Abstract
BACKGROUND
Blood banks have large altruistic donor populations and existing infrastructure that make them attractive sites for genetic epidemiologic research, but donors’ willingness to participate and the impact on blood donation are unknown.
STUDY DESIGN AND METHODS
A total of 2162 blood donors in Northern California responded to a cross-sectional questionnaire in August and September 2007. Participants were asked their likelihood of participation and future blood donation under three different scenarios: identity-linked genetic research, identity-unlinked genetic research, and genetic testing as a service.
RESULTS
The majority of blood donors indicated that they would be likely or very likely to participate in identity-linked genetic research (67%) and in identity-unlinked genetic research (54%). While older donors and more frequent donors were more likely to participate in identity-linked research, younger, Caucasian, more educated, and more frequent donors were more likely to participate in identity-unlinked research. Less than 10% of donors indicated they would be less likely to donate blood in the future if genetic research was conducted at blood banks. More than 75% of donors would be interested in genetic testing as an optional service at the blood bank, but more than 20% of donors would be less likely to donate if such a service was offered.
CONCLUSION
Overall, we found that the majority of blood donors would be likely to participate in genetic research and that less than 10% would be less inclined to donate if such research was conducted by blood banks.
Blood banks may be uniquely positioned to conduct large genetic epidemiologic studies. They have a broad donor base, collect blood samples frequently, test them for infectious agents using nucleic acid technology, and routinely collect demographic (phenotype) information into large databases.1 Large repositories of banked specimens from blood donors exist.2 At the same time, blood donor return rates are of increasing concern to blood banks and the potential adverse impact of genetic studies on these rates are unknown.3,4
Researchers have examined participation rates in genetic studies in other US populations and have found that the acceptability of allowing future unlimited research on stored specimens varies widely. In a recent review, Sterling and coworkers5 found that consent rates for genetic studies among individuals who had previously participated in health research ranged from 21% to 85%. Consent rates varied by demographic characteristics such as sex and race and/or ethnicity. The REDS-II study recently reported that 91% of donors who consented for a study of HLA antibodies were also willing to have their blood samples stored in a biorepository for future research use to “improve our understanding of transfusion biology and transfusion safety.”6 Odds of repository participation were lower for subjects who were African American or Hispanic, were 35 to 44 years old, or had not completed high school and were lowest at one geographic location, regardless of other variables.
But to our knowledge, there have been no studies specifically examining US blood donor opinions on participation in genetic research or testing and intention of ongoing donation if such research were undertaken by the blood center. We therefore conducted a survey among blood donors with three goals: to assess blood donor interest in participating in genetic studies through blood banks, to measure the potential impact of genetic studies on future blood donations, and to estimate donor interest in genetic testing offered by blood banks as an optional service.
MATERIALS AND METHODS
Study design and population
This was a cross-sectional, anonymous survey of blood donors. In August and September 2007, we administered a brief anonymous survey to blood donors at eight fixed collection sites and four mobile drives at a single blood center in the San Francisco Bay Area. All whole blood and apheresis donors passing donor eligibility criteria were eligible to participate. They were given a questionnaire at the time of registration at the collection site and asked to return it anonymously into a box at the postdonation canteen area. Individuals unable to read or understand English or Spanish were not included in the study. The Committee on Human Research at the University of California, San Francisco, approved the study protocol.
Instrument
In the questionnaire (see Appendix S1, available as supporting information in the online version of this paper), participants were asked how likely they would be to participate in three different scenarios at the blood bank: genetic research where donor identities would be linked to their samples, genetic research where donor identities would not be linked to their samples, and genetic testing as a service. Donor intent to participate was assessed by 5-point Likert scales ranging from “very likely” to “very unlikely.” In addition, we asked participants whether they would be more or less likely to donate in the future if they were asked to participate in each of the above scenarios at the blood bank. We assessed likelihood of future donation by a 5-point Likert scale ranging from “much more likely” to “much less likely.” In addition, we collected information on participant demographics as well as information on which diseases participants would consider for genetic testing and how much, if anything, they would pay for the service. Before administration of the survey, we conducted pilot testing with 10 donors using cognitive testing techniques.7 Pilot participants were asked to think aloud as they completed each item in the self-administered questionnaire. At the completion of each item, participants were asked a series of scripted and unscripted probes to determine if they understood the questions and interpreted the items as was intended by the research team. The questionnaire was revised based on pilot participant responses.
