Abstract
We sought to estimate the risk of seizure-related events associated with refilling prescriptions for antiepileptic drugs (AEDs) and to estimate the effect of switching between brand and generic, or two generic versions, of the same drug. We conducted a case-crossover study using healthcare databases from British Columbia, Canada, among AED users who had an emergency room visit or hospitalization for seizure (index event) between 1997 and 2005. AED refilling was associated with 2.3-fold elevated odds of seizure-related events when the refill occurred within 21 days before the index event (odds ratio [OR], 2.31; 95% confidence interval [CI], 1.56–3.44). The OR was 2.75 (95% CI, 0.88–8.64) for switching, yielding a refill-adjusted ORs for switching of 1.19 (95% CI, 0.35–3.99). Refilling the same AED was associated with an elevated risk of seizure-related events whether or not the refill involved switching from a branded to a generic product.
INTRODUCTION
Generic medications contain the same amount of the same active ingredient as their brand name counterparts, and must demonstrate bioequivalence to approved brand products according to pharmacokinetic parameters such as peak drug concentrations (Cmax) and area under the curve (AUC) of drug concentration over time (1–3). Some authors have hypothesized that small differences in between-product bioavailability, even those within the narrow bounds of allowable variation that determine bioequivalence, can lead to adverse clinical outcomes when switching between brand and generic versions of narrow therapeutic index drugs, such as antiepileptic drugs (4–6). Several surveys have documented widespread negative perceptions of antiepileptic generic substitution among patients, physicians, and pharmacists (7–9) and the American Academy of Neurology opposes antiepileptic generic substitution without physician approval (6). Others have argued that within-person variation in drug use patterns and non-adherence are at least as likely to explain variability in drug response as are pharmacokinetics (10).
For more than two decades (11), the hypothesis that switching between brand and generic antiepileptic drugs (generic substitution) can lead to breakthrough seizures has been sustained by theoretical concerns, case reports, and data from small pharmacokinetic and bioequivalence studies (12). Only recently have observational studies emerged that examine the clinical consequences of antiepileptic drug switching (13–16). All but one of these studies suggest that switching between brand and generic antiepileptic drugs, or between generic antiepileptic drugs from different manufactures, increases the odds of seizure-related outcomes by about 80% as compared to not switching. However, clinical differences between brand and generic antiepileptic drugs have not been consistently borne out by clinical trial data (17).
Prescription switching can be viewed as a special type of prescription refilling with additional potential variation caused by between-manufacturer variability (in both bioavailability and peripheral drug features) that does not exist with refilling the same drug from the same manufacturer. However, no study has examined whether there is an elevated risk of seizure associated with refilling the same antiepileptic drug from the same manufacturer. We hypothesized that refilling the same manufacturer’s antiepileptic drug might itself be associated with risk of seizure-related events, possibly due to between-lot differences in bioavailability, delays in refilling, neurological symptoms prompting the refill, or temporary non-adherence or minor changes in use patterns and that these confounders may partly account for observed associations between antiepileptic drug switching and seizure outcomes. The purpose of this study was to define the risk of seizure-related events associated with refilling of a prescription for the same antiepileptic medication, and to estimate the additional risk associated with switching between the same antiepileptic drugs from different manufacturers.
RESULTS
We identified 1,762 patients with an index seizure-related event requiring emergency treatment between 1997 and 2005 and who were eligible as cases for this study (Table 1). The mean age of patients was 35 (s.d. = 23.8) years, 53% were female, and most seizures were recorded as other (i.e. not generalized or partial) or unspecified. On average, patients were dispensed 1.6 (s.d. = 0.9) different antiepileptic medications in the 43 days preceding the index event (Table 1), where day 1 preceding the event is the induction period, days 2–22 are the case-period, and days 23–43 are the control period. Because only those with variation in exposure status between the case-and control- periods contribute to the analysis of case-crossover studies (18), only a fraction of those eligible for the study were included in primary analyses (Table 2).
Table 1.
Characteristics of patients with seizure requiring hospitalization eligible for study inclusion (n=1,762)
| Characteristic | |
|---|---|
| Age at index date, mean (s.d.) | 35.2 (23.8) |
| Female, n (%) | 936 (53.1) |
| Primary seizure diagnosis type, n (%) | |
| Generalized | 291 (16.5) |
| Partial | 157 (8.9) |
| Other/unspecified | 1314 (74.6) |
| No. different antiepileptic drugs dispensed during primary 43-day study period, mean (s.d.) | 1.6 (0.9) |
| No. patients dispensed each antiepileptic drug during primary 43-day study period, n (% of all patients) | |
| Carbamazepine | 845 (48.0) |
| Phenytoin | 698 (39.6) |
| Valproic acid | 460 (26.1) |
| Clobazam | 309 (17.5) |
| Clonazepam | 180 (10.2) |
| Lamotrigine | 129 (7.3) |
| Gabapentin | 123 (7.0) |
| Topiramate | 96 (5.4) |
Table 2.
Odds ratiosa (95% confidence intervals) for the association between antiepileptic refilling and switching and seizure-related outcomes for primary analyses
| No. days in case/control periods | Refill of the same drug, strength and dosage form from the same manufacturerb | Switch between the same drug, strength and dosage form but from different manufacturersc | Refill-adjusted odds ratio for switching | ||
|---|---|---|---|---|---|
| nd | Odds ratio (95% CI) | nd | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| 21 | 116 | 2.31 (1.56–3.44) | 15 | 2.75 (0.88–8.64) | 1.19 (0.35–3.99) |
| 28 | 151 | 2.08 (1.48–2.93) | 19 | 2.17 (0.82–5.70) | 1.04 (0.37–2.90) |
In case-crossover studies, odds ratios are inherently adjusted for time-invariant patient factors
Includes refilling of brand products and refilling of generic products
included switching between brand and generic products, generic and brand products, and two generic products from different manufacturers
n represents the number of index events that contributed to the estimation of the odds ratio
The distributions of refills and switches in the 56 days preceding the index event dates included in the analyses are displayed in Figure 2. The odds of seizure-related events were 2.3-times higher when a refill occurred in the 21-day case-period rather than in the 21-day control-period in the primary analysis (odds ratio [OR], 2.31; 95% confidence interval [CI], 1.56–3.44). Using 28-day case- and control-periods, we observed a 2.1-fold increase in odds (OR, 2.08; 95% CI, 1.48–2.93). When we considered switching between the same antiepileptic medications from different manufacturers (with dose and dosage form held constant) as the exposure, ORs were 2.75 (0.88–8.64) and 2.17 (0.82–5.70) for 21- and 28-day case- and control-periods, respectively (Table 2). The refill-adjusted analysis yielded ORs of 1.19 (95% CI, 0.35–3.99) and 1.04 (95% CI, 0.37–2.90) for the primary 21- and 28-day analyses, respectively (Table 2).
Figure 2.
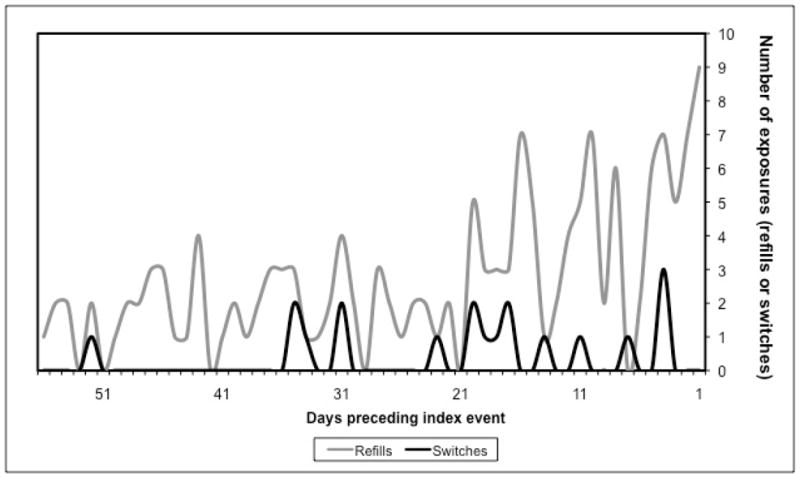
Distributions of exposures (refills and switches) in the 8 weeks preceding the index event dates.
ORs were similar regardless of whether patients refilled a brand or a generic medication (Table 3). Switching between brand and generic versions was associated with a higher event risk than switching between generic products from different manufacturers in the 21-day analysis, but these were based on few cases and the 95% CIs for ORs were widely overlapping (Table 3).
Table 3.
Odds ratios (95% confidence intervals) for the association between antiepileptic refilling and switching and seizure-related outcomes stratified by type of refill and switch
| Exposure type | na | Odds ratio (95% CI) |
|---|---|---|
| 21-day case- and control-periods | ||
| Refill | ||
| Brand | 79 | 2.29 (1.42–3.70) |
| Generic | 37 | 2.36 (1.17–4.78) |
| Switch | ||
| Generic-generic | 10 | 2.33 (0.60–9.02) |
| Brand-generic or generic-brand | 5 | 4.00 (0.45–35.79) |
| 28-day case- and control-periods | ||
| Refill | ||
| Brand | 105 | 1.92 (1.28–2.87) |
| Generic | 46 | 2.54 (1.34–4.82) |
| Switch | ||
| Generic-generic | 13 | 2.25 (0.69–7.31) |
| Brand-generic or generic-brand | 6 | 2.00 (0.37–10.92) |
n = number of index events that contributed to the estimation of the odds ratio
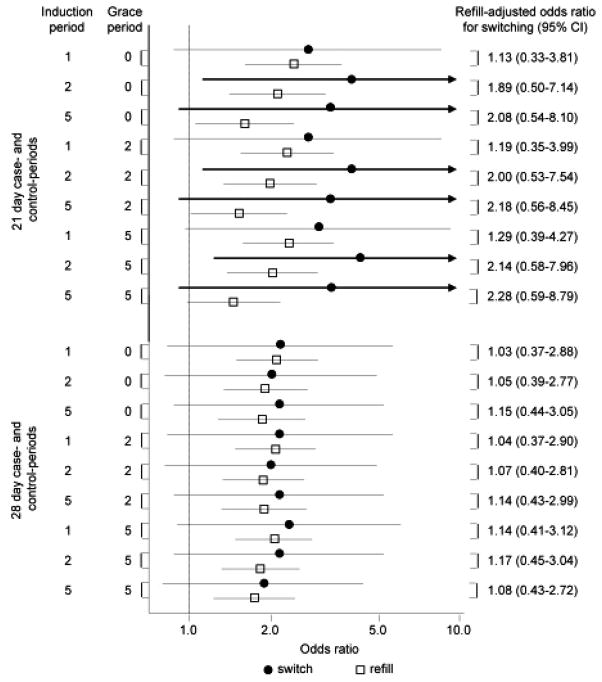
Adjusted analyses and sensitivity analyses yielded results similar to primary analyses (Figure 3 and Table 4).
Figure 3.
Results of sensitivity analyses (odds ratios and 95% confidence intervals) for the association between refilling or switching antiepileptic medications and seizure-related outcomes, along with difference in differences results, varying the case- and control-periods, the induction period, and the grace period.
Table 4.
Odds ratiosa (95% confidence intervals) for the association between antiepileptic refilling and switching and seizure-related outcomes for sensitivity analysesb
| No. days in case/control periods | Refill of the same drug, strength and dosage form from the same manufacturerc | Switch between the same drug, strength and dosage form but from different manufacturersd | Refill-adjusted odds ratio for switching |
|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Adjusted for use of other medications and visits to general practitioners and neurologists | |||
| 21 | 2.31 (1.56–3.44) | 2.65 (0.68–10.34) | 1.15 (0.28–4.73) |
| 28 | 2.08 (1.48–2.93) | 3.05 (0.84–11.04) | 1.46 (0.39–5.55) |
| Excluding diagnosis code 780.3x | |||
| 21 | 2.18 (1.23–3.87) | 3.00 (0.81–11.08) | 1.38 (0.33–5.74) |
| 28 | 1.71 (1.08–2.73) | 2.20 (0.76–6.33) | 1.28 (0.40–4.07) |
| Excluding clobazam and clonazepam | |||
| 21 | 2.23 (1.45–3.44) | 3.33 (0.92–12.11) | 1.49 (0.38–5.82) |
| 28 | 2.07 (1.43–3.01) | 3.00 (0.97–9.30) | 1.45 (0.44–4.76) |
| Excluding clobazam, clonazepam, gabapentin, topiramate | |||
| 21 | 2.00 (1.29–3.10) | 3.00 (0.81–11.08) | 1.50 (0.38–5.95) |
| 28 | 1.95 (1.33–2.87) | 2.75 (0.88–8.64) | 1.41 (0.42–4.72) |
| Excluding those with one or more visit to a general practitioner nor neurologist | |||
| 21 | 2.31 (1.56–3.44) | 2.00 (0.18–22.05) | 0.86 (0.08–9.85) |
| 28 | 2.08 (1.48–2.93) | 4.00 (0.45–35.79) | 1.92 (0.21–17.65) |
In case-crossover studies, odds ratios are inherently adjusted for time-invariant patient factors
These sensitivity analyses assume a 1-day induction period and a 2-day grace period consistent with primary analyses
Includes refilling of brand products and refilling of generic products
included switching between brand and generic products, generic and brand products, and two generic products from different manufacturers
DISCUSSION
This is the first known study to find that antiepileptic drug refilling itself may be associated with an elevated risk of seizure-related outcomes. We observed that, with dose and dosage form held constant, refilling the same antiepileptic from the same manufacture was associated with an approximately 2.1- to 2.3-fold increase in odds of emergency treatment related to seizure, and that points estimates for switching between antiepileptic drugs from different manufacturers were similar, but with wide confidence intervals. The refill-adjusted effect of switching between products from different manufacturers was small, with a 4% to 19% increase in odds of seizure-related outcomes.
While the exact mechanisms underlying these phenomena have not been determined, we propose two possible explanations. First, our results are consistent with hypotheses that suggest that variability in antiepileptic bioavailability can lead to a small increased risk of breakthrough seizures. A review of more than 2,000 clinical bioequivalence studies of orally administered generic products approved by FDA found that the average difference in Cmax and AUC between generic and innovator products was 4.4% and 3.6%, respectively (19). Given inherent variability in manufacturing processes, similar fluctuations in bioavailability between lots produced by the same manufacturer are expected (20–22) and have been observed (23).
A second possible explanation is that refilling behavior may result in lapses in pharmacotherapy continuity which may lead to breakthrough seizures. The prescription refilling process (and switching process, to the extent that switching is a special case of refilling) involves many time-sensitive steps, often beginning with a visit or telephone call to a prescribing physician or to a dispensing pharmacy, to picking up the medication from the dispensing pharmacy, to beginning the new refill on the right day. A failure or delay at any point along this chain of events could result in a brief lapse in antiepileptic drug adherence, which may cause breakthrough seizures (24, 25). Labels on newly dispensed prescriptions may also differ from the previous dispensing, and confusion about dosing or administration may result (26). Between-manufacturer variation in peripheral features of the drug product may further contribute to such behavioral explanations. For example, patients stable on particular drug products of given size, shape, and color, may regard new prescriptions that vary in one or more of these features with caution, perhaps going so far as to not take the medication for fear of having received the wrong drug from the pharmacy, thus increasing the likelihood of lapses in pharmacotherapy continuity. Some have suggested that variation in drug use patterns is at least as important as pharmacokinetics in explaining variation in drug response (10, 27, 28).
Regardless of the mechanism, we observed that both refilling and switching of antiepileptic medications were associated with an elevated risk of seizure-related events. While this is an important finding, the refill-adjusted analysis allowed us to ascertain the extent to which switching between antiepileptic drugs is associated with seizure-related outcomes after accounting for within-manufacturer variability and aspects of patient behavior associated with breakthrough seizure inherent in the prescription refilling/switching process.
The results of our refill-adjusted analyses are consistent with a recent meta-analysis of bioequivalence trials that reported seizure outcomes (17) and with a recent case-control study (16). Other case-control studies suggest that the increased risk of seizure-related outcomes associated with switching versus not switching ranges between 78% and 84% (13–15). However, these studies did not consider the effect of within-manufacturer variability in bioavailability or other factors inherent in the refilling and switching process, which likely inflated their findings. Furthermore, confounding by epilepsy severity may limit these studies since epilepsy that is not well controlled would predispose to seizure and would also be associated with use of multiple antiepileptic medications, thus increasing the probability that there would be at least one medication switch. Hansen et al partly adjusted for this confounding bias by adjusting for number of antiepileptic medications dispensed, which reduced the primary effect from 1.78 (95% CI, 1.35–2.36) to 1.57 (95% CI, 1.17–2.10) and indicated number of antiepileptic medications was a strong predictor of seizure-related events (15). Indeed, the one case-control study that was consistent with our findings adjusted for several potential confounders, including total number of antiepileptic medications and use of interacting medications, although conditioning on intermediates may be a concern in that study (16). The case-crossover approach is valid regardless of the number of drugs used since the frequency of refills and switches would be expected to be uniformly distributed across case- and control-periods of short duration, in the absence of a true effect and of bias.
To assess the validity of the duration of the pre-defined case- and control-periods, we plotted the distributions of exposures (both refills and switches) over the 8 weeks immediately preceding the index event for each patient. Both refills and switches were largely clustered within the 21 days preceding the index events, substantiating the use of the 21-day periods and suggesting that the risk of seizure-related events may be greatest in the 3 weeks following an antiepileptic refill or switch. Furthermore, case periods of greater than 21 days would lead to misclassification of the exposure. This explains why the effect estimates for analyses using 28-day periods are smaller than those using 21-day periods.
Several important limitations of this study should be noted. First, we identified seizures using inpatient and ER ICD codes. The accuracy of ICD codes for identifying seizure is not known and many patients do not seek emergency treatment for seizure so our study population represents a select sample of patients who experience a seizure of severity great enough to warrant hospitalization. While this highly specific outcome definition may limit the generalizability of the results, it preserves the internal validity of ratio effect measures (29). We likely further improved the specificity of the outcome by requiring current antiepileptic drug use and an outpatient diagnosis of epilepsy in the year prior to the outcome.
A second limitation is that, should a bioavailability mechanism be implicated, focusing on seizure-related outcomes ignores the other end of the adverse event spectrum – namely, toxicities resulting from exceeding the upper limit of the therapeutic window for drug plasma levels. Nevertheless, such adverse events would be difficult to capture in administrative data and are less important than breakthrough seizures given the clinical ramifications of seizures (30). Another limitation of this analysis is the small number of cases on which it is based, particularly in subgroups of refilling or switching type. However, because seizure-related emergency treatment is fairly rare and because only those with an exposure in either the case- or the control-period, but not both, contribute to the analyses of case-crossover studies, this represents a rare disease – rare exposure scenario. That a sizeable proportion of prescription dispensings in BC are for 90-days supply, rather than 30 days, further reduces the total number of possible exposures.
We expect that the implications of this study can be extended beyond Canada and that the association between antiepileptic switching and seizure-related events may be smaller in other countries since EMA and US FDA bioequivalence requirements are more stringent than those of Health Canada (1–3). Requirements established by FDA, which are identical to those of EMA, are intended to ensure that differences in bioavailability between bioequivalent products are no greater than between-lot variation from a single manufacturer (31). Indeed, this study is the first to describe an association between refilling the same antiepileptic medication and seizure-related events and we also found that this association is of similar magnitude as that for prescription switching. Nevertheless, some advocacy groups (32), professional organizations (6), and legislators (33) oppose antiepileptic drug substitution and at least one US state prevents substitution by pharmacists (34).
While our results do not completely rule out the possibility that switching between antiepileptic medications produced by different manufacturers may contribute to loss of seizure control in some patients, our findings indicate that after adjustment for the risk associated with refilling the same agent, the residual harmful effect of switching between two generic formulations of the same medication or between a brand and a generic (or vice versa) of the same drug is either negligible or much smaller than that reported in earlier studies that did not adjust properly for the effect of prescription refilling per se.
METHODS
Data source
We used data from British Columbia (BC), Canada, covering the period 1996 to 2005. Individuals were identified in the Ministry of Health inpatient administrative database, which contains data, such as diagnostic codes, admission dates, and dates of service, on all hospitalizations in BC. Patients were linked from the hospital data to data on physician services and to the PharmaNet database by a personal health number unique to each BC resident. The PharmaNet database captures all records of prescriptions dispensed at community pharmacies in BC. The Institutional Review Board of Brigham and Women’s Hospital approved this study.
Design
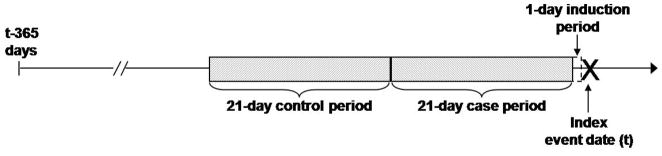
We used a case-crossover design to assess the relationship between seizure-related outcomes and antiepileptic drug refilling with and without switching to the same product from a different manufacturer (35). Case-crossover studies are the observational analogue to crossover trials used to experimentally test bioequivalence of two drug preparations, and can be conceptualized as case-control studies which, rather than selecting separate individuals as controls, use an antecedent period in each case’s history (i.e. the control-period) to ascertain the exposure distribution in the person-time that gave rise to the case-defining events (Figure 1).
Figure 1.
Case-crossover design to study the association between seizure-related outcomes and refilling and switching of antiepileptic medications.
Note: In primary analyses, the induction period was defined as 1 day (i.e. day t-1) and 21 days were used for the case- (i.e. days t-22 to t-2) and the control-period (i.e. days t-43 to t-23).
By using each subject as his or her own control, the case-crossover design inherently adjusts for potential confounding (both measured and unmeasured) by time-invariant factors (35, 36). Case-crossover studies are well suited to study relationships between transient exposures such as medication switching or refilling, and acute outcomes such as seizure-related events, using short study periods where confounding by time-varying factors is limited or unlikely (35).
Case ascertainment
Cases were identified, using the inpatient data file, as individuals with an emergency room visit or hospitalization related to seizure between 1997 and 2005. We used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) codes 345.xx (epilepsy and recurrent seizures) or 780.3x (convulsions) to define seizure-related events, but excluded code 345.6x, which indicates infantile spasms. The index date (Figure 1) was defined as the date of first occurrence in the inpatient file of one of the codes of interest in the primary diagnosis position. We further required individuals to have at least one diagnosis of epilepsy or seizure recorded in their outpatient file in the year preceding the index date. Thus, we excluded events that occurred in 1996 to ensure that exposure and covariate data were available for the 365 days prior to the index date for each case. If an individual experienced more than one seizure-related event during the study period, only the first event was included in the analysis.
Exposure and covariate assessment
We defined etiologically relevant exposure risk windows and induction periods that precede the index event (37). For both refilling and switching, we chose a 1-day induction period and a 21-day exposure window (i.e. “case-period”) for primary analyses (Figure 1), consistent with observations and hypotheses surrounding the pharmacokinetics that govern the association of interest (38, 39). The control-period was then defined as the 21 days immediately preceding the case-period in primary analyses (Figure 1). As with matched case-control studies, only discordant pairs contribute information to the analysis of case-crossover studies (18), so that only those with variation in exposure status between the case- and control- periods contribute information to the analysis of this study.
We operationalized the exposure definitions by identifying only those cases with 2 or more antiepileptic drug dispensings in the 365 days prior to the index date, as a minimum of 2 dispensings is required to define at least one refill or switch (Figure 1). Brand and generic drugs are distinguishable in the PharmaNet database using the drug identification numbers (DINs), which are specific to each drug product marketed by each manufacturer; however, DINs cannot distinguish between individual lots. We included the following antiepileptic drugs for which both brand and generic versions were or became available in BC during the study period: carbamazepine, clobazam, clonazepam, gabapentin, lamotrigine, phenytoin, topiramate, and valproic acid.
A refill of the same product was defined as a prescription dispensing for a given antiepileptic drug that was preceded by a prescription record for the same DIN, indicating dispensing of the same dose of the same drug product manufactured by the same company. A switch was defined as a prescription record for a drug with a given DIN that was preceded by a record for the same medication but with a different DIN, indicating a different manufacturer. We excluded switches between DINs with different strengths or dosage forms (e.g. immediate versus extended release carbamazepine formulations) and excluded those who had a subsequent dispensing of the first DIN, which would also indicate a dose change by the addition of the second prescription. The date of the refill or switch was defined as the date of the second prescription in the respective sequence, which must have occurred within the coverage days of the first prescription, defined by the prescription fill date plus the days supply for that dispensing, plus a 2-day grace period.
In addition to antiepileptic drug refills and switches, other drug-related covariates were collected for those individuals included in the analysis. We ascertained whether patients initiated other medications during either the case- or control-period to adjust for the possibility of drug-drug interactions. We also collected the number of physician visits to general practitioners and neurologists during both periods.
Statistical analysis
Data were analyzed using standard methods for matched case-crossover data (40, 41). ORs and 95% CIs for seizure-related events were estimated using separate conditional logistic regression models for both exposure types: refilling and switching. OR estimates were based on the ratio of the observed frequency of refills or switches in the case-period compared to the respective frequency in the control-period. We adjusted the conditional logistic regression models for the time-varying factors, number of visits to general practice physicians or neurologists in either the case- or control-period and addition of other medications during either period, under the assumption that these factors were not affected by previous exposures (42).
We conducted analyses stratified by the type of refill or switch. Specifically, we examined whether outcomes varied depending on whether patients refilled a brand or a generic product, and examined switches from brand to generic products and generic to brand products as well as switches between generics from different manufacturers.
We used the case-crossover analogue to the difference-in-differences study design to define the risk of seizure-related outcomes associated with antiepileptic drug switching, relative to that associated with refilling of the same antiepileptic medication. Difference-in-differences, or ratio-of-ratios, analyses have been used for many years in the economics literature (43) and are commonly used in controlled time-series analyses to evaluate drug policy changes (44). We conducted a conditional logistic regression analysis among all cases, including those in both the refilling and the switching analyses. Independent variables included a binary indicator for exposure status (“exposure”), defined as exposure = 1 for a refill or a switch and exposure = 0 otherwise, and a product term for “exposure*group”, where group was a binary indicator for switching agents (group = 1) or refilling the same agent (group = 0). The antilogarithm of the coefficient for the product term obtained from the model can be interpreted as the increase in odds of seizure-related outcome associated with switching between antiepileptic drugs beyond that associated with refilling an antiepileptic drug. This adjusted for inherent within-manufacturer between-lot variability and factors involved in the refilling or switching process (the “refill-adjusted OR for switching”). Our implementation of this approach is similar to the case-time-control design for adjusting for confounding due to exposure time trends (45, 46) but avoids certain assumptions by using other cases that experience the event of interest, rather than controls that did not experience the event, to ascertain the magnitude of effect by which to adjust the primary effect measure (47). The supplementary information demonstrates the calculation of the crude OR from the case-crossover analyses and the refill-adjusted OR from the difference-indifference analysis.
Sensitivity analyses
We conducted sensitivity analyses to assess the robustness of the results by varying the duration of the case- and control-periods, the induction period, and the grace period used in primary analyses. We extended case- and control-periods to 28 days and extended induction periods to 2 days and 5 days. In primary analyses, we allowed a 2-day grace period at the end of the days supply of the first dispensing in the sequence to define coverage days. In sensitivity analyses, we considered grace periods of 0 and 5 days added to the days supply, but did not consider longer grace periods since these likely indicate gaps in pharmacotherapy which may themselves be risk factors for seizure (24).
We also conducted two separate sensitivity analyses by excluding from the primary analyses refilling and switching of drugs that are frequently used for other indications. The first excluded users of the anxiolytics clobazam and clonazepam, and the second excluded users of clobazam, clonazepam, gabapentin, and topiramate. Additionally, we excluded cases with primary diagnoses of 780.3x (convulsions), which have been excluded in some(13–15) but not all(24, 48) studies that have used claims databases to identify seizure-related outcomes. Finally, we excluded those with one or more general practitioner or neurologist visits during either the case-or control-period, under the assumption that these factors were not affected by previous exposures (41).
Supplementary Material
Acknowledgments
Dr. Gagne is supported by a National Institute on Aging training grant (T32 AG000158). We thank Claire Canning for help in data management and programming.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
Dr. Gagne participated in a research fellowship administered by Jefferson Medical College and sponsored by Ortho-McNeil Janssen Scientific Affairs, L.L.C., which ended in 2007. Dr. Schneeweiss received an investigator initiated grant from Pfizer to study the safety of coxibs.
References
- 1.Guidance for industry: Conduct and analysis of bioavailability and bioequivalence studies: Part A: Oral dosage formulations used for systemic effects. Health Canada; 1997. [Accessed on October 10, 2009]. Available at http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/bio/bio-a-eng.php. [Google Scholar]
- 2.Guidance for industry: Bioavailability and bioequivalence studies for orally administered drug products – general considerations. US Food and Drug Administration; 2003. [Accessed on May 20, 2008]. Available at http://www.fda.gov/cder/guidance/5356fnl.htm. [Google Scholar]
- 3.Guideline on the investigation of bioequivalence. European Medicines Agency; 2008. [Accessed on October 10, 2009]. Available at http://www.emea.europa.eu/pdfs/human/qwp/140198enrev1.pdf. [DOI] [PubMed] [Google Scholar]
- 4.Andermann F, Duh MS, Gosselin A, Paradis PE. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia. 2007;48:464–469. doi: 10.1111/j.1528-1167.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 5.Berg MJ. What’s the problem with generic antiepileptic drugs?: A call to action. Neurology. 2007;68:1245–1246. doi: 10.1212/01.wnl.0000262876.37269.8b. [DOI] [PubMed] [Google Scholar]
- 6.Liow K, Barkley GL, Pollard JR, Harden CL, Bazil CW. American Academy of Neurology. Position statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology. 2007;68:1249–1250. doi: 10.1212/01.wnl.0000259400.30539.cc. [DOI] [PubMed] [Google Scholar]
- 7.Berg MJ, Gross RA, Haskins LS, Zingaro WM, Tomaszewski KJ. Generic substitution in the treatment of epilepsy: patient and physician perceptions. Epilepsy Behav. 2008;13:693–699. doi: 10.1016/j.yebeh.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Papsdorf TB, Ablah E, Ram S, Sadler T, Liow K. Patient perception of generic antiepileptic drugs in the Midwestern United States. Epilepsy Behav. 2009;14:150–153. doi: 10.1016/j.yebeh.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 9.McAuley JW, Chen AY, Elliott JO, Shneker BF. An assessment of patient and pharmacist knowledge of and attitudes toward reporting adverse drug events due to formulation switching in patients with epilepsy. Epilepsy Behav. 2009;14:113–117. doi: 10.1016/j.yebeh.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Urquhart J. The odds of the three nons when an aptly prescribed medicine isn’t working: non-compliance, non-absorption, non-response. Br J Clin Pharmacol. 2002;54:212–220. doi: 10.1046/j.1365-2125.2002.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald JT. Breakthrough seizure following substitution of Depakene capsules (Abbott) with a generic product. Neurology. 1987;37:1885. doi: 10.1212/wnl.37.12.1885. [DOI] [PubMed] [Google Scholar]
- 12.Besag FM. Is generic prescribing acceptable in epilepsy? Drug Saf. 2000;23:173–182. doi: 10.2165/00002018-200023030-00001. [DOI] [PubMed] [Google Scholar]
- 13.Zachry WM, 3rd, Doan QD, Clewell JD, Smith BJ. Case-control analysis of ambulance, emergency room, or inpatient hospital events for epilepsy and antiepileptic drug formulation changes. Epilepsia. 2009;50:493–500. doi: 10.1111/j.1528-1167.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 14.Rascati KL, Richards KM, Johnsrund MT, Mann TA. Effects of antiepileptic drug substitutions on epileptic events requiring acute care. Pharmacotherapy. 2009;29:769–774. doi: 10.1592/phco.29.7.769. [DOI] [PubMed] [Google Scholar]
- 15.Hansen RN, Campbell JD, Sullivan SD. Association between antiepileptic drug switching and epilepsy-related events. Epilepsy Behav. 2009;15:481–485. doi: 10.1016/j.yebeh.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Devine ST, Weisbart E, Barron J, Behm A. Acute epilepsy exacerbations in patients switched between A-rated anti-epileptic drugs. Curr Med Res Opin. 2010;26:455–463. doi: 10.1185/03007990903488704. [DOI] [PubMed] [Google Scholar]
- 17.Kesselheim AS, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs. 2010;70:605–621. doi: 10.2165/10898530-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Stürmer T, Maclure M. Case-crossover and case-time-control designs as alternatives in pharmacoepidemiologic research. Pharmacoepidemiol Drug Saf. 1997;6:S51–59. doi: 10.1002/(SICI)1099-1557(199710)6:3+<S51::AID-PDS301>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Davit BM, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583–1597. doi: 10.1345/aph.1M141. [DOI] [PubMed] [Google Scholar]
- 20.Perucca E, Albani F, Capovilla G, Bernardina BD, Michelucci R, Zaccara G. Recommendations of the Italian League against Epilepsy working group on generic products of antiepileptic drugs. Epilepsia. 2006;47:16–20. doi: 10.1111/j.1528-1167.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 21.Bialer M. Generic products of antiepileptic drugs (AEDs): Is it an issue? Epilepsia. 2007;48:1825–1832. doi: 10.1111/j.1528-1167.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 22.Boylan LS. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy (Letter) Neurology. 2009;72:1876. doi: 10.1212/01.wnl.0000349653.30398.e8. [DOI] [PubMed] [Google Scholar]
- 23.Meyer MC, et al. Variability in the bioavailability of phenytoin capsules in males and females. Pharm Res. 2001;18:394–397. doi: 10.1023/a:1011075502215. [DOI] [PubMed] [Google Scholar]
- 24.Manjunath R, Davis KL, Candrilli SD, Ettinger AB. Association of antiepileptic drug adherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14:372–378. doi: 10.1016/j.yebeh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Cramer JA, Glassman M, Rienzi V. The relationship between poor medication compliance and seizures. Epilepsy Behav. 2002;3:338–342. doi: 10.1016/s1525-5050(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 26.Shrank WH, et al. The variability and quality of medication container labels. Arch Intern Med. 2007;167:1760–1765. doi: 10.1001/archinte.167.16.1760. [DOI] [PubMed] [Google Scholar]
- 27.Harter JG, Peck CC. Chronobiology. Suggestions for integrating it into drug development. Ann N Y Acad Sci. 1991;618:563–571. doi: 10.1111/j.1749-6632.1991.tb27276.x. [DOI] [PubMed] [Google Scholar]
- 28.Burnier M, Schneider MP, Choléro A, Stubi CL, Brunner HR. Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens. 2001;19:335–341. doi: 10.1097/00004872-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Kelsey JL, Whittemore AS, Evans AS, Thompson WD, editors. Methods in Observational Epidemiology. 2. New York, NY: Oxford University Press; 1996. Measurement error; pp. 341–363. [Google Scholar]
- 30.Liow K. Understanding patients’ perspective in the use of generic antiepileptic drugs: compelling lessons for physicians to improve physician/patient communication. BMC Neurol. 2009;9:11. doi: 10.1186/1471-2377-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams RL. [Accessed on October 12, 2009];Therapeutic equivalence of generic drugs: response to the National Association of Boards of Pharmacy (letter) 1997 April 16; Available at www.fda.gov/cder/news/ntiletter.htm.
- 32.Epilepsy Foundation. [Accessed on October 12, 2009];Statement on generic substitution of AEDs. Available at: www.epilepsyfoundation.org/advocacy/care/genedrev.cfm.
- 33.National Black Caucus of State Legislators. Resolution HHS-07–19. [Accessed on October 12, 2009];Epilepsy patient prescription drug safety. Available at www.nbcsl.org.
- 34. [Accessed on October 12, 2009];Hawaii revised statutes. www.capitol.hawaii.gov/hrscurrent/Vol06_Ch0321-0344/HRS0328/HRS_0328-0092.HTM.
- 35.Maclure M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 36.Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther. 2007;82:143–156. doi: 10.1038/sj.clpt.6100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Di Bonaventura C, Fattouch J, Fabbrini G, Manfredi M, Prencipe M, Giallonardo TA. Switching from branded to generic antiepileptic drugs as a confounding factor and unpredictable diagnostic pitfall in epilepsy management. Epileptