Abstract
In epithelial cells, E-cadherin plays a key role in cell-cell adhesion, and loss of E-cadherin is a hallmark of tumor progression fostering cancer cell invasion and metastasis. To examine E-cadherin loss in squamous cell cancers, we used primary human esophageal epithelial cells (keratinocytes) as a platform and retrovirally transduced wild-type and dominant-negative forms of E-cadherin into these cells. We found decreased cell adhesion in the cells expressing dominant-negative E-cadherin, thereby resulting in enhanced migration and invasion. To analyze which molecular pathway(s) may modulate these changes, we conducted microarray analysis and found up-regulation of transforming growth factor β receptor II (TβRII) in the wild-type E-cadherin-overexpressing cells, which was confirmed by real-time PCR and Western blot analyses. To investigate the in vivo relevance of this finding, we analyzed tissue microarrays of paired esophageal squamous cell carcinomas and adjacent normal esophagus, and we could show a coordinated loss of E-cadherin and TβRII in ~80% of tumors. To determine if there may be an E-cadherin-dependent regulation of TβRII, we show the physical interaction of E-cadherin with TβRII and that this is mediated through the extracellular domains of E-cadherin and TβRII, respectively. In addition, TβRI is recruited to this complex. When placed in the context of three-dimensional cell culture, which reflects the physiologic microenvironment, TβRII-mediated cell signaling is dependent upon intact E-cadherin function. Our results, which suggest that E-cadherin regulates TβRII function, have important implications for epithelial carcinogenesis characterized through the frequent occurrence of E-cadherin and TβRII loss.
Introduction
E-cadherin belongs to a family of cell surface glycoproteins, called the cadherins, which mediate calcium-dependent intercellular adhesion (1). The pivotal role for E-cadherin in epithelial morphogenesis and maintenance is illustrated by E-cadherin null mice that display embryonic lethality (2). The adhesive function of E-cadherin depends on interaction with regulatory proteins of the catenin family (i.e., β-catenin, plakoglobin, and α-catenin), which establish linkage to the cytoskeleton (1). Deletions of the extracellular domain and the cytoplasmic tail of E-cadherin have been used in Xenopus models and in human cancer cell lines with successful disruption of cell adhesion (3, 4). In particular, mutations and deletions in the cytoplasmic tail of E-cadherin, which contains the catenin-binding sites, result in disruption of the cadherin/catenin complex and loss of cellular adhesion (5). Furthermore, mutations in the region of E-cadherin that binds p120-catenin lead to the uncoupling of the E-cadherin-p120 complex and result in disruption of strong adhesion, although interaction with the other catenins remains intact (6).
The designation of E-cadherin as a “caretaker” of the epithelial phenotype is supported by its role in epithelial-mesenchymal transition (EMT; ref. 7). Disturbances in epithelial cell adhesion that lead to a more invasive and metastatic phenotype are a hallmark of tumor progression. A direct role for E-cadherin in suppression of tumor invasion has been shown by the reversion of undifferentiated, invasive tumors to a differentiated phenotype after transfection of E-cadherin cDNA in cell culture (8, 9). Although somatic mutations have been observed in a variety of human epithelial tumors (10), down-regulation of E-cadherin may be due also to transcriptional repression. The transcriptional suppression of E-cadherin through the zinc finger proteins Snail, Slug, and SIP-1 is observed in vitro and in vivo (11, 12). Transforming growth factor β1 (TGFβ1) has been shown to stimulate expression of Snail and SIP-1, thereby resulting in E-cadherin loss (7). Although E-cadherin is down-regulated frequently in response to TGFβ1, how the two molecules and the pathways they regulate are linked remains to be elucidated.
TGFβ signaling is known to activate cellular responses during growth, differentiation, and specification of developmental fate (13–15). TGFβ proteins signal through binding TGFβ receptor type II (TβRII), which in turn phosphorylates TGFβ receptor type I (TβRI). The latter then binds and phosphorylates cytoplasmic mediators of the Smad family. On phosphorylation, they form heteromeric complexes that are translocated to the nucleus and play a regulatory role in transcription (16–18). The transcriptional activation by Smads, through their physical interaction and functional cooperativity with other transcription factors, permits cross-talk with other signaling pathways (13, 19–21).
Although it is clear that TGFβ1 is a potent growth inhibitor in most cell types (18, 22), TGFβ1 has been shown to induce a transformed phenotype in normal rat kidney fibroblasts (23). Nevertheless, several components of the TGFβ pathway have been shown to act as tumor suppressors (24). Mutations in the Smad2 and Smad4 genes have been detected in several carcinomas (25, 26) but overall represent infrequent genetic alterations in all cancers. Despite its role as a tumor suppressor, tumor cells often show increased production of TGFβ1, which influences tumor cell motility and invasion with changes in the tumor microenvironment, leading to EMT (7, 27–29). This morphologic transition is characterized by extensive changes in the expression of cell adhesion molecules and cytokeratins to the expression of vimentin and S-100 (28, 30, 31). The ability of epithelial cells to undergo EMT in cell culture correlates with cell changes that facilitate invasion and metastasis in vivo (30, 31).
We describe herein that loss of E-cadherin function mediates enhanced esophageal cell migration and invasion. Intact E-cadherin induces an up-regulation of TβRII, and we show that the two molecules physically interact. In the context of three-dimensional culture systems, cells with intact E-cadherin are sensitive to the effects of TGFβ, whereas abrogation of E-cadherin function renders the cells resistant to TGFβ stimulation. This newly described interplay between E-cadherin and TβRII sheds new insights into the frequent occurrence of E-cadherin and TβRII loss in esophageal squamous cancers and, perhaps, other epithelially derived cancers.