Statistical analysis
We calculated frequencies for questions on potential participation in genetic studies and/or testing and likelihood of future blood donation, as well as for demographic information among all 2162 participants. To determine whether likeliness to participate in future research and to donate in the future differed by demographic characteristics, we restricted our analysis to the 1992 donors who provided information on their age, sex, education, race and/or ethnicity, and donation frequency. We constructed separate multivariate logistic regression models for identity-linked genetic research, identity-unlinked genetic research, and genetic testing to calculate multivariate adjusted odds ratios (ORs). Demographic variables were thought to be potential confounders for each other a priori and therefore were included in all multivariate models. All statistical analyses were conducted using computer software (SAS, Version 9.1, SAS Institute, Cary, NC).
RESULTS
A total of 2162 allogeneic whole blood and apheresis donors participated; their characteristics are displayed in Table 1. Approximately half of participating donors were age 50 or older, and almost 70% were 40 years of age or older. One-fourth of participants were non-Caucasian and participants were evenly split between men and women. The vast majority of participants (90%) had attended some college and approximately 60% had a bachelor’s degree or higher. Only 5% of participants were first-time donors and more than 60% indicated that they had donated six or more times in the past 5 years. Compared to all donors at the blood center in 2007, participants were of similar sex, but older, more likely to be of Caucasian race and/or ethnicity and higher educational attainment, and less likely to be first-time donors.
TABLE 1.
Participant characteristics*
| Characteristic | Participants | 2007 donors |
|---|---|---|
| Age (years) | ||
| 16–19 | 127 (6) | 15,123 (20) |
| 20–29 | 252 (12) | 12,087 (16) |
| 30–39 | 265 (13) | 11,501 (15) |
| 40–49 | 435 (21) | 14,845 (19) |
| 50–59 | 571 (27) | 14,310 (18) |
| 60 or more | 461 (22) | 9,619 (12) |
| Missing | 51 | 38 |
| Sex | ||
| Male | 1050 (51) | 38,274 (51) |
| Female | 1025 (49) | 39,249 (49) |
| Missing | 87 | 0 |
| Race and/or ethnicity | ||
| Caucasian | 1598 (76) | 52,252 (67) |
| Non-Caucasian | 508 (24) | 25,267 (33) |
| Missing | 56 | 4 |
| Education | ||
| High school or less | 181 (9) | 21,115 (27) |
| Some college or trade school | 646 (31) | 20,675 (27) |
| Completed college (bachelor’s degree) | 688 (33) | 19,722 (25) |
| Graduate school or higher | 600 (28) | 11,336 (15) |
| Missing | 47 | 4,675 |
| Number of donations in past 5 years | ||
| First-time | 113 (5) | 21,633 (28) |
| 1–3 times | 362 (17) | 17,487 (23) |
| 4–5 times | 277 (13) | 9,145 (12) |
| 6–10 times | 396 (19) | 11,514 (15) |
| 11–20 times | 427 (20) | 9,720 (13) |
| More than 20 times | 515 (25) | 8,024 (10) |
| Missing | 72 | 0 |
Data are reported as number (%).
