incorporation and migration could be tracked using 1.5-T MR imaging following i.v. injection of SPION/green fluorescent protein-labeled cells. MSCs distribution throughout the tumor on day 7 (B′) was shown by a well-defined dark hypointense region. After 14 days, most MSCs were found at the tumor border (hypointense region in [D′]), (C′, F′). 3D reconstructions show the SPION-labeled MSCs as yellow structures indicated by the yellow arrows. This study demonstrates that systemically transplanted MSCs migrated toward glioma with high specificity in a temporal–spatial pattern. Reproduced from [49], with permission from ©1944-2009 by the American Association of Neurosurgeons. Abbreviations: MRI. magnetic resonance imaging.
Targeting Cancer and Cellular MRI.
In cancer, to date, cellular MRI has primarily been explored in glioma models with most studies employing EnSC or NSC. MRI of SPION-labeled endothelial progenitor cells demonstrated their tumor tropic migration and differentiation into neovasculature within intracranial glioma (Fig. 2C) [135,140]. Given their neuronal bias, NSC carriers are the most explored for targeting glioma, (NSC literature for reference: [9,35,25,63,99,141–143]). Indeed, through MRI of magnetically labeled NSC, their seeding, migration, homing to invading tumor cells has been evaluated successfully clearly indicating the promise of such combinational approaches [9,144–151] for tracking cellular carriers to cancer lesions. In that, MSCs have generated recent interest as unlike NSCs, these are readily expandable with minimal ethical issues. MRI of intravenously infused SPION-labeled MSCs demonstrated specific migration toward glioma MRI [49] in a temporal-spatial pattern showing initial distribution throughout the tumor with subsequent concentration at the periphery (Fig. 2D) [49]. MRI at 1.5 T could detect the signal for over a week with ensuing decay after 14 days, but could be improved by MRI at higher magnetic field strengths. As glioma has a diffuse distribution and spreads beyond the original site [152], the ability of MSCs to home toward metastatic glioma highlights their potential to “track” the migration of cancer. The translation of such noninvasive imaging techniques toward a broader repertoire of cancers is now being explored.
Approaches to Improve MRI Sensitivity and Duration
Magnetic-nanoparticle-labeled cells face limitations typical of exogenously labeled cells such as the dilution of signal with cell division limits the duration of MRI tracking; attenuation of signal down to 42% of the original after 8 weeks was observed [153]. Further, asymmetric sequestration of label during cell division may compromise detection accuracy [154]. The accuracy of MRI data is also compromised by the inability to distinguish viable and nonviable cells and the generation of false signals from dead cells or those engulfed by macrophages [155]. This has initiated interactive research to improve cellular MRI sensitivity and accuracy; a discussion of some of the approaches follows.
Efficient labeling of MSCs can improve detection by magnifying MRI signals [102,156]. Given that spontaneous SPION uptake is minimal in virtually all cell types apart from those of the RES [157], attempts have been made to increase iron loading into cells through SPION derivatization with peptides [158], dendrimer coatings [159], combination with transfection agents (TAs) [131,160–162], and electroporation [163] with variable success (Table 3). Currently, the most widely accepted protocols involve combining SPION with TAs such as poly-L-lysine and protamine-sulfate, achieving high labeling efficiency approaching 100% [161,162]. However, the narrow therapeutic index for titration of these TAs raises the chance of cytotoxicity, changes in the gene expression or their migratory ability [168,180,181]. Furthermore, while SPION-TA-transduced MSCs display unaltered adipogenic and osteogenic differentiation, there is continued debate regarding their deleterious effects on chondrogenesis [131,170]. Thus, a special emphasis on new ways to maximize iron internalization in MSCs while limiting toxicity and impact on normal MSCs properties is needed (Table 3).
Table 3.
Potential strategies to increase MSC uptake of magnetic nanoparticles
 |
Use of Other Nanolabels Can Improve MRI Detection of SCs.
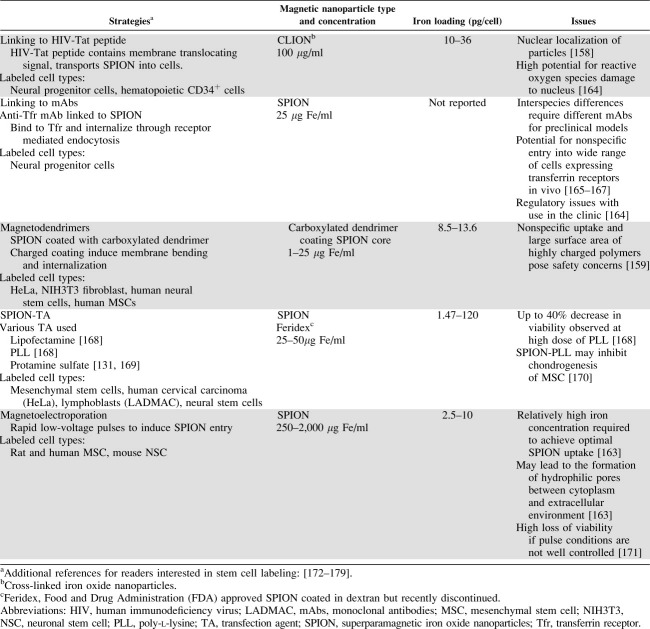
Given that signal gain (T1 contrast) is more specific and easier to interpret than signal loss (T2 contrast), paramagnetic manganese-oxide- or gadolinium-oxide-based nanoparticles (T1 contrast enhancers) may provide an attractive alternative [99,101,182]. Gd-oxide nanoparticles have appeal because Gd-chelates are approved for clinical MRI and have been used to trace human NSC or MSC [183]. However, potential mitochondrial toxicity [184,185] compounded by a requirement of greater molar quantities for optimal imaging has limited the interest in their use for MRI. Novel paramagnetic fluorinated nanoparticles have recently been shown to display high specificity with both clinical and high field MRI. Given the absence of endogenous fluorine (F) in the body, hot spot 19F MRI images of labeled cells were generated with negligible background. Ahrens et al., tracked 19F-nanoparticle-labeled dendritic cells (using cationic perfluoropolyether) to the regional lymph-node after injections into the foot pad of mice [186]. For a complete picture, though, the hot spot image requires overlaying with a simultaneous proton image (standard1H MRI) [150,187] (Fig. 3B). Despite the benefit of quantifying the labeled cells [191], sensitivity is low as the signal comes only from the labeled cells, while the proton signal draws from a much larger pool within the body and hence, is currently the preferred choice.
Figure 3.
Approaches to improve stem cell tracking by MRI. (A): PET-MRI dual modality imaging using multimodal nanoparticles: (magnetic nanoparticles + radionuclide, 124I), brachial (3 mm; A′, B′, C′) and axillary lymph nodes (D′, E′, F′) could be detected by superimposition (C′, F′) of anatomical MRI images (A′, D′) with the intense red signal images obtained with PET (B′, E′). Reproduced from [188], with permission from ©Wiley-VCH Verlag GmbH & Co. KGaA. (B): 19F Rapid imaging of labeled mononuclear cells (human umbilical cord blood) at both research (11.7 Tesla) and clinical field (1.5 Tesla) strengths: Using multiple perfluorocarbon nanoparticles, green (PFOB) or red (CE), hot spot 19F images (B′: PFOB) and (C′: CE) were generated and could be superimposed with 1H MRI (11.7 T) for anatomical localizations to the mouse legs (D′). Similar results were obtained using 1.5 T MR (E′: 19F image and F′: superimposed 19F and 1H image). The authors were able to detect as few as 2,000 CE-labeled and 10,000 PFOB-labeled cells with 19F MR spectroscopy and 6,000 CE-labeled cells with 19F MRI in vitro. Reproduced from [187], with permission from FEDN of AM Societies for Expreimental Bio (FASEB) Journal via copyright clearance center, © 2006 by FASEB. (C): Detection of Ultra Small Superparamagnetic Iron Oxide Nanoparticles (USPION)-labeled cells using fast imaging employing steady state acquisition pulse sequence on a 1.5 T clinical MRI scanner. (A′): Single USPION-labeled cells could be detected using a custom built gradient RF coil and optimized pulse technology. (a): fluorescent image of Dil/superparamagnetic iron-oxide nanoparticles (SPION)-labeled cells localized between two layers of gelatin in an ELISA well, (b) MR image, (c) fusion image, (d) Axial MR showing the localization of cells in a plane. Reprinted from [189], with permission from ©2003Wiley-Liss, Inc. a subsidiary of John Wiley & Sons, Inc. (B′): In vivo MR images detecting SPION-labeled macrophages (signal voids shown by arrows) injected into the mouse brain frontal cortex (a) and cerebellum (b). (c, d) represent the corresponding images of a control mouse. Reproduced from [190], with permission from ©2006 Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. Abbreviations: CE, perfluoro15-crown-5 ether; MRI, magnetic resonance imaging; PET, positron emission tomography; PFC, Perfluorocarbon; PFOB, perfluorooctylbromide.
Other Approaches.
One approach is to utilize gene technology to introduce magnetic susceptibility enhancing genes [192], efficient transduction of these cells would be key to success of such an approach. Toward that end, the magnetic properties of virus or plasmid DNA-conjugated SPION have been harnessed through “magnetofection” to provide superior transduction (up to 500-fold increase) of “hard to transduce” cells with shorter incubation periods [193–195]. We have shown that Ad-conjugated SPION and magnetofection markedly improves the transduction of MSCs (low CAR expression) ex vivo while minimizing vector toxicity through a reduction in vector dose and incubation time (unpublished data).
Additionally, notable success of approaches employing modification of hardware and imaging protocols as well as synergizing different imaging modalities has pioneered new innovations for future research [105,114,196–202]. Some of these approaches are summarized in Table 4 and Figure 3.
Table 4.
Approaches to improve detection of labeled stem cells by MRI
 |
Thus, recent developments in SC, gene technology, and nanotechnology platforms against cancer have reached a junction where there is enormous potential to synergize their individual advantages to achieve concomitant tumor-targeted therapy and imaging.
CONCLUSIONS AND PERSPECTIVES
The potential synergism between MSCs, gene-therapy, and magnetic nanoparticles offers an exciting innovation that may offer cancer patients greater treatment and disease management options and ultimately better quality of life. The advances in nanotechnology may be combined with MSCs to facilitate their tracking and provide accurate details about their location, viability and survival. For effective cellular therapy of cancer, the carriers need to target cancer deposits irrespective of their size and location, should be traceable and should survive long enough to deliver the therapeutic payload. This will require real-time imaging ability with high spatial and temporal resolution as well as stringent target specificity.
Current clinical probes generally cater to a single imaging modality, however, it is now clear that combining the attributes of multiple modalities will be required to provide a comprehensive assessment of events as they occur [114]. Indeed, this concept, now explored by various research groups will soon be a preferred choice for clinical application. Again, the flexibility of nanotechnology platforms may be a great ally in imaging cell-based therapies. For example, the imaging potential of MSCs labeled with magnetic nanoparticles conjugated with radionuclides will allow the combined advantages of short-term PET sensitivity and the long-term signal persistence of MRI. Further, development of multimodal smart nanoparticles that can simultaneously image and treat cancer with real time monitoring of associated events [114,188] is now feasible through the versatility of nanotechnology. However, these particles need to be exhaustively assessed for their biocompatibility and intracellular or in vivo toxicity before they achieve widespread applicability in the clinic.
Given a relatively poor understanding and ability to control MSCs in vivo behavior, their application as carriers of contrast agents may not be safe [227]. This may be resolved to some extent by combining imaging with a backup suicide gene technology to eradicate misbehaving cells. This can be achieved through smart combinations with tools of gene therapy, for example, through introduction of suicide genes with regulatable promoters. For example, the use of Herpes Simplex Virus (HSV)/tk Gene Directed Enzyme Prodrug Therapy (GDEPT) and radioactive substrate (18F-9-(4-[18F]Fluoro-3-Hydroxymethylbutyl) Guanine (18F-FHBG)) has been successful in both human and animal studies for PET imaging [228]; the presence of HSV/tk suicide gene can serve as an additional control to eliminate the transduced cell by treatment with the prodrug (Ganciclovir and Acyclovir) which is then converted to a toxic drug by tk. Thus, magnetically labeled MSCs with HSV/tk GDEPT would allow MRI-PET along with the control of cell survival as needed. Both nanoparticles and MSCs can carry gene vectors, hence, there is scope for endogenous expression of reporter genes under tissue or lineage specific promoters [229] or expression of magnetic susceptibility enhancing genes to enhance the accuracy of the imaging data. Such adjuncts may allow additional assessment of cell viability, survival and fate. Use of reporter genes green fluorescent protein or luciferase [192] regulated by lineage-specific promoters may help detect SCs following differentiation, for example, use of cardiac-specific α myosin heavy chain promoter to detect SC conversion to cardiac myocytes [230,231], Tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE) promoter [229] to detect endothelial differentiation, and osteopontin or osteocalcin promoters to detect osteogenic changes [232].
Taking into account the limitations and attributes of different types of SCs, MSCs offer a feasible option in clinical oncology. New sources of MSCs are under development, including those from adipose tissue [30,76,77] and umbilical cord blood [233,234] and show similar tumor homing and functional capacity as BM-derived MSCs. The wide availability of MSCs from these sources and development of well-characterized immortalized clonal stem cell lines may also ease the practical application of MSCs in the clinic. In particular, the potential for MSCs to be transplanted across MHC barriers in humans [71] can be further explored to facilitate ease of donation in future clinical contexts. It must be noted that the specificity of this system is highly conditional on the tumor homing abilities of MSCs and their in vivo behavior, making this a priority research area. Enhanced insight into the mediators of homing will allow for active targeting of tumors by inducing MSCs to overexpress target receptors for homing. Also, with increasing knowledge of mechanisms or pathways involved in SCs migration, efforts are being directed toward developing specific ligands to target lesions, for example, to direct cells to CXCR4/SDF1 axis to facilitate MSCs tumor tropism [59]. Recent evidence of increased MSCs engraftment in tumors following irradiation (releases chemotactic signals) [235,236] also indicates the potential of using MSC-based gene therapy as an adjuvant following radiotherapy to maximize the removal of residual disease. Finally, highly specific delivery and individualized therapy may be achieved by the choice of therapeutic genes and manipulation of the vectors depending on the cancer type and degree of aggressive therapy required.
Overall, it is clear that there is no single magic bullet to overcome the complexity and heterogeneity of cancer. Multifaceted approaches that exploit the best attributes of MSC biology, nanotechnology, gene-technology, and gene therapy have the potential to overcome hurdles encountered when each is used alone. However, the possibility that such multidimensional modifications may also enhance the danger of unwanted changes in MSCs functional phenotype such as gain of tumorigenic potential or loss of specific migration, cannot be ignored. A rigorous characterization of modified cells with focus toward addressing the potential regulatory issues would be crucial to achieve their speedy translation to the clinic.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Acknowledgments
This work is supported by funds from Cancer Australia Prioritydriven Collaborative Cancer Research Scheme, Prostate Cancer Foundation, Australia and University of New South Wales Faculty Research Grants Funding Scheme, Australia.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Available at http://www.who.int/mediacentre/factsheets/fs297/en/index.html Accessed July 2008.
- 3.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima J, Tanimoto A, Kikuchi E, et al. Clinical implications of tumor size and local extent of primary prostatic lesions in prostate cancer patients with metastases: Value of endorectal magnetic resonance imaging in patients with metastases. Urology. 2007;70:86–90. doi: 10.1016/j.urology.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Majhen D, Ambriovic-Ristov A. Adenoviral vectors–How to use them in cancer gene therapy? Virus Res. 2006;119:121–133. doi: 10.1016/j.virusres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain G, Fox J, Ashton B, et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 7.Sykova E, Jendelova P. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ. 2007;14:1336–1342. doi: 10.1038/sj.cdd.4402140. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Chun BG, Kim YK, et al. In vivo tracking of human mesenchymal stem cells in experimental stroke. Cell Transplant. 2008;16:1007–1012. [PubMed] [Google Scholar]
- 9.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult braEvidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabatabai G, Bahr O, Mohle R, et al. Lessons from the bone marrow: How malignant glioma cells attract adult haematopoietic progenitor cells. Brain. 2005;128:2200–2211. doi: 10.1093/brain/awh563. [DOI] [PubMed] [Google Scholar]
- 11.Corsten MF, Shah K. Therapeutic stem-cells for cancer treatment: Hopes and hurdles in tactical warfare. Lancet Oncol. 2008;9:376–384. doi: 10.1016/S1470-2045(08)70099-8. [DOI] [PubMed] [Google Scholar]
- 12.Hoehn M, Kustermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ittrich H, Lange C, Togel F, et al. In vivo magnetic resonance imaging of iron oxide-labeled, arterially-injected mesenchymal stem cells in kidneys of rats with acute ischemic kidney injury: Detection and monitoring at 3T. J Magn Reson Imaging. 2007;25:1179–1191. doi: 10.1002/jmri.20925. [DOI] [PubMed] [Google Scholar]
- 14.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Fan X, Houghton J. Tumor microenvironment: The role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 17.Chantrain CF, Henriet P, Jodele S, et al. Mechanisms of pericyte recruitment in tumour angiogenesis: A new role for metalloproteinases. Eur J Cancer. 2006;42:310–318. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth—Bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Oh MC, Lim DA. Novel treatment strategies for malignant gliomas using neural stem cells. Neurotherapeutics. 2009;6:458–464. doi: 10.1016/j.nurt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khanna A, Shin S, Rao MS. Stem cells for the treatment of neurological disorders. CNS Neurol Disord Drug Target. 2008;7:98–109. doi: 10.2174/187152708783885183. [DOI] [PubMed] [Google Scholar]
- 22.Konopleva M, Tabe Y, Zeng Z, et al. Therapeutic targeting of microenvironmental interactions in leukemia: Mechanisms and approaches. Drug Resist Update. 2009;12:103–113. doi: 10.1016/j.drup.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markowicz S. Harnessing stem cells and dendritic cells for novel therapies. Acta Polym Pharm. 2008;65:625–632. [PubMed] [Google Scholar]
- 24.Biffi A, Cesani M. Human hematopoietic stem cells in gene therapy: Pre-clinical and clinical issues. Curr Gene Ther. 2008;8:135–146. doi: 10.2174/156652308784049381. [DOI] [PubMed] [Google Scholar]
- 25.Altaner C. Glioblastoma and stem cells. Neoplasma. 2008;55:369–374. [PubMed] [Google Scholar]
- 26.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 27.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 28.Sykova E, Jendelova P. Magnetic resonance tracking of implanted adult and embryonic stem cells in injured brain and spinal cord. Ann N Y Acad Sci. 2005;1049:146–160. doi: 10.1196/annals.1334.014. [DOI] [PubMed] [Google Scholar]
- 29.Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 30.Gimble J, Guilak F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 31.Vaananen HK. Mesenchymal stem cells. Ann Med. 2005;37:469–479. doi: 10.1080/07853890500371957. [DOI] [PubMed] [Google Scholar]
- 32.Owen M, Friedenstein AJ. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 33.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- 36.Pittenger MF, Mosca JD, McIntosh KR. Human mesenchymal stem cells: Progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- 37.Chan J, O'Donoghue K, de la Fuente J, et al. Human fetal mesenchymal stem cells as vehicles for gene delivery. Stem Cells. 2005;23:93–102. doi: 10.1634/stemcells.2004-0138. [DOI] [PubMed] [Google Scholar]
- 38.Lee K, Majumdar MK, Buyaner D, et al. Human mesenchymal stem cells maintain transgene expression during expansion and differentiation. Mol Ther. 2001;3:857–866. doi: 10.1006/mthe.2001.0327. [DOI] [PubMed] [Google Scholar]
- 39.Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8–18. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 41.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 43.Ishii G, Sangai T, Oda T, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 44.Direkze NC, Hodivala-Dilke K, Jeffery R, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 45.Hurwitz DR, Kirchgesser M, Merrill W, et al. Systemic delivery of human growth hormone or human factor IX in dogs by reintroduced genetically modified autologous bone marrow stromal cells. Hum Gene Ther. 1997;8:137–156. doi: 10.1089/hum.1997.8.2-137. [DOI] [PubMed] [Google Scholar]
- 46.Krebsbach PH, Zhang K, Malik AK, et al. Bone marrow stromal cells as a genetic platform for systemic delivery of therapeutic proteins in vivo: Human factor IX model. J Gene Med. 2003;5:11–17. doi: 10.1002/jgm.292. [DOI] [PubMed] [Google Scholar]
- 47.Brooke G, Cook M, Blair C, et al. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007;18:846–858. doi: 10.1016/j.semcdb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Hall B, Dembinski J, Sasser AK, et al. Mesenchymal stem cells in cancer: Tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 49.Wu X, Hu J, Zhou L, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 50.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 51.Stoff-Khalili MA, Rivera AA, Mathis JM, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 52.Mohr A, Lyons M, Deedigan L, et al. Mesenchymal Stem Cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J Cell Mol Med. 2008;12:2628–2643. doi: 10.1111/j.1582-4934.2008.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dwyer RM, Potter-Beirne SM, Harrington KA, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 54.Komarova S, Kawakami Y, Stoff-Khalili MA, et al. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 55.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 56.Menon LG, Picinich S, Koneru R, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–528. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- 57.Forte G, Minieri M, Cossa P, et al. Hepatocyte growth factor effects on mesenchymal stem cells: Proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 58.Schichor C, Birnbaum T, Etminan N, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 59.Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 60.Spaeth E, Klopp A, Dembinski J, et al. Inflammation and tumor microenvironments: Defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 61.Birnbaum T, Roider J, Schankin CJ, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 62.Li SC, et al. Therapeutic Window, a Critical Developmental Stage for Stem Cell Therapies. Curr Stem Cell Res Ther. 2010 Epub ahead of print. [PMC free article] [PubMed] [Google Scholar]
- 63.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 64.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 65.Chirmule N, Propert K, Magosin S, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 66.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: Impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Douglas JT. Adenoviral vectors for gene therapy. Mol Biotechnol. 2007;36:71–80. doi: 10.1007/s12033-007-0021-5. [DOI] [PubMed] [Google Scholar]
- 68.Yang TC, Millar JB, Grinshtein N, et al. T-cell immunity generated by recombinant adenovirus vaccines. Expert Rev Vaccines. 2007;6:347–356. doi: 10.1586/14760584.6.3.347. [DOI] [PubMed] [Google Scholar]
- 69.Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 71.Le Blanc K, Pittenger M. Mesenchymal stem cells: Progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 72.Pereboeva L, Komarova S, Mikheeva G, et al. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells. 2003;21:389–404. doi: 10.1634/stemcells.21-4-389. [DOI] [PubMed] [Google Scholar]
- 73.Kurozumi K, Nakamura K, Tamiya T, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Xin H, Kanehira M, Mizuguchi H, et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 75.Hakkarainen T, Sarkioja M, Lehenkari P, et al. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum Gene Ther. 2007;18:627–641. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- 76.Kucerova L, Altanerova V, Matuskova M, et al. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 77.Kucerova L, Matuskova M, Pastorakova A, et al. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008;10:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- 78.Kawabata K, Sakurai F, Koizumi N, et al. Adenovirus vector-mediated gene transfer into stem cells. Mol Pharm. 2006;3:95–103. doi: 10.1021/mp0500925. [DOI] [PubMed] [Google Scholar]
- 79.Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000;28:382–390. doi: 10.1016/s0301-472x(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 80.Mizuguchi H, Sasaki T, Kawabata K, et al. Fiber-modified adenovirus vectors mediate efficient gene transfer into undifferentiated and adipogenic-differentiated human mesenchymal stem cells. Biochem Biophys Res Commun. 2005;332:1101–1106. doi: 10.1016/j.bbrc.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 81.Djouad F, Bony C, Apparailly F, et al. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 2006;82:1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed] [Google Scholar]
- 82.Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miletic H, Fischer Y, Litwak S, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 84.Bieback K, Kern S, Kocaomer A, et al. Comparing mesenchymal stromal cells from different human tissues: Bone marrow, adipose tissue and umbilical cord blood. Biomed Mater Eng. 2008;18:S71–S76. [PubMed] [Google Scholar]
- 85.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 86.Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Chen X. Imaging mesenchymal stem cell migration and the implications for stem cell-based cancer therapies. Future Oncol. 2008;4:623–628. doi: 10.2217/14796694.4.5.623. [DOI] [PubMed] [Google Scholar]
- 89.Barrett T, Brechbiel M, Bernardo M, et al. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007;26:235–249. doi: 10.1002/jmri.20991. [DOI] [PubMed] [Google Scholar]
- 90.Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fass L. Imaging and cancer: A review. Mol Oncol. 2008;2:115–152. doi: 10.1016/j.molonc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schnall M, Rosen M. Primer on imaging technologies for cancer. J Clin Oncol. 2006;24:3225–3233. doi: 10.1200/JCO.2006.06.5656. [DOI] [PubMed] [Google Scholar]
- 94.Iyer M, Sato M, Johnson M, et al. Applications of molecular imaging in cancer gene therapy. Curr Gene Ther. 2005;5:607–618. doi: 10.2174/156652305774964695. [DOI] [PubMed] [Google Scholar]
- 95.Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293:855–862. doi: 10.1001/jama.293.7.855. [DOI] [PubMed] [Google Scholar]
- 96.Love Z, Wang F, Dennis J, et al. Imaging of mesenchymal stem cell transplant by bioluminescence and PET. J Nucl Med. 2007;48:2011–2020. doi: 10.2967/jnumed.107.043166. [DOI] [PubMed] [Google Scholar]
- 97.MacLaren DC, Toyokuni T, Cherry SR, et al. PET imaging of transgene expression. Biol Psychiatry. 2000;48:337–348. doi: 10.1016/s0006-3223(00)00970-7. [DOI] [PubMed] [Google Scholar]
- 98.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 99.Long CM, Bulte JW. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin Biol Ther. 2009;9:293–306. doi: 10.1517/14712590802715723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Modo M. Noninvasive imaging of transplanted cells. Curr Opin Organ Transplant. 2008;13:654–658. doi: 10.1097/MOT.0b013e328317a43c. [DOI] [PubMed] [Google Scholar]
- 101.Budde MD, Frank JA. Magnetic tagging of therapeutic cells for MRI. J Nucl Med. 2009;50:171–174. doi: 10.2967/jnumed.108.053546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kraitchman DL, Bulte JW. Imaging of stem cells using MRI. Basic Res Cardiol. 2008;103:105–113. doi: 10.1007/s00395-008-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kalish H, Arbab AS, Miller BR, et al. Combination of transfection agents and magnetic resonance contrast agents for cellular imaging: Relationship between relaxivities, electrostatic forces, and chemical composition. Magn Reson Med. 2003;50:275–282. doi: 10.1002/mrm.10556. [DOI] [PubMed] [Google Scholar]
- 104.Dodd CH, Hsu HC, Chu WJ, et al. Normal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. J Immunol Methods. 2001;256:89–105. doi: 10.1016/s0022-1759(01)00433-1. [DOI] [PubMed] [Google Scholar]
- 105.Muja N, Bulte J. Magnetic resonance imaging of cells in experimental models. Prog Nucl Reson Spectrosc. 2009;55:61–77. doi: 10.1016/j.pnmrs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waters EA, Wickline SA. Contrast agents for MRI. Basic Res Cardiol. 2008;103:114–121. doi: 10.1007/s00395-008-0711-6. [DOI] [PubMed] [Google Scholar]
- 107.Fukumori Y, Ichikawa H. Nanoparticles for cancer therapy and diagnosis. Adv Powder Technol. 2006;17:1–28. [Google Scholar]
- 108.Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 109.Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 110.Tang M. Promise of Novel Magnetic Nanoparticles in Enhancement of Adenoviral Gene Delivery. Sydney: Faculty of Medicine, University of New South Wales; 2007. [Google Scholar]
- 111.Jones CF, Grainger DW. In vitro assessments of nanomaterial toxicity. Adv Drug Deliv Rev. 2009;61:438–456. doi: 10.1016/j.addr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee HM, Shin DM, Song HM, et al. Nanoparticles up-regulate tumor necrosis factor-alpha and CXCL8 via reactive oxygen species and mitogen-activated protein kinase activation. Toxicol Appl Pharmacol. 2009;238:160–169. doi: 10.1016/j.taap.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 113.Alexis F, Rhee JW, Richie JP, et al. New frontiers in nanotechnology for cancer treatment. Urol Oncol. 2008;26:74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 114.Cheon J, Lee JH. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc Chem Res. 2008 doi: 10.1021/ar800045c. [DOI] [PubMed] [Google Scholar]
- 115.Cao YC. Nanomaterials for biomedical applications. Nanomedicine. 2008;3:467–469. doi: 10.2217/17435889.3.4.467. [DOI] [PubMed] [Google Scholar]
- 116.Lee JH, Huh YM, Jun YW, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 117.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 118.Shubayev VI, Pisanic TR, II, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao J, Gu H, Xu B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc Chem Res. 2009;42:1097–1107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 120.Seneterre E, Taourel P, Bouvier Y, et al. Detection of hepatic metastases: Ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996;200:785–792. doi: 10.1148/radiology.200.3.8756932. [DOI] [PubMed] [Google Scholar]
- 121.Mack MG, Balzer JO, Straub R, et al. Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology. 2002;222:239–244. doi: 10.1148/radiol.2221010225. [DOI] [PubMed] [Google Scholar]
- 122.Harisinghani MG, Barentsz JO, Hahn PF, et al. MR lymphangiography for detection of minimal nodal disease in patients with prostate cancer. Acad Radiol. 2002;9(suppl 2):S312–S313. doi: 10.1016/s1076-6332(03)80213-1. [DOI] [PubMed] [Google Scholar]
- 123.Feldman AS, McDougal WS, Harisinghani MG. The potential of nanoparticle-enhanced imaging. Urol Oncol. 2008;26:65–73. doi: 10.1016/j.urolonc.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 124.Sadek H, Latif S, Collins R, et al. Use of ferumoxides for stem cell labeling. Regen Med. 2008;3:807–816. doi: 10.2217/17460751.3.6.807. [DOI] [PubMed] [Google Scholar]
- 125.Neuberger T, Schöpf B, Hofmann H, et al. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater. 2005;293:483–496. [Google Scholar]
- 126.Gupta AK, Gupta M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials. 2005;26:1565–1573. doi: 10.1016/j.biomaterials.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 127.Duguet E, Vasseur S, Mornet S, et al. Magnetic nanoparticles and their applications in medicine. Nanomedicine. 2006;1:157–168. doi: 10.2217/17435889.1.2.157. [DOI] [PubMed] [Google Scholar]
- 128.Gupta AK, Naregalkar RR, Vaidya VD, et al. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- 129.Gurzau ES, Neagu C, Gurzau AE. Essential metals–case study on iron. Ecotoxicol Environ Saf. 2003;56:190–200. doi: 10.1016/s0147-6513(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 130.Thorek DL, Chen AK, Czupryna J, et al. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 131.Arbab AS, Yocum GT, Rad AM, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 132.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 133.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 134.Stuckey DJ, Carr CA, Martin-Rendon E, et al. Iron particles for noninvasive monitoring of bone marrow stromal cell engraftment into, and isolation of viable engrafted donor cells from, the heart. Stem Cells. 2006;24:1968–1975. doi: 10.1634/stemcells.2006-0074. [DOI] [PubMed] [Google Scholar]
- 135.Anderson SA, Glod J, Arbab AS, et al. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105:420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- 136.Carr CA, Stuckey DJ, Tatton L, et al. Bone marrow-derived stromal cells home to and remain in the infarcted rat heart but fail to improve function: An in vivo cine-MRI study. Am J Physiol Heart Circ Physiol. 2008;295:H533–H542. doi: 10.1152/ajpheart.00094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jendelova P, Herynek V, Urdzikova L, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 139.Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–383. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- 140.Moore XL, Lu J, Sun L, et al. Endothelial progenitor cells' “homing” specificity to brain tumors. Gene Ther. 2004;11:811–818. doi: 10.1038/sj.gt.3302151. [DOI] [PubMed] [Google Scholar]
- 141.Huszthy PC, Giroglou T, Tsinkalovsky O, et al. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. Plos One. 2009;4:e6314. doi: 10.1371/journal.pone.0006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao D, Najbauer J, Garcia E, et al. Neural stem cell tropism to glioma: Critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- 143.Yip S, Aboody KS, Burns M, et al. Neural stem cell biology may be well suited for improving brain tumor therapies. Cancer J. 2003;9:189–204. doi: 10.1097/00130404-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 144.Delcroix GJ, Jacquart M, Lemaire L, et al. Mesenchymal and neural stem cells labeled with HEDP-coated SPIO nanoparticles: In vitro characterization and migration potential in rat brain. Brain Res. 2009;1255:18–31. doi: 10.1016/j.brainres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 145.Zhu W, Li X, Tang Z, et al. Superparamagnetic iron oxide labeling of neural stem cells and 4.7T MRI tracking in vivo and in vitro. J Huazhong Univ Sci Technol Med Sci. 2007;27:107–110. doi: 10.1007/s11596-007-0130-1. [DOI] [PubMed] [Google Scholar]
- 146.Shah K. Imaging neural stem cell fate in mouse model of glioma. Curr Protoc Stem Cell Biol. 2009 doi: 10.1002/9780470151808.sc05a01s8. Chapter 5:Unit 5A 1. [DOI] [PubMed] [Google Scholar]
- 147.Waerzeggers Y, Klein M, Miletic H, et al. Multimodal imaging of neural progenitor cell fate in rodents. Mol Imaging. 2008;7:77–91. [PubMed] [Google Scholar]
- 148.Brekke C, Williams SC, Price J, et al. Cellular multiparametric MRI of neural stem cell therapy in a rat glioma model. Neuroimage. 2007;37:769–782. doi: 10.1016/j.neuroimage.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 149.Song M, Kim Y, Kim Y, et al. MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci Res. 2009;64:235–239. doi: 10.1016/j.neures.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 150.Ruiz-Cabello J, Walczak P, Kedziorek DA, et al. In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med. 2008;60:1506–1511. doi: 10.1002/mrm.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Z, Jiang Q, Jiang F, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004;23:281–287. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 152.Pereboeva L, Curiel DT. Cellular vehicles for cancer gene therapy: Current status and future potential. Biodrugs. 2004;18:361–385. doi: 10.2165/00063030-200418060-00003. [DOI] [PubMed] [Google Scholar]
- 153.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walczak P, Kedziorek DA, Gilad AA, et al. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: The case of the shiverer dysmyelinated mouse brain. Magn Reson Med. 2007;58:261–269. doi: 10.1002/mrm.21280. [DOI] [PubMed] [Google Scholar]
- 155.Pawelczyk E, Arbab AS, Chaudhry A, et al. In vitro model of bromodeoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: Implications for cellular therapy. Stem Cells. 2008;26:1366–1375. doi: 10.1634/stemcells.2007-0707. [DOI] [PubMed] [Google Scholar]
- 156.Frangioni JV, Hajjar RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation. 2004;110:3378–3383. doi: 10.1161/01.CIR.0000149840.46523.FC. [DOI] [PubMed] [Google Scholar]
- 157.Matuszewski L, Persigehl T, Wall A, et al. Cell tagging with clinically approved iron oxides: Feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005;235:155–161. doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]
- 158.Lewin M, Carlesso N, Tung CH, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 159.Bulte JW, Douglas T, Witwer B, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 160.Arbab AS, Bashaw LA, Miller BR, et al. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: Methods and techniques. Transplantation. 2003;76:1123–1130. doi: 10.1097/01.TP.0000089237.39220.83. [DOI] [PubMed] [Google Scholar]
- 161.Arbab AS, Yocum GT, Kalish H, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 162.Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–7. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 163.Walczak P, Kedziorek DA, Gilad AA, et al. Instant MR labeling of stem cells using magnetoelectroporation. Magn Reson Med. 2005;54:769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 164.Bulte JW, Arbab AS, Douglas T, et al. Preparation of magnetically labeled cells for cell tracking by magnetic resonance imaging. Methods Enzymol. 2004;386:275–299. doi: 10.1016/S0076-6879(04)86013-0. [DOI] [PubMed] [Google Scholar]
- 165.Daniels TR, Delgado T, Rodriguez JA, et al. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 166.Gatter KC, Brown G, Trowbridge IS, et al. Transferrin receptors in human tissues: Their distribution and possible clinical relevance. J Clin Pathol. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Daniels TR, Delgado T, Helguera G, et al. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121:159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 168.Arbab AS, Yocum GT, Wilson LB, et al. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004;3:24–32. doi: 10.1162/15353500200403190. [DOI] [PubMed] [Google Scholar]
- 169.Janic B, Rad AM, Jordan EK, et al. Optimization and validation of FePro cell labeling method. Plos One. 2009;4:e5873. doi: 10.1371/journal.pone.0005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kostura L, Kraitchman DL, Mackay AM, et al. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 171.Daldrup-Link HE, Meier R, Rudelius M, et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15:4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 172.Himmelreich U, Hoehn M. Stem cell labeling for magnetic resonance imaging. Minim Invasive Ther Allied Technol. 2008;17:132–142. doi: 10.1080/13645700801969873. [DOI] [PubMed] [Google Scholar]
- 173.Kustermann E, Himmelreich U, Kandal K, et al. Efficient stem cell labeling for MRI studies. Contrast Media Mol Imaging. 2008;3:27–37. doi: 10.1002/cmmi.229. [DOI] [PubMed] [Google Scholar]
- 174.Montet-Abou K, Montet X, Weissleder R, et al. Cell internalization of magnetic nanoparticles using transfection agents. Mol Imaging. 2007;6:1–9. [PubMed] [Google Scholar]
- 175.Anderson CJ, Bulte JW, Chen K, et al. Design of targeted cardiovascular molecular imaging probes. J Nucl Med. 2010;51(Suppl 1):3S–17S. doi: 10.2967/jnumed.109.068130. Epub 2010 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song M, Moon WK, Kim Y, et al. Labeling efficacy of superparamagnetic iron oxide nanoparticles to human neural stem cells: Comparison of ferumoxides, monocrystalline iron oxide, cross-linked iron oxide (CLIO)-NH2 and tat-CLIO. Korean J Radiol. 2007;8:365–371. doi: 10.3348/kjr.2007.8.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bulte JW, Ben-Hur T, Miller BR, et al. MR microscopy of magnetically labeled neurospheres transplanted into the Lewis EAE rat brain. Magn Reson Med. 2003;50:201–205. doi: 10.1002/mrm.10511. [DOI] [PubMed] [Google Scholar]
- 178.Gamarra LF, Pavon LF, Marti LC, et al. In vitro study of CD133 human stem cells labeled with superparamagnetic iron oxide nanoparticles. Nanomedicine. 2008;4:330–339. doi: 10.1016/j.nano.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 179.Bulte JW, Douglas T, Witwer B, et al. Monitoring stem cell therapy in vivo using magnetodendrimers as a new class of cellular MR contrast agents. Acad Radiol. 2002;9(suppl 2):S332–S335. doi: 10.1016/s1076-6332(03)80221-0. [DOI] [PubMed] [Google Scholar]
- 180.Schafer R, Kehlbach R, Muller M, et al. Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy. 2009;11:68–78. doi: 10.1080/14653240802666043. [DOI] [PubMed] [Google Scholar]
- 181.Schafer R, Kehlbach R, Wiskirchen J, et al. Transferrin receptor upregulation: In vitro labeling of rat mesenchymal stem cells with superparamagnetic iron oxide. Radiology. 2007;244:514–523. doi: 10.1148/radiol.2442060599. [DOI] [PubMed] [Google Scholar]
- 182.Gilad AA, Walczak P, McMahon MT, et al. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn Reson Med. 2008;60:1–7. doi: 10.1002/mrm.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Modo M, Mellodew K, Cash D, et al. Mapping transplanted stem cell migration after a stroke: A serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21:311–317. doi: 10.1016/j.neuroimage.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 184.Anderson SA, Lee KK, Frank JA. Gadolinium-fullerenol as a paramagnetic contrast agent for cellular imaging. Invest Radiol. 2006;41:332–338. doi: 10.1097/01.rli.0000192420.94038.9e. [DOI] [PubMed] [Google Scholar]
- 185.Brekke C, Morgan SC, Lowe AS, et al. The in vitro effects of a bimodal contrast agent on cellular functions and relaxometry. NMR Biomed. 2007;20:77–89. doi: 10.1002/nbm.1077. [DOI] [PubMed] [Google Scholar]
- 186.Ahrens ET, Feili-Hariri M, Xu H, et al. Receptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imaging. Magn Reson Med. 2003;49:1006–1013. doi: 10.1002/mrm.10465. [DOI] [PubMed] [Google Scholar]
- 187.Partlow KC, Chen J, Brant JA, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 188.Choi JS, Park JC, Nah H, et al. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew Chem Int Ed Engl. 2008;47:6259–6262. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]
- 189.Foster-Gareau P, Heyn C, Alejski A, et al. Imaging single mammalian cells with a 1.5 T clinical MRI scanner. Magn Reson Med. 2003;49:968–971. doi: 10.1002/mrm.10417. [DOI] [PubMed] [Google Scholar]
- 190.Heyn C, Ronald JA, Mackenzie LT, et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med. 2006;55:23–29. doi: 10.1002/mrm.20747. [DOI] [PubMed] [Google Scholar]
- 191.Srinivas M, Morel PA, Ernst LA, et al. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med. 2007;58:725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- 192.Gilad AA, Ziv K, McMahon MT, et al. MRI reporter genes. J Nucl Med. 2008;49:1905–1908. doi: 10.2967/jnumed.108.053520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Plank C, Schillinger U, Scherer F, et al. The magnetofection method: Using magnetic force to enhance gene delivery. Biol Chem. 2003;384:737–747. doi: 10.1515/BC.2003.082. [DOI] [PubMed] [Google Scholar]
- 194.Scherer F, Anton M, Schillinger U, et al. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 195.Bhattarai SR, Kim SY, Jang KY, et al. N-hexanoyl chitosan-stabilized magnetic nanoparticles: Enhancement of adenoviral-mediated gene expression both in vitro and in vivo. Nanomedicine. 2008;4:146–154. doi: 10.1016/j.nano.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 196.Pichler BJ, Wehrl HF, Judenhofer MS. Latest advances in molecular imaging instrumentation. J Nucl Med. 2008;49(suppl 2):5S–23S. doi: 10.2967/jnumed.108.045880. [DOI] [PubMed] [Google Scholar]
- 197.Pichler BJ, Wehrl HF, Kolb A, et al. Positron emission tomography/magnetic resonance imaging: The next generation of multimodality imaging? Semin Nucl Med. 2008;38:199–208. doi: 10.1053/j.semnuclmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dousset V, Ballarino L, Delalande C, et al. Comparison of ultrasmall particles of iron oxide (USPIO)-enhanced T2-weighted, conventional T2-weighted, and gadolinium-enhanced T1-weighted MR images in rats with experimental autoimmune encephalomyelitis. AJNR Am J Neuroradiol. 1999;20:223–227. [PMC free article] [PubMed] [Google Scholar]
- 199.Judenhofer MS, Wehrl HF, Newport DF, et al. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 200.Catana C, Procissi D, Wu Y, et al. Simultaneous in vivo positron emission tomography and magnetic resonance imaging. Proc Natl Acad Sci USA. 2008;105:3705–3710. doi: 10.1073/pnas.0711622105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pichler BJ, Judenhofer MS, Pfannenberg C. Multimodal imaging approaches: PET/CT and PET/MRI. Handb Exp Pharmacol. 2008:109–132. doi: 10.1007/978-3-540-72718-7_6. [DOI] [PubMed] [Google Scholar]
- 202.Lee Z, Dennis JE, Gerson SL. Imaging stem cell implant for cellular-based therapies. Exp Biol Med (Maywood) 2008;233:930–940. doi: 10.3181/0709-MR-234. [DOI] [PubMed] [Google Scholar]
- 203.Aisen P. Transferrin, the transferrin receptor, and the uptake of iron by cells. Met Ions Biol Syst. 1998;35:585–631. [PubMed] [Google Scholar]
- 204.Aisen P. Ferritin receptors and the role of ferritin in iron transport. Target Diagn Ther. 1991;4:339–354. [PubMed] [Google Scholar]
- 205.Ikuta K, Zak O, Aisen P. Recycling, degradation and sensitivity to the synergistic anion of transferrin in the receptor-independent route of iron uptake by human hepatoma (HuH-7) cells. Int J Biochem Cell Biol. 2004;36:340–352. doi: 10.1016/s1357-2725(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 206.Bulte JW, Zhang S, van Gelderen P, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: Magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci USA. 1999;96:15256–15261. doi: 10.1073/pnas.96.26.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Genove G, DeMarco U, Xu H, et al. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 208.Zurkiya O, Chan AW, Hu X. MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn Reson Med. 2008;59:1225–1231. doi: 10.1002/mrm.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Alfke H, Stoppler H, Nocken F, et al. In vitro MR imaging of regulated gene expression. Radiology. 2003;228:488–492. doi: 10.1148/radiol.2282012006. [DOI] [PubMed] [Google Scholar]
- 210.Ramadan SS, Heyn C, Mackenzie LT, et al. Ex-vivo cellular MRI with b-SSFP: Quantitative benefits of 3T over 1.5 T. Magn Reson Mater Phys Biol Med. 2008;21:251–259. doi: 10.1007/s10334-008-0118-2. [DOI] [PubMed] [Google Scholar]
- 211.Heyn C, Ronald JA, Mackenzie LT, et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med. 2006;55:23–29. doi: 10.1002/mrm.20747. [DOI] [PubMed] [Google Scholar]
- 212.Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 213.Shapiro EM, Skrtic S, Sharer K, et al. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kircher MF, Mahmood U, King RS, et al. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63:8122–8125. [PubMed] [Google Scholar]
- 215.Josephson L, Kircher MF, Mahmood U, et al. Near-infrared fluorescent nanoparticles as combined MR/optical imaging probes. Bioconjug Chem. 2002;13:554–560. doi: 10.1021/bc015555d. [DOI] [PubMed] [Google Scholar]
- 216.Wang S, Jarrett BR, Kauzlarich SM, et al. Core/shell quantum dots with high relaxivity and photoluminescence for multimodality imaging. J Am Chem Soc. 2007;129:3848–3856. doi: 10.1021/ja065996d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huber MM, Staubli AB, Kustedjo K, et al. Fluorescently detectable magnetic resonance imaging agents. Bioconjug Chem. 1998;9:242–249. doi: 10.1021/bc970153k. [DOI] [PubMed] [Google Scholar]
- 218.Zhang Z, Liang K, Bloch S, et al. Monomolecular multimodal fluorescence-radioisotope imaging agents. Bioconjug Chem. 2005;16:1232–1239. doi: 10.1021/bc050136s. [DOI] [PubMed] [Google Scholar]
- 219.Zielhuis SW, Seppenwoolde JH, Mateus VA, et al. Lanthanide-loaded liposomes for multimodality imaging and therapy. Cancer Biother Radiopharm. 2006;21:520–527. doi: 10.1089/cbr.2006.21.520. [DOI] [PubMed] [Google Scholar]
- 220.Hill JM, Dick AJ, Raman VK, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 222.Park KS, Tae J, Choi B, et al. Characterization, in vitro cytotoxicity assessment, and in vivo visualization of multimodal, RITC-labeled, silica-coated magnetic nanoparticles for labeling human cord blood-derived mesenchymal stem cells. Nanomedicine. 2009 doi: 10.1016/j.nano.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 223.Jackson J, Chapon C, Jones W, et al. In vivo multimodal imaging of stem cell transplantation in a rodent model of Parkinson's disease. J Neurosci Methods. 2009;183:141–148. doi: 10.1016/j.jneumeth.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 224.Chapon C, Jackson JS, Aboagye EO, et al. An in vivo multimodal imaging study using MRI and PET of stem cell transplantation after myocardial infarction in rats. Mol Imaging Biol. 2009;11:31–38. doi: 10.1007/s11307-008-0174-z. [DOI] [PubMed] [Google Scholar]
- 225.Zinn KR, Chaudhuri TR, Szafran AA, et al. Noninvasive bioluminescence imaging in small animals. ILAR J. 2008;49:103–115. doi: 10.1093/ilar.49.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Myhr G. MR guided cancer treatment system for an elevated therapeutic index—A macroscopic approach. Med Hypotheses. 2008;70:665–670. doi: 10.1016/j.mehy.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 227.Cao F, Drukker M, Lin S, et al. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007;9:107–117. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- 228.Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.De Palma M, Venneri MA, Roca C, et al. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 230.Kang JH, Lee DS, Paeng JC, et al. Development of a sodium/iodide symporter (NIS)-transgenic mouse for imaging of cardiomyocyte-specific reporter gene expression. J Nucl Med. 2005;46:479–483. [PubMed] [Google Scholar]
- 231.Choi SC, Shim WJ, Lim DS. Specific monitoring of cardiomyogenic and endothelial differentiation by dual promoter-driven reporter systems in bone marrow mesenchymal stem cells. Biotechnol Lett. 2008;30:835–843. doi: 10.1007/s10529-007-9631-z. [DOI] [PubMed] [Google Scholar]
- 232.Kumar S, Mahendra G, Ponnazhagan S. Determination of osteoprogenitor-specific promoter activity in mouse mesenchymal stem cells by recombinant adeno-associated virus transduction. Biochim Biophys Acta. 2005;1731:95–103. doi: 10.1016/j.bbaexp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 233.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 234.Kim SM, Lim JY, Park SI, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 235.Klopp AH, Spaeth EL, Dembinski JL, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zielske SP, Livant DL, Lawrence TS. Radiation increases invasion of gene-modified mesenchymal stem cells into tumors. Int J Radiat Oncol Biol Phys. 2009;75:843–853. doi: 10.1016/j.ijrobp.2008.06.1953. Epub 2008 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]