Materials and Methods
Cell lines
Primary esophageal epithelial cells (keratinocytes), designated as EPC, from normal human esophagus were established and infected with filtered (0.45-μm pore size) retroviral supernatant from an overnight culture of Phoenix-Ampho cells (32), producing the pFB-neo retroviruses encoding full-length E-cadherin (designated as Ecad) and a dominant-negative mutant of E-cadherin lacking the cytoplasmic tail (designated as Ecyto). In addition, Ecad and Ecyto were expressed in EPC cells that were previously transduced with retrovirus encoding hTERT and shown to be immortalized (designated as EPC-hTERT; ref. 33). Additionally, a dominant-negative mutant of TβRII (a gift of Dr. H. Moses, Vanderbilt University, Nashville, TN) was subcloned in a retroviral vector, and Ecad and Ecyto cells were infected (designated as Ecad-dnTβRII and Ecyto-dnTβRII). hTERT cell lines overexpressing full-length TβRII were generated through subcloning of TβRII and designated Ecad-TβRII and Ecyto-TβRII. EPC2 and EPC-hTERT cells were grown at 37°C and 5% CO2 in keratinocyte serum-free medium (KSFM) supplemented with 40 μg/mL bovine pituitary extract, 1.0 ng/mL epidermal growth factor, 100 units/mL penicillin, and 100 μg/mL streptomycin. For TGFβ1 stimulation, 5 μmol/L TGFβ1 (R&D Systems, Minneapolis, MN) was added to the cell culture medium overnight at 37°C. E-cadherin neutralizing antibody (Sigma, St. Louis, MO) was added to the cell culture medium overnight at 1:2,000 concentration at 37°C.
Cell proliferation assays were done by plating the same number of cells from different cell lines in triplicate and harvested at different time points as indicated and counted using a Beckman Coulter particle counter (Beckman, Miami, FL).
Three-dimensional culture and immunofluorescence
Cells were resuspended in 2% Matrigel and then cultured on a Matrigel layer in chamber slides (Nalge Nunc, Naperville, IL) for 7 to 21 days (34). Cells were fixed in 4% paraformaldehyde (Fisher Scientific, Hampton, NJ) overnight at 4°C. After fixation, cells were treated with 0.1% Triton X-100 in PBS without calcium and magnesium for 20 minutes. Cells were washed in PBS and blocked with 1% bovine serum albumin (BSA; Sigma) for 1 hour. Incubation with tetramethylrhodamine isothiocyanate (TRITC)–conjugated phalloidin was overnight at 4°C. Stained spheroids were examined with a Zeiss (Thornwood, NY) confocal microscope at the Bioimaging Core Facility of the University of Pennsylvania (Philadelphia, PA).
RNA microarrays and tissue microarrays
RNA microarray analysis was done comparing the E-cadherin-overexpressing cells versus dominant-negative E-cadherin cells. RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) and converted to first-strand cDNA using SuperScript II reverse transcriptase primed by a poly(T) oligomer that incorporated the T7 promoter (Invitrogen, Carlsbad, CA). Hybridization to the Affymetrix (Santa Clara, CA) GeneChip U133A was done by the University of Pennsylvania Microarray facility.
A tissue microarray with 20 matched esophageal normal and tumor tissues was produced in the Morphology Core facility of the University of Pennsylvania. An additional tissue microarray with 84 spotted esophageal tissues, AccuMax Tissue Microarray, was purchased from ISU Abxis (distributed by Accurate Chem, Westbury, NY).
Immunohistochemistry
Immunohistochemistry for E-cadherin, β-catenin, TβRII, and pSmad2 in the tissue microarrays was done using the Vectastain Elite kit (Vector Laboratories, Burlingame, CA) following the manufacturer’s protocol. In brief, paraffin sections were pretreated with xylene and then placed in a microwave oven in 10 mmol/L citric acid buffer. Endogenous peroxidases were quenched using hydrogen peroxide before sections were incubated in avidin D blocking reagent and biotin blocking reagent. Sections were incubated with primary and secondary antibodies, and the signal was developed using the 3,3′-diaminobenzidine substrate kit for peroxidases.
Cell migration and invasion assays
Migration assays were done using Boyden chambers (8-μm pore size membranes), and invasion assays were done using Biocoat Matrigel invasion chambers (BD Biosciences, Franklin Lakes, NJ). Inserts were placed in a 24-well plate containing KSFM medium, including all supplements, which stimulates cell migration. Primary esophageal epithelial cells (5 × 104 per chamber) were resuspended in starvation medium, keratinocyte basal medium, and added into each insert.
For TGFβ1 treatment, 5 μmol/L TGFβ1 was added to the lower chamber during the time of incubation to serve as a chemoattractant. For E-cadherin neutralizing antibody studies, DECMA was added at a concentration of 1:2,000 to the upper chamber and incubated at 37°C for 24 hours. Then, cells attached to the upper side of the membrane were removed gently with a cotton swab and rinsed. Cells that migrated through the membrane and attached to the bottom of the membrane were fixed and stained with reagents from the Diff-Quik staining kit (Dade Behring, Newark, DE). Membranes were cut out and photographed such that migrated cells could be counted. There was no evidence of cell death. All experiments were done at least thrice in triplicate. The Student’s t test was done, and P < 0.05 was considered statistically significant.
Antibodies
The FLAG tag antibody (M2), actin, and E-cadherin neutralizing antibody (DECMA) were purchased from Sigma. Antibodies against pSmad2, pSmad3, and total Smad2 were obtained from Cell Signaling (Beverly, MA). The TβRII and TβRI antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and a second anti-TβRII antibody (2411) was from R&D Systems. β-Catenin, p120, and E-cadherin antibodies were obtained from Transduction Laboratories (Lexington, KY). Anti-mouse and anti-rabbit horseradish peroxidase (HRP)–conjugated antibodies were purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Immunoprecipitation
Preconfluent cells were washed with PBS and incubated with 700 μL lysis buffer [1% Triton X-100, 1% NP40, 50 mmol/L Tris (pH 8), proteinase inhibitors 2 μg/mL aprotinin, 1 mmol/L phenyl-methylsulfonyl fluoride, 10 mmol/L NaF, 2 mmol/L Na3VO4, 5 mmol/L sodium pyrophosphate] for 30 minutes on ice. BSA (4%, 70 μL) and 140 μL of 1.5 mol/L NaCl were added to the extracts, which were then preabsorbed with 10 μL recombinant protein G (rProtein G) agarose (Life Technologies, Gaithersburg, MD) for 1 hour at 4°C. Preabsorbed extracts were incubated with antibodies against pSmad2, TβRII, E-cadherin, and β-catenin. After 1-hour incubation at 4°C, the antigen-antibody complex was incubated with 10 μL rProtein G agarose for 1 hour at 4°C. The precipitates were washed thrice with 1 mL wash buffer [50 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS] and boiled with 100 μL lithium dodecyl sulfate (LDS) buffer (Invitrogen) containing reducing agent for 10 minutes. Supernatants were used for Western blotting as described below. Experiments were done in triplicate.
Western blotting
Subconfluent cells were harvested in lysis buffer [10 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1% NP40, 0.1% sodium deoxycholate, 0.1% SDS, 1 mmol/L EDTA, 2 mmol/L sodium orthovanadate, protease inhibitor mixture tablet (Roche Molecular Biochemicals, Indianapolis, IN)]. All stimulants and the neutralizing antibody were added to the culture 12 hours before harvesting to maintain the conditions used for the migration assays. Protein concentration was determined by the Bio-Rad protein assay (Bio-Rad, Hercules, CA). The solution was subsequently solubilized in NuPAGE LDS sample buffer (Invitrogen) containing reducing agent. Total protein samples (10 μg) were separated on a 4% to 12% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA). The membrane was blocked in 5% nonfat milk (Bio-Rad, Melville, NY) in TBS [10 mmol/L Tris, 150 mmol/L NaCl (pH 8.0), 0.1% Tween 20] for 1 hour at room temperature. Membranes were probed with the primary antibody diluted in 5% milk in TBS overnight at 4°C, washed thrice in TBS-Tween 20, incubated with anti-mouse or anti-rabbit HRP-conjugated antibody diluted 1:3,000 in TBS for 1 hour at room temperature, and then washed thrice in TBS. The signal was visualized by an enhanced chemiluminescence solution (ECL Plus, Amersham Pharmacia Biotech) and exposed to Kodak X-Omat LS film (Kodak, New York, NY). Experiments were repeated at least thrice.
ELISA
To determine the concentration of secreted human TGFβ1, the DuoSet ELISA kit from R&D Systems was used. In brief, conditioned media were activated using 0.1 mL 1 N HCl and then neutralized before addition to 96-well plates prepared with the capture antibody, washed, and blocked with 5% Tween in PBS. The samples and standard were loaded in duplicate. After overnight incubation, the plate was washed and the detection antibody was added followed by incubation with the streptavidin-HRP complex. The developing reaction with substrate was stopped, and the plates were analyzed subsequently.
Results
Dominant-negative E-cadherin alters cell adhesion and migration
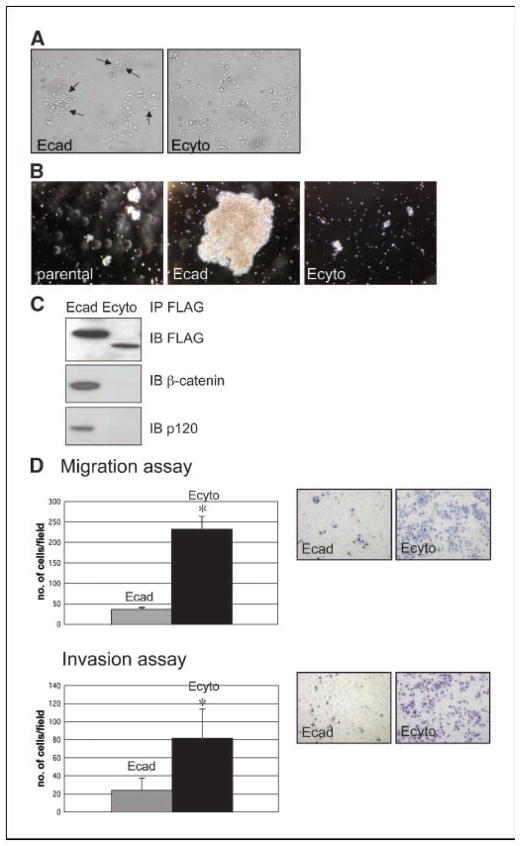
To model E-cadherin loss in esophageal epithelial cells or keratinocytes, we generated retroviral vectors encoding full-length E-cadherin (Ecad) and a dominant-negative mutant of E-cadherin lacking the cytoplasmic tail (Ecyto), both containing a COOH-terminal FLAG epitope tag. Overexpression of E-cadherin in primary esophageal keratinocytes results in cell clustering (Fig. 1A) and strong cell adhesion as measured by aggregation assays, whereas Ecyto inhibits cell adhesion (Fig. 1B). Coimmunoprecipitation with an antibody against FLAG shows that Ecyto cannot associate with β-catenin and therefore no longer mediates attachment to the actin cytoskeleton (Fig. 1C).
Figure 1.
Loss of E-cadherin-mediated cell adhesion results in increased cell migration and invasion. A, phase-contrast microscopic images of primary esophageal epithelial cells (keratinocytes) after retroviral transduction with full-length (wild-type) E-cadherin (Ecad) and cytoplasmic-deleted (dominant-negative) E-cadherin (Ecyto) reveal strong clustering in Ecad cells. B, there is strong adhesion in Ecad cells compared with parental and Ecyto cells based on cell aggregation assays. C, coimmunoprecipitation and Western blot assays with a FLAG antibody show disrupted interaction between E-cadherin and β-catenin/p120-catenin in Ecyto cells. D, cell migration (Boyden chamber) and invasion assays (Matrigel-coated Boyden chambers) reveal increased cell migration/invasion in Ecyto cells compared with Ecad cells. Gray column, Ecad cells; black column, Ecyto cells. *, P < 0.05 is statistically significant.
The disruption of E-cadherin-mediated adhesion in Ecyto cells did result in increased cell migration and invasion (Fig. 1D).
E-cadherin up-regulates members of the TGFβ signaling family
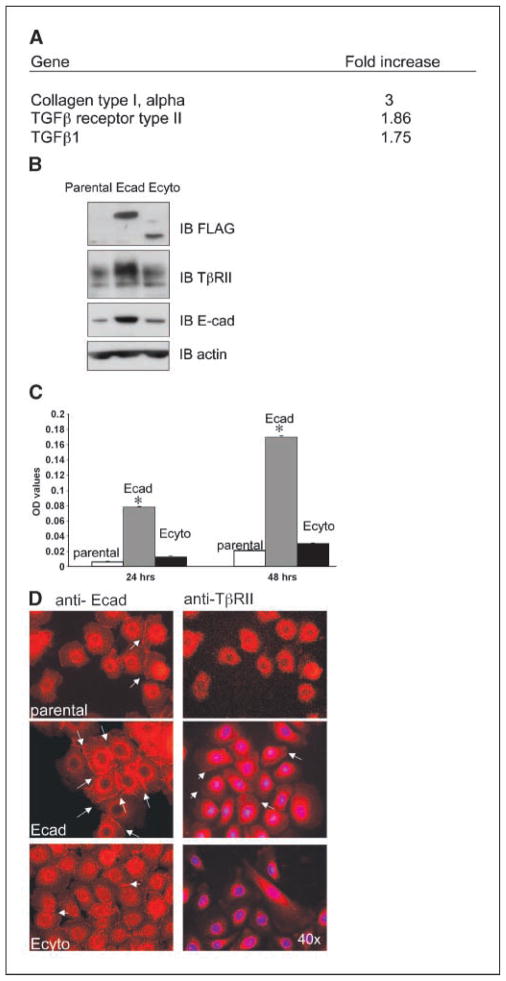
To determine the role of E-cadherin-mediated signaling through its cytoplasmic domain and thus provide potential insight into how E-cadherin modulates cell migration and invasion, we did RNA microarray analysis comparing Ecad versus Ecyto cells. Among the genes differentially expressed under these conditions, notably, multiple members of the TGFβ signaling family were up-regulated in response to E-cadherin overexpression (Fig. 2A). Of particular interest was the overexpression of TβRII, which was confirmed by real-time PCR (data not shown) as well as by Western blotting (Fig. 2B). Additionally, there was evidence of increased TGFβ1 secretion in Ecad cells compared with Ecyto cells as measured by ELISA (Fig. 2C).
Figure 2.
TGFβ gene family members are up-regulated in response to E-cadherin overexpression. A, selective representation of gene microarray results in Ecad versus Ecyto cells. B, Western blotting shows that TβRII is up-regulated as is endogenous E-cadherin in Ecad versus Ecyto and parental cells. An antibody against the FLAG epitope shows the expression of dominant-negative mutant and wild-type E-cadherin. Actin serves as a loading control. C, TGFβ1 secretion is increased in Ecad cells compared with Ecyto cells as measured by ELISA. White column, control cells; gray column, Ecad cells; black column, Ecyto cells. *, P < 0.05 is statistically significant. D, white arrows, E-cadherin and TβRII localize to the cell membrane in Ecad cells as determined by immunofluorescence staining. Parental and Ecyto cells show reduced expression of E-cadherin and TβRII. Magnification, ×40.
Given that parental esophageal epithelial cells are maintained in low calcium conditions or otherwise terminal differentiation would be triggered under high calcium conditions, we find that there is low endogenous E-cadherin in these cells (Fig. 2B) without induction of TβRII. Regardless of the particular genotype of the cells, nevertheless, both endogenous E-cadherin and TβRII localize to the cell membrane (Fig. 2D).
Overall, these unexpected results highlighting the induction of TβRII with enhanced E-cadherin expression prompted us to explore the potential relationship between E-cadherin and TβRII.
E-cadherin and TβRII interact through the extracellular domain of E-cadherin
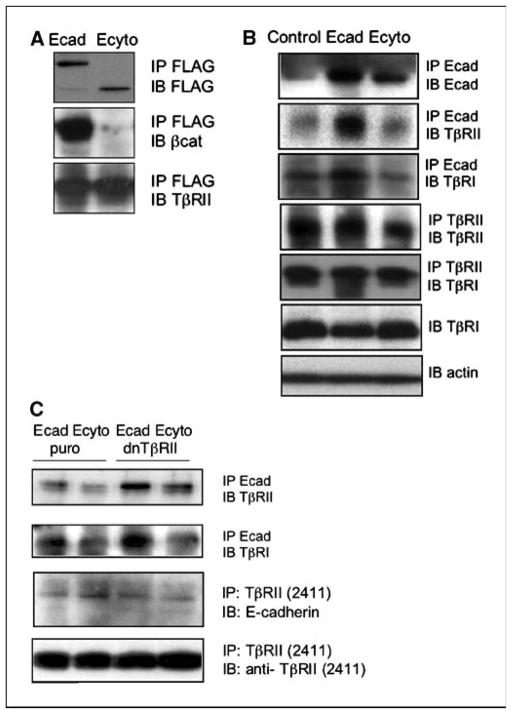
Coimmunoprecipitation with FLAG-tagged E-cadherin and TβRII was done to analyze if there might be physical interaction between E-cadherin and TβRII. E-cadherin and TβRII could be coprecipitated even in the absence of the cytoplasmic tail of E-cadherin, suggesting that the interaction is not mediated through cytoplasmic linker molecules (Fig. 3A). Furthermore, this interaction is present in parental cells as shown with antibodies against endogenous E-cadherin, apart from cells in which there is E-cadherin or mutant E-cadherin overexpression (Fig. 3B). Interestingly, this complex involves the recruitment of TβRI (Fig. 3B), suggesting the existence of a trimeric complex of E-cadherin, TβRII, and TβRI (Fig. 3B). The expression of TβRI remains unchanged in the presence of high levels of E-cadherin (Fig. 3B). To delineate further the domain(s) of TβRII required for interaction with E-cadherin, hTERT-Ecad and hTERT-Ecyto esophageal cells were transduced stably with a retroviral vector harboring TβRII with deletion of the cytoplasmic tail. Indeed, immunoprecipitations/immunoblots reveal that the extracellular domains of E-cadherin and TβRII mediate binding to each other (Fig. 3C).
Figure 3.
The extracellular domains of E-cadherin and TβRII mediate their physical interaction. A, coimmunoprecipitation and Western blot assays reveal physical interaction between FLAG epitope-tagged E-cadherin and TβRII in Ecad and Ecyto cells. B, coimmunoprecipitation of endogenous E-cadherin in parental, Ecad, and Ecyto cells showed that this interaction not only occurs after overexpression of E-cadherin but is also present in parental and Ecyto cells. Furthermore, TβRI is recruited to this complex. C, transduction of a dominant-negative TβRII mutant lacking the cytoplasmic tail identifies the extracellular domain of TβRII to be sufficient to mediate interaction with endogenous E-cadherin and TβRII.
TGFβ1 function is dependent on E-cadherin
To evaluate the effects of increased TGFβ1 secretion in Ecad cells, we tested the consequences of TGFβ1 stimulation of Ecad and Ecyto cells. TGFβ1 is known to induce the transcription of E-cadherin repressors, such as Snail and SIP-1, thereby resulting in E-cadherin loss and EMT. In addition, TGFβ1 has been suggested to disrupt adherens junctions, thus inducing a shift of β-catenin to an association with the Smads rather than E-cadherin (35). We therefore did migration and invasion assays in the presence of TGFβ1 stimulation.
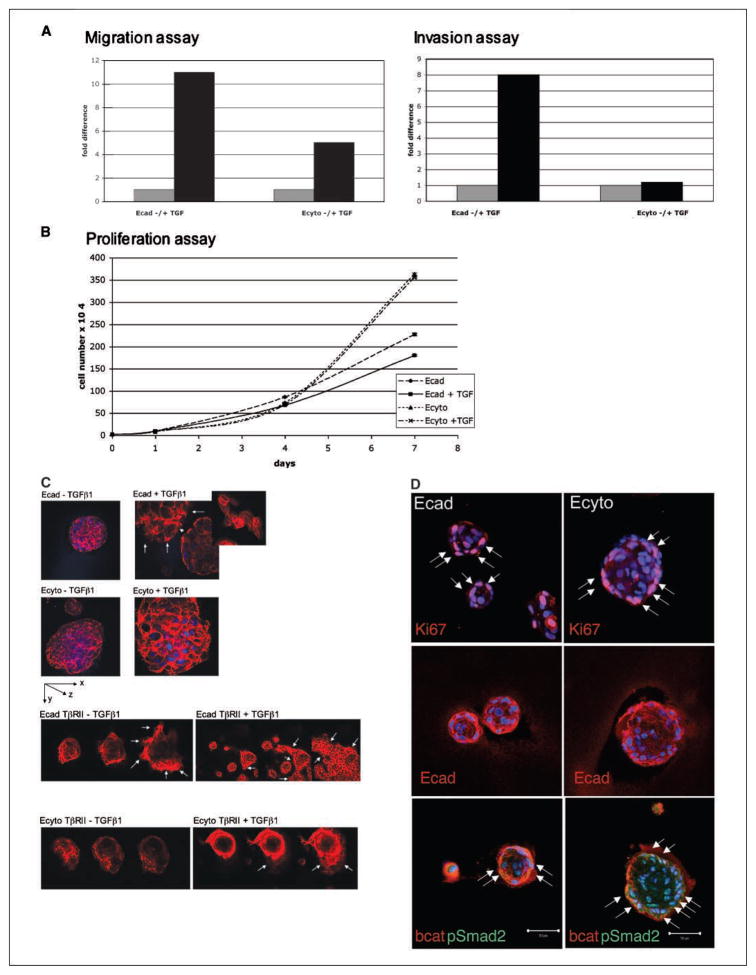
Although the ability to migrate and invade was increased in both Ecad and Ecyto cells in the presence of TGFβ1, the increase was more pronounced in Ecad when compared with Ecyto cells (Fig. 4A), suggesting that full-length E-cadherin may be required to mediate the full repertoire of effects of TGFβ1 on cell migration and invasion. Ecyto cells proliferate faster than Ecad cells, and this is preserved in the presence of TGFβ1 stimulation (Fig. 4B), whereas TGFβ1 exerts growth-inhibitory effects on cells with functional E-cadherin. However, TGFβ1 stimulation does not lead to any changes in the physical interaction of full-length E-cadherin with β-catenin or with TβRII as shown by coimmunoprecipitation and Western blotting (data not shown).
Figure 4.
Three-dimensional cultures of Ecad and Ecyto cells (spheroids) display differences in overall architecture and response to TGFβ1. A, TGFβ1 stimulation results in increased migration and invasion in both Ecad and Ecyto cells. Gray column, without TGFβ1 stimulation; black column, with TGFβ1 stimulation. B, proliferation assays show growth-inhibitory effects of TGFβ1 in Ecad cells but no changes in proliferation of Ecyto cells. C, Ecad spheroids are smaller compared with Ecyto spheroids, reflecting differences in cell-cell adhesion. TGFβ1 stimulation results in disruption of the integrity of the Ecad spheroids (arrows, cells in monolayer after TGFβ1 stimulation), which is not evident in Ecyto spheroids. Inset, cells in monolayer after TGFβ1 stimulation as well as stress fiber formation. White arrows, Ecad-TβRII spheroids disperse even in the absence of TGFβ1 and form monolayers to a greater extent in the presence of TGFβ1. This response is partially restored but delayed in Ecyto-TβRII spheroids in contrast to Ecyto spheroids that are resistant to the effects of TGFβ1. Spheroids are stained with TRITC-conjugated phalloidin. D, immunofluorescence of Ecad and Ecyto spheroids with antibody against Ki-67 (white arrows) shows fewer positive cells in the smaller Ecad spheroids. E-cadherin antibody shows localization of endogenous E-cadherin to the cell membrane in Ecad spheroids but only punctates cytoplasmic staining in Ecyto spheroids. White arrows, pSmad2 is localized to the nuclei of both Ecad and Ecyto spheroids. Bar, 50 μm.
TGFβ1 induces disruption of E-cadherin-dependent spheroid cysts in three-dimensional culture
Three-dimensional cell culture systems permit the investigation of how cells communicate with each other and how fundamental processes (i.e., proliferation, differentiation, and apoptosis) are modulated by the microenvironment (36). These culture models also reveal how tissue architecture can dramatically influence the response of a cell to exogenous stimuli. To test the effects of TGFβ1 in the three-dimensional model, we cultured Ecad and Ecyto cells embedded in 2% Matrigel and then placed the cells on a Matrigel layer (34). Immunofluorescence staining of these spheroids with TRITC-conjugated phalloidin, a marker for the actin cytoskeleton, revealed differences in size of the spheroids with Ecyto spheroids being larger than Ecad spheroids (Fig. 4C) In addition, there was a difference in response to TGFβ1 by Ecad cells compared with Ecyto cells. Stimulation with TGFβ1 disrupts Ecad spheroids, and the cells form monolayers and display stress fibers (Fig. 4C). These data suggest that the effects of TGFβ1 on migration and invasion may be dependent on intact E-cadherin structure and function in the physiologic microenvironment as revealed in three-dimensional cultures. To investigate further the role of TβRII, full-length TβRII was overexpressed through retroviral transduction in Ecad and Ecyto cells to determine if TβRII expression could restore a response to TGFβ1 in Ecyto cells. Ecad-TβRII cells show strong disruption of the spheroids that leads to monolayer formation that is enhanced by TGFβ1 treatment (Fig. 4C). Ecyto-TβRII cells, in contrast to Ecyto cells, respond to TGFβ1 stimulation with spheroid disruption, but this is delayed in the absence of TGFβ1 stimulation. To determine consequences apart from EMT, there is evidence of robust Ki-67 staining in both Ecad and Ecyto spheroids (Fig. 4D), although more so in Ecyto spheroid cysts because they have more cells. Endogenous E-cadherin staining is mislocalized in Ecyto spheroid cysts (Fig. 4D), which may reflect internalization and possible degradation of endogenous E-cadherin in the face of ectopically stable expression of mutant E-cadherin (Fig. 4D). In spite of the differences in E-cadherin in Ecad versus Ecyto spheroid cysts, pSmad2 is nuclear in both settings, suggesting that pSmad2 is influenced not only by the interplay between E-cadherin and TβRII but more so in a manner autonomous from that interaction.
Down-regulation of E-cadherin alters availability of TβRII
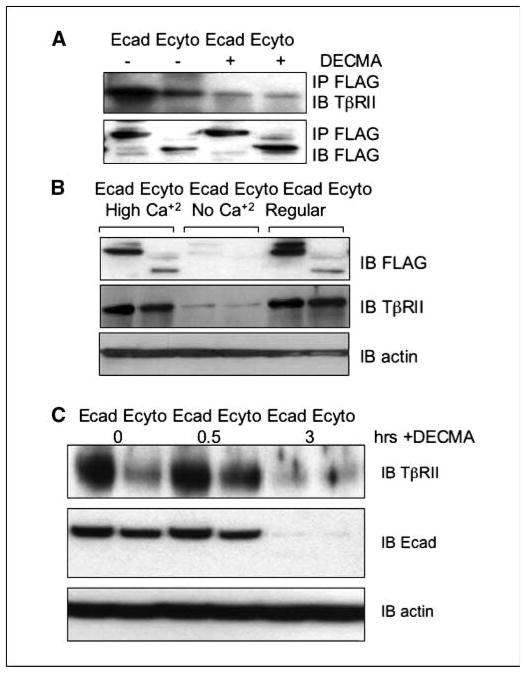
To confirm if the extracellular domain of E-cadherin is sufficient for the interaction with TβRII, we did coimmunoprecipitation and Western blotting in the presence of an E-cadherin neutralizing antibody (DECMA). After addition of the neutralizing antibody, the complex formation of E-cadherin and TβRII is essentially disrupted (Fig. 5A). Interestingly, in the face of E-cadherin loss with the neutralizing antibody, the expression of TβRII was also decreased (data not shown).
Figure 5.
Functional E-cadherin is necessary for the detection of TβRII. A, addition of an E-cadherin neutralizing antibody (DECMA) disrupts the interaction between E-cadherin and TβRII in both Ecad and Ecyto cells as shown by coimmunoprecipitation with antibody against FLAG. B, cells cultured without calcium show lower levels of E-cadherin, which is associated with lower TβRII expression levels. C, cells cultured in the presence of neutralizing antibody show a coordinated decrease of E-cadherin and TβRII levels after 3 hours of treatment. Actin serves as a loading control.
E-cadherin is a calcium-dependent glycoprotein with four calcium-binding domains in its extracellular domain. A switch to a low or calcium-free environment renders E-cadherin nonfunctional. We therefore cultivated Ecad and Ecyto cells in tissue culture medium without calcium supplementation in contrast to standard culture conditions (0.09 mmol/L calcium) and under a high calcium concentration (1.2 mmol/L), the latter of which induces terminal differentiation. The lack of calcium leads to low E-cadherin and TβRII levels as detected by Western blot (Fig. 5B), and this may be due to rapid turnover of E-cadherin on endocytosis (37, 38). Time course kinetic analysis of E-cadherin and TβRII shows coordinated decreased expression 3 hours after treatment with neutralizing antibody against E-cadherin (Fig. 5C) and destabilization of E-cadherin under low calcium conditions (data not shown) possibly due to increased degradation after endocytosis.
Loss of E-cadherin and TβRII is correlated in an esophageal squamous cancer tissue microarray
The finding that TβRII is up-regulated in E-cadherin-overexpressing cells led us to investigate whether this association could play a role in carcinogenesis, especially in light of frequent E-cadherin loss in later stages of epithelial-derived tumors. This provided a platform for a more rigorous basis to investigate a potential correlation between E-cadherin and TβRII expression. To that end, we generated tissue microarrays of 20 matched normal esophageal and squamous cell carcinoma samples and included a commercially available tissue microarrays spotted with 80 cores of esophageal tumors. We found a statistically significant correlation between loss of E-cadherin and TβRII; additionally, in tumors with retained expression of E-cadherin, there was also retained TβRII (Supplementary Fig. S1). Furthermore, we found a statistically significant correlation between the loss of E-cadherin and either loss of β-catenin or cytoplasmic localization of β-catenin (Supplementary Fig. S1). Importantly, we found statistically significant correlations of lost or retained expression of E-cadherin and TβRII as well as β-catenin and TβRII in ~80% of the tumors (Supplementary Fig. S1).
Discussion
E-cadherin is a critical calcium-dependent transmembrane molecule that mediates cell-cell adhesion through homophilic interactions. In turn, E-cadherin interacts with β-catenin and p120-catenin, which link to the actin cytoskeleton to foster cell adhesion. We show that cell adhesion is disrupted through deletion of the cytoplasmic tail of E-cadherin, and such cells are conferred an advantage in cell migration and invasion, which are critical events in the ability of malignant cells to navigate in their tumor microenvironment. Microarray analysis reveals that members of the TGFβ1 family, especially TβRII, are up-regulated in cells with intact E-cadherin compared with cells with a deletion of the cytoplasmic tail of E-cadherin. Moreover, cells with intact E-cadherin produce more TGFβ1 as well, suggesting an autocrine loop for ligand secretion.
What is the consequence of increased TGFβ1 and TβRII? It would seem that the physical interaction between E-cadherin and TβRII, one that is mediated by their respective extracellular domains, may contribute to the cellular effects of TGFβ1. This complex also involves the recruitment of TβRI and is not dependent on levels of TβRI. TGFβ1-mediated effects on cell migration and invasion are more pronounced in cells with intact E-cadherin compared with cells with a deletion of the cytoplasmic tail of E-cadherin. Because complete repertoire of TGFβ1 on modulating migration and invasion seemed to depend on intact E-cadherin, we used innovative three-dimensional culture systems to model the in vivo microenvironment and to study the response to TGFβ1. The disruption of the spheroids after stimulation with TGFβ1 is reminiscent of other three-dimensional culture systems that model EMT (39), and we show that there is a dependency of TGFβ1 signaling on intact E-cadherin. This dependency is emphasized by the fact that overexpression of TβRII in E-cadherin-overexpressing cells has a much stronger effect on spheroid disruption even in the absence of TGFβ1 than in Ecyto cells. The delayed response of Ecyto spheroids in dispersing and growing as monolayers seems to indicate that the full spectrum of TβRII function is supported by the presence of E-cadherin.
Disturbances in epithelial cell adhesion that lead to a more invasive and metastatic phenotype are a hallmark of tumor progression. Although somatic mutations in E-cadherin have been observed in a variety of human epithelial tumors (10), down-regulation of E-cadherin is mostly due to transcriptional repression. As we show here, E-cadherin is lost in the majority of esophageal squamous cell carcinomas, and this correlates with loss of TβRII.
Loss of TβRII has been associated with the induction of tumor formation. Patients with hereditary nonpolyposis colorectal cancer may have somatic mutations in the TβRII gene (40). Loss of TβRII has been modeled in mice through a dominant-negative approach in skin (41) and mammary gland and with the conditional knockout of TβRII (42, 43). In both models, mice develop tumors and have high potential for metastasis, thereby supporting the tumor-suppressive function of TβRII and intact TGFβ1 signaling. Furthermore, loss of other members of the TGFβ1 signaling pathway is involved in carcinogenesis.
The influence exerted by molecules involved in cell migration and invasion, such as E-cadherin, on TβRII and the accompanying signaling cascade remains unknown. The opportunity for the coordinated regulation of TβRII by E-cadherin is suggested by the following lines of evidence from our studies. First, there is up-regulation of TβRII in esophageal epithelial cells that harbor E-cadherin overexpression. Second, there is a statistically significant loss of both E-cadherin and TβRII in esophageal tumor tissues. Third, there is loss of TβRII under conditions, such as low calcium or with a neutralizing antibody, which destabilize E-cadherin as revealed in time course kinetics.
In addition, it is conceivable that the E-cadherin/TβRII complex may be internalized into the cytoplasm from the cell surface after ligand stimulation. It is known that nonfunctional E-cadherin is rapidly internalized into the cytoplasm, which could result in the coordinated endocytosis of E-cadherin and TβRII as indicated by our data and described for other adhesion molecule/signaling receptor complexes (44, 45). The complex formation of adhesion molecules and receptor tyrosine kinases is now believed to influence the regulation of signal transduction (46–49). TβRII has been shown to have signaling functions autonomous from its localization to the cell surface. Recently, the promyelocytic leukemia (PML) tumor suppressor of acute PML, which was only known to accumulate in nuclear PML bodies, was shown to have a cytoplasmic pool regulating TGFβ signaling through interaction with Smad2/3 and SARA (50). Additionally, it has been shown that TβRI and TβRII are internalized into the early endosome through the clathrin-dependent pathway (51, 52). However, instead of targeting TβRII for degradation, the clathrin/early endosome pathway is critical for TGFβ signaling transduction. As we show the presence of pSmad2 and pSmad3 in the E-cadherin/TβRII complex by coimmunoprecipitation, it is possible that the endosomal pathway plays a role in regulating the signaling cascade observed in our experimental system.
Taken together, our data suggest a new role of E-cadherin in tumor suppression by enhancing TGFβ signaling, in addition to its classic role in suppressing cell migration and invasion. We speculate that, in normal cells, E-cadherin supports cell adhesion and promotes TGFβ1 signaling potentially to suppress uncontrolled cell proliferation. Thus, there could be an exquisite equilibrium between TGFβ1-inhibited cell proliferation, as regulated in part by E-cadherin, and TGFβ1-mediated repression of E-cadherin presumably to foster EMT. Under normal conditions, this could be conducive to maintaining the normal epithelial phenotype and constraining hyperproliferation. However, with disruption of the E-cadherin and TβRII interaction and loss of these molecules, the balance is tilted in favor of proliferation and tumor invasion.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute grant PO1-CA098101 (C.D. Andl, B.B. Fargnoli, H. Nakagawa, M. Takaoka, T. Okawa, A. Klein-Szanto, M. Herlyn, and A.K. Rustgi) and its core facilities, NIH/National Institutes of Diabetes, Digestive and Kidney Diseases Center for Molecular Studies in Digestive and Liver Diseases P30-DK50306, Morphology Core (Greg Enders, Gary Swain, Kelly Dempsey, and Shukriyyah Mitchell), Molecular Biology Core (Gary Wu and Sue Keilbaugh), Biomedical Imaging Core (Qian-Chun Yu and Xinyu Zhao), Microarray Core (Don Baldwin), and Cell Culture Core (Carl June and Richard Carroll) facilities; NIH grant F32-CA108657 and the American Gastroenterological Association/Foundation for Digestive Health and Nutrition Research Scholar Award (C.D. Andl); and NIH grant K01-DK066205 (H. Nakagawa).
We thank Rebecca Wells, Wafik El-Deiry, Cameron Johnstone, Therese Deramaudt, Ben Rhoades, Carmen Michaylira, and Doug Stairs for discussions.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–21. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 2.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–7. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development. 1994;120:901–9. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- 4.Nieman MT, Kim JB, Johnson KR, Wheelock MJ. Mechanism of extracellular domain-deleted dominant negative cadherins. J Cell Sci. 1999;112:1621–32. doi: 10.1242/jcs.112.10.1621. [DOI] [PubMed] [Google Scholar]
- 5.Stappert J, Kemler R. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes Commun. 1994;2:319–27. doi: 10.3109/15419069409014207. [DOI] [PubMed] [Google Scholar]
- 6.Thoreson MA, Anastasiadis PZ, Daniel JM, et al. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 8.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–11. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 9.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–19. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 10.Berx G, Becker KF, Hofler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226–37. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 12.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 13.Massague J. How cells read TGF-β signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 14.Patterson GI, Padgett RW. TGFβ-related pathways. Roles in Caenorhabditis elegans development. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- 15.Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 17.ten Dijke P, Hill CS. New insights into TGF-β-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Roberts AB, Wakefield LM. The two faces of transforming growth factor β in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piek E, Heldin CH, Ten Dijke P. Specificity, diversity, and regulation in TGF-β superfamily signaling. FASEB J. 1999;13:2105–24. [PubMed] [Google Scholar]
- 20.Zhang Y, Derynck R. Regulation of Smad signalling by protein associations and signalling crosstalk. Trends Cell Biol. 1999;9:274–9. doi: 10.1016/s0962-8924(99)01579-2. [DOI] [PubMed] [Google Scholar]
- 21.ten Dijke P, Miyazono K, Heldin CH. Signaling inputs converge on nuclear effectors in TGF-β signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- 22.Coffey RJ, Jr, Bascom CC, Sipes NJ, Graves-Deal R, Weissman BE, Moses HL. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor β. Mol Cell Biol. 1988;8:3088–93. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts AB, Flanders KC, Heine UI, et al. Transforming growth factor-β: multifunctional regulator of differentiation and development. Philos Trans R Soc Lond B Biol Sci. 1990;327:145–54. doi: 10.1098/rstb.1990.0050. [DOI] [PubMed] [Google Scholar]
- 24.Akhurst RJ, Derynck R. TGF-β signaling in cancer—a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 25.Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 26.Hata A, Shi Y, Massague J. TGF-β signaling and cancer: structural and functional consequences of mutations in Smads. Mol Med Today. 1998;4:257–62. doi: 10.1016/s1357-4310(98)01247-7. [DOI] [PubMed] [Google Scholar]
- 27.Bhowmick NA, Ghiassi M, Bakin A, et al. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–36. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(β) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112:4557–68. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- 30.Oft M, Heider KH, Beug H. TGFβ signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–52. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- 31.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–77. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 32.Andl CD, Mizushima T, Nakagawa H, et al. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–30. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 33.Harada H, Nakagawa H, Oyama K, et al. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res. 2003;1:729–38. [PubMed] [Google Scholar]
- 34.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 35.Tian YC, Phillips AO. Interaction between the transforming growth factor-β type II receptor/Smad pathway and β-catenin during transforming growth factor-β1-mediated adherens junction disassembly. Am J Pathol. 2002;160:1619–28. doi: 10.1016/s0002-9440(10)61109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahir N, Weaver VM. Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev. 2004;14:71–80. doi: 10.1016/j.gde.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–32. [PMC free article] [PubMed] [Google Scholar]
- 38.Kartenbeck J, Schmelz M, Franke WW, Geiger B. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J Cell Biol. 1991;113:881–92. doi: 10.1083/jcb.113.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates RC, Mercurio AM. Tumor necrosis factor-α stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003;14:1790–800. doi: 10.1091/mbc.E02-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markowitz S, Wang J, Myeroff L, et al. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–8. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 41.Bottinger EP, Jakubczak JL, Haines DC, Bagnall K, Wakefield LM. Transgenic mice overexpressing a dominant-negative mutant type II transforming growth factor β receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7,12-dimethylbenz-[a]-anthracene. Cancer Res. 1997;57:5564–70. [PubMed] [Google Scholar]
- 42.Forrester E, Chytil A, Bierie B, et al. Effect of conditional knockout of the type II TGF-β receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 43.Gorska AE, Jensen RA, Shyr Y, Aakre ME, Bhowmick NA, Moses HL. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-β receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am J Pathol. 2003;163:1539–49. doi: 10.1016/s0002-9440(10)63510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryant DM, Wylie FG, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol Biol Cell. 2005;16:14–23. doi: 10.1091/mbc.E04-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 46.Davies G, Jiang WG, Mason MD. HGF/SF modifies the interaction between its receptor c-Met, and the E-cadherin/catenin complex in prostate cancer cells. Int J Mol Med. 2001;7:385–8. doi: 10.3892/ijmm.7.4.385. [DOI] [PubMed] [Google Scholar]
- 47.Kamei T, Matozaki T, Sakisaka T, et al. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells—regulation by Rho, Rac, and Rab small G proteins. Oncogene. 1999;18:6776–84. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- 48.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–53. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 49.Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L. IGF-II induces rapid β-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene. 2001;20:4942–50. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 50.Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-β signalling. Nature. 2004;431:205–11. doi: 10.1038/nature02783. [DOI] [PubMed] [Google Scholar]
- 51.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 52.Hayes S, Chawla A, Corvera S. TGFβ receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol. 2002;158:1239–49. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.