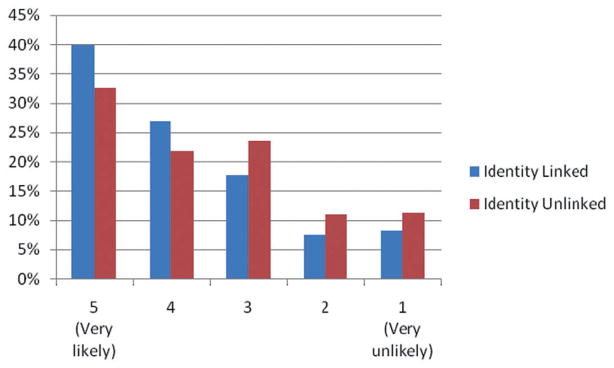
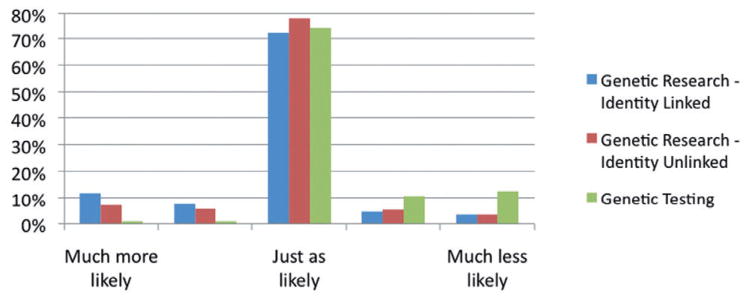
In the scenario with identity-linked genetic research, 67% of donors indicated that they were very likely or likely to participate (Fig. 1). In a multivariate model, younger donors aged 20 to 49 were less inclined to participate in identity-linked research compared to donors aged 60 or older (OR, 0.45–0.66; p < 0.05; Table 2). In addition, participants who were first-time donors were less inclined to participate than donors who had donated more than 20 times in the past 5 years (OR, 0.54; 95% confidence interval [CI], 0.33–0.86). Only 8% of participants believed that they would be less likely or much less likely to donate blood in the future if they were asked to participate in identity-linked research when they donated blood (Fig. 2). In the multivariate model, donors age 60 or older, those who had donated blood three times or less in the past 5 years, non-Caucasian donors, and donors with less than a bachelor’s degree indicated that they were less likely to donate blood in the future if they were asked to participate in identity-linked research at the blood bank (Table 3).
Fig. 1.
Likelihood of blood donor participation in genetic research.
TABLE 2.
Multivariate logistic regression: likely to participate in genetic research and/or testing*
| Characteristic | Identities linked | Identities not linked | Genetic testing as optional service |
|---|---|---|---|
| Age (years) | |||
| 16–19 | 0.84 (0.50–1.43) | 1.78 (1.06–2.97)† | 0.93 (0.53–1.61) |
| 20–29 | 0.66 (0.45–0.96)† | 1.62 (1.14–2.30)† | 1.03 (0.68–1.55) |
| 30–39 | 0.45 (0.32–0.64)† | 1.32 (0.95–1.84) | 0.91 (0.61–1.34) |
| 40–49 | 0.54 (0.40–0.73)† | 1.54 (1.15–2.05)† | 0.84 (0.61–1.18) |
| 50–59 | 0.80 (0.60–1.08) | 1.30 (1.00–1.69)† | 0.88 (0.65–1.20) |
| 60 or more | 1.00 | 1.00 | 1.00 |
| Wald p value | <0.01† | 0.04† | 0.89 |
| Sex | |||
| Male | 1.00 | 1.00 | 1.00 |
| Female | 0.98 (0.81–1.20) | 1.01 (0.84–1.21) | 0.82 (0.67–1.02) |
| Wald p value | 0.87 | 0.95 | 0.07 |
| Race/ethnicity | |||
| Caucasian | 1.00 | 1.00 | 1.00 |
| Non-Caucasian | 0.85 (0.67–1.08) | 0.67 (0.53–0.84)† | 0.96 (0.74–1.24) |
| Wald p value | 0.18 | <0.01† | 0.74 |
| Education | |||
| High school or less | 0.76 (0.50–1.17) | 0.33 (0.22–0.50)† | 0.53 (0.35–0.83)† |
| Some college or trade school | 0.94 (0.72–1.22) | 0.55 (0.43–0.71)† | 0.88 (0.66–1.17) |
| Completed college (bachelor’s degree) | 0.96 (0.74–1.23) | 0.79 (0.62–1.00) | 1.02 (0.77–1.35) |
| Graduate school or higher | 1.00 | 1.00 | 1.00 |
| Wald p value | 0.67 | <0.01† | 0.02† |
| Number of donations in past 5 years | |||
| First-time | 0.54 (0.33–0.86)† | 0.45 (0.28–0.73)† | 0.65 (0.39–1.08) |
| 1–3 times | 0.74 (0.54–1.02) | 0.68 (0.50–0.92)† | 0.78 (0.55–1.10) |
| 4–5 times | 0.76 (0.54–1.06) | 0.61 (0.44–0.84)† | 0.98 (0.68–1.43) |
| 6–10 times | 0.87 (0.64–1.17) | 0.77 (0.58–1.02) | 0.97 (0.70–1.36) |
| 11–20 times | 0.89 (0.66–1.20) | 0.81 (0.61–1.06) | 0.95 (0.69–1.31) |
| More than 20 times | 1.00 | 1.00 | 1.00 |
| Wald p value | 0.14 | 0.01† | 0.46 |
Data are reported as OR (95% CI).
Significant at p < 0.05.
Fig. 2.
Likelihood of donating blood in the future.
TABLE 3.
Multivariate logistic regression: less likely to donate in future if asked to participate in genetic research and/or testing at the blood bank*
| Characteristic | Identities linked | Identities not linked | Genetic testing as optional service |
|---|---|---|---|
| Age (years) | |||
| 16–19 | 0.41 (0.23–0.74)† | 0.38 (0.19–0.77)† | 1.03 (0.60–1.79) |
| 20–29 | 0.54 (0.35–0.84)† | 0.52 (0.31–0.89)† | 1.12 (0.75–1.67) |
| 30–39 | 0.35 (0.22–0.56)† | 0.70 (0.42–1.16) | 0.85 (0.57–1.28) |
| 40–49 | 0.57 (0.40–0.82)† | 0.85 (0.56–1.29) | 1.01 (0.72–1.43) |
| 50–59 | 0.54 (0.38–0.76)† | 0.72 (0.48–1.07) | 0.81 (0.58–1.12) |
| 60 or more | 1.00 | 1.00 | 1.00 |
| Wald p value | <0.01† | 0.06 | 0.51 |
| Sex | |||
| Male | 1.00 | 1.00 | 1.00 |
| Female | 1.08 (0.85–1.37) | 0.98 (0.74–1.29) | 0.92 (0.74–1.15) |
| Wald p value | 0.54 | 0.87 | 0.47 |
| Race and/or ethnicity | |||
| Caucasian | 1.00 | 1.00 | 1.00 |
| Non-Caucasian | 2.73 (2.08–3.57)† | 2.21 (1.62–3.02)† | 1.68 (1.31–2.16)† |
| Wald p value | <0.01† | <0.01† | <0.01† |
| Education | |||
| High school or less | 2.55 (1.59–4.11)† | 2.31 (1.34–3.99)† | 1.70 (1.07–2.69)† |
| Some college or trade school | 1.61 (1.16–2.22)† | 1.71 (1.18–2.47)† | 1.26 (0.94–1.71) |
| Completed college (bachelor’s degree) | 1.26 (0.91–1.75) | 1.16 (0.79–1.70) | 1.37 (1.02–1.84)† |
| Graduate school or higher | 1.00 | 1.00 | 1.00 |
| Wald p value | <0.01† | <0.01† | 0.07 |
| Number of donations in past 5 years | |||
| First-time | 1.90 (1.11–3.24)† | 1.73 (0.95–3.14) | 2.84 (1.72–4.68)† |
| 1–3 times | 1.81 (1.24–2.63)† | 1.31 (0.85–2.01) | 2.41 (1.70–3.43)† |
| 4–5 times | 1.28 (0.84–1.93) | 0.72 (0.43–1.21) | 1.49 (1.01–2.19)† |
| 6–10 times | 1.03 (0.70–1.51) | 0.91 (0.59–1.40) | 0.89 (0.62–1.29) |
| 11–20 times | 1.00 (0.68–1.46) | 0.85 (0.55–1.30) | 0.92 (0.74–1.15) |
| More than 20 times | 1.00 | 1.00 | 1.00 |
| Wald p value | <0.01† | 0.06 | <0.01† |
Data are reported as OR (95% CI).
Significant at p < 0.05.
In the scenario where donor identities would not be linked to their samples, 54% of participants indicated that they were likely or very likely to participate (Fig. 1). In a multivariate model, older donors, non-Caucasian donors, donors with less than a graduate school education, and participants who had donated five times or less in the past 5 years were less inclined to participate in identity-unlinked research (Table 2). Nine percent of participants believed that they would be less likely or much less likely to donate blood in the future if asked to participate in identity-unlinked research at the blood bank (Fig. 2). In a multivariate model, donors aged 60 or older, non-Caucasian donors, and donors with less than a bachelor’s degree were less likely to donate blood in the future if asked to participate in identity-unlinked research (Table 3).
Finally, we asked donors whether or not they would be interested in being tested if the blood bank offered genetic testing as an optional service. Approximately three-fourths of participants indicated that they would be interested in genetic testing, 9% indicated that they would not be interested, and 15% stated that they did not know if they would want to be tested. In a multivariate model, high school–educated donors were less interested in genetic testing as a service compared to donors who had attended graduate school (OR, 0.53; 95% CI, 0.35–0.83; Table 2). Among donors who were interested in genetic testing or did not know if they would be interested, one-quarter indicated that they would not pay for genetic testing at the blood bank. Thirty-one percent were willing to pay up to $49 for genetic testing, 12% would pay between $50 and $99, whereas only 9% were willing to pay $100 or more. Almost one-quarter of these donors did not know how much they would be willing to pay for genetic testing. Among those who stated they would be interested in genetic testing or were undecided, 90% stated that they were interested in being tested for heart disease, 83% would be interested in being tested for colon cancer, and 82% would be interested in being tested for diabetes. More than 60% of those participants indicated that they would be interested in being tested for breast and ovarian cancer and prostate cancer. Twenty-three percent of all participants indicated that they would be less likely or much less likely to donate blood in the future if genetic testing was offered as an optional service at the blood bank (Fig. 2). This was a higher proportion of participants than in identity-linked and identity-unlinked research. Non-Caucasian donors, donors with less than a graduate education, and donors who had donated five times or less in the past 5 years were less likely to donate blood in the future if genetic testing was offered as an optional service (Table 3).
Participants were able to provide narrative comments at the end of the questionnaire, and these provide some additional insights. Both donors who stated that they would be likely to participate in identity-linked studies and those who stated that they would be unlikely to participate in such studies expressed concerns about the potential loss of privacy. Some donors were concerned that insurance companies would become aware of their genetic information. However, several donors commented that they would prefer to know individual results of genetic research, which could not occur in the identity-unlinked scenario. Consistent with the altruistic nature of blood donors, several donors stated they would participate in either scenario because they believed that health research is very important and others could benefit from their participation.
DISCUSSION
The main finding of this study was that the majority of blood donors would be likely to participate in genetic studies and that less than 10% of donors would be less inclined to donate in the future if genetic studies were conducted in blood banks. Contrary to expectations, more donors indicated that they would be likely to participate in identity-linked research compared to identity-unlinked research. Finally, there was interest in the provision of genetic testing as a service at the blood center, although this scenario was accompanied by a higher potential for discouraging future donation.
Our main finding that the majority of blood donors expressed a willingness to participate in genetic research is consistent with the altruistic nature of blood donation and with some, but not all, of the findings from other populations. Among individuals who had previously participated in health research, actual consent rates for genetic studies have ranged from 21% to 85%.5 Researchers recently conducted an online survey of US adults, asking about their potential participation in a large cohort study examining genetic, environmental, and lifestyle factors. Sixty percent of participants indicated that they would participate in such a study.8
We observed some demographic and donation status differences in willingness to participate in genetic research. Older donors were more likely than younger donors to participate in identity-linked research; however, younger donors were more likely to participate in identity-unlinked research. In general, more frequent donors would be more likely to participate in future genetic studies. We suspect that more frequent donors have established a greater degree of trust with the blood bank that counterbalances concerns about confidentiality, resulting in a greater willingness to participate in even identity-linked genetic research.
While a previous study examining consent rates for genetic research reported lower consent rates among women,9 we did not detect any differences by sex. Non-Caucasian donors were less likely than Caucasian donors to indicate they would participate in genetic studies, although the difference was only significant for identity-unlinked research. In a recent review, Sterling and coworkers5 noted significantly lower participation rates among African Americans compared to Caucasians in several studies. In a National Health and Nutrition Examination Survey (NHANES) report, both females and non-Hispanic African Americans were less likely to consent to having their biologic samples stored in a national repository.9 In an NIH clinical center study, African Americans were less likely to permit future research, although 75% still authorized unlimited future research with their samples.10 On the other hand, Wendler and colleagues11 reviewed enrollment data from 20 health research studies and found little overall difference in participation rates between African Americans or Hispanics compared to non-Hispanic Caucasians. However, none of the studies of medical or surgical intervention reviewed by Wendler and colleagues included a primary genetic research question.
The finding of a greater acceptability of identity-linked versus unlinked research was unanticipated. We predicted that the better protection of confidentiality in the identity-unlinked scenario would be more attractive to donors. A possible explanation is that donors are interested in receiving the individual results of genetic research. This desire may temper privacy concerns that participants noted. Interestingly, in the survey of US adults about participation in a cohort study of genetic, environmental, and lifestyle factors, 75% of participants indicated they would be less inclined to participate if they did not receive research results.8 Others have noted greater than anticipated public interest in more public sharing of genetic information similar to the concept of the Personal Genome Project.12
The survey found that less than 10% of donors would be discouraged from donating if their blood center participated in genetic research and that more than 10% would be more likely to donate in the future. These results are encouraging, since donor loss is frequently mentioned by blood center personnel as a concern when genetic research is proposed. However, a donor loss of 8% to 9% without a concomitant increase in blood donation due to genetic research could be detrimental to blood banks. Under both identity-linked and -unlinked scenarios, donors aged 60 or older, non-Caucasian donors, and donors with less than a bachelor’s degree were more likely to be among this small group. We did not ask why donors would be less likely to donate in the future; however, we suspect that older donors view genetic studies as distracting from the blood center’s primary mission. In addition, comments on the questionnaire indicated that a few donors were unsure if participation in the study would be optional for blood donors, potentially contributing to the observed differences by level of education. In a previous study, we found that non-Caucasian donors had 30% to 40% lower odds of donating blood again within 1 year compared to Caucasian donors.13 This observation was also evident when we examined return after donor deferral regardless of first-time or repeat donor status.14 The racial disparity we observed in this study may be indicative of the larger pattern of lower repeat donation rates among non-Caucasian donors. Previously, we found that non-Caucasian donors were more likely than Caucasian donors to cite poor staff skills and bad treatment as factors influencing their decision not to donate blood in the future.15 Another study found that the race of the research physician, knowledge of the Tuskegee study, and belief that minorities bore most of the risks in medical research influenced African American subjects’ willingness to participate in medical research.16 These findings will have relevance to the design of future genetic studies in the blood donor setting, particularly in the design of educational and consent materials as well as staff training.
More than 70% of donors indicated that they would be interested in genetic testing as an optional service, with more than 80% of interested or undecided donors indicating that they would be interested in being tested for heart disease, colon cancer, and diabetes. However, more than 20% of participants indicated they would be less likely or much less likely to donate blood in the future if genetic testing was offered (as a service as opposed to research). During pilot test interviews, some donors indicated that genetic testing would make the donation process more complicated and introduce new processes. It is also possible that some participants did not understand that genetic testing would be optional rather than required for blood donation. Pilot test interviews indicated that even if the disease was not relevant to their personal health, some participants would be interested in being tested for genetic traits that may be passed to their children. Therefore, we specified that genetic testing could be for diseases the participant may develop or for diseases that the participant may pass on to their children. As a result, some men responded they would be interested in genetic testing for breast and ovarian cancer and some women indicated they would be interested in testing for prostate cancer.
There are limitations to our study. First, we used a convenience sample of predominantly repeat donors at one Northern California center who were willing to participate. Due to response bias, their opinions may not be representative of other blood donors at this center nor of all US blood donors. Questions were complex and required detailed descriptions of genetic studies, genetic testing, and linked or unlinked samples, so it is possible that some individuals, particularly those with less education, did not comprehend the scenarios presented. However, we conducted detailed pilot testing before our study to revise the instrument and maximize comprehension of the three scenarios. In addition, participants were highly educated, with more than 90% indicating that they at least had some college education. Another limitation is that donors were asked to indicate what they would do in a hypothetical scenario. However, we have previously found that donor intention to return is predictive of actual return.15
In conclusion, our study reveals that factors about the research study design, such as whether samples are linked to donor information, as well as donor demographics and donation status, will impact donor consent rates in genetic studies. The finding that the majority of donors would be interested in participating in genetic studies is encouraging with regard to the feasibility of conducting genetic research in the blood bank setting. However, donor loss due to genetic research in the blood bank remains a potential concern. Therefore, we suggest that if genetic research is implemented in blood banks, studies should be implemented slowly and actual participation and refusal rates as well as subsequent donation trends should be monitored.
Supplementary Material
Acknowledgments
The authors wish to thank the staff at Blood Centers of the Pacific for assistance in questionnaire administration. Funded in part by NIH midcareer award K24-HL-75036 to Dr Murphy and by NIH training grant T32-CA-09001 to the Harvard School of Public Health.
Footnotes
CONFLICT OF INTEREST
No conflict of interests to disclose.
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Your opinions about genetic research?
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Busch M, Custer B. Health outcomes research using large donor-recipient databases: a new frontier for assessing transfusion safety and contributing to public health. Vox Sang. 2006;91:282–4. doi: 10.1111/j.1423-0410.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 2.Busch MP, Glynn SA. Use of blood-donor and transfusion-recipient biospecimen repositories to address emerging blood-safety concerns and advance infectious disease research: the National Heart, Lung, and Blood Institute Biologic Specimen Repository. J Infect Dis. 2009;199:1564–6. doi: 10.1086/598860. [DOI] [PubMed] [Google Scholar]
- 3.Ownby HE, Kong F, Watanabe K, Tu Y, Nass CC. Analysis of donor return behavior. Retrovirus Epidemiology Donor Study. Transfusion. 1999;39:1128–35. doi: 10.1046/j.1537-2995.1999.39101128.x. [DOI] [PubMed] [Google Scholar]
- 4.Notari EP, Zou S, Fang CT, Eder AF, Benjamin RJ, Dodd RY. Age-related donor return patterns among first-time blood donors in the United States. Transfusion. 2009;49:2229–36. doi: 10.1111/j.1537-2995.2009.02288.x. [DOI] [PubMed] [Google Scholar]
- 5.Sterling R, Henderson GE, Corbie-Smith G. Public willingness to participate in and public opinions about genetic variation research: a review of the literature. Am J Public Health. 2006;96:1971–8. doi: 10.2105/AJPH.2005.069286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott EA, Schlumpf KS, Mathew SM, Mast AE, Busch MP, Gottschall JL National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II) Biospecimen repositories: do blood donors want to participate? Transfusion. 2010;50:1943–50. doi: 10.1111/j.1537-2995.2010.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks (CA): Sage Publications Inc; 2005. [Google Scholar]
- 8.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008;10:831–9. doi: 10.1097/GIM.0b013e31818bb3ab. [DOI] [PubMed] [Google Scholar]
- 9.McQuillan GM, Pan Q, Porter KS. Consent for genetic research in a general population: an update on the National Health and Nutrition Examination Survey experience. Genet Med. 2006;8:354–60. doi: 10.1097/01.gim.0000223552.70393.08. [DOI] [PubMed] [Google Scholar]
- 10.Chen DT, Rosenstein DL, Muthappan P, Hilsenbeck SG, Miller FG, Emanuel EJ, Wendler D. Research with stored biological samples: what do research participants want? Arch Intern Med. 2005;165:652–5. doi: 10.1001/archinte.165.6.652. [DOI] [PubMed] [Google Scholar]
- 11.Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? Plos Med. 2006;3:e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Personal Genome Project. Volunteers from the general public working together with researchers to advance personal genomics. Boston (MA): Personal Genome Project; 2009. [cited 2009 Nov 1]. Available from: URL: http://www.personalgenomes.org. [Google Scholar]
- 13.Schlumpf KS, Glynn SA, Schreiber GB, Wright DJ, Randolph Steele W, Tu Y, Hermansen S, Higgins MJ, Garratty G, Murphy EL. Factors influencing donor return. Transfusion. 2008;48:264–72. doi: 10.1111/j.1537-2995.2007.01519.x. [DOI] [PubMed] [Google Scholar]
- 14.Custer B, Chinn A, Hirschler NV, Busch MP, Murphy EL. The consequences of temporary deferral on future whole blood donation. Transfusion. 2007;47:1514–23. doi: 10.1111/j.1537-2995.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber GB, Schlumpf KS, Glynn SA, Wright DJ, Tu Y, King MR, Higgins MJ, Kessler D, Gilcher R, Nass CC, Guiltinan AM. Convenience, the bane of our existence, and other barriers to donating. Transfusion. 2006;46:545–53. doi: 10.1111/j.1537-2995.2006.00757.x. [DOI] [PubMed] [Google Scholar]
- 16.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–56. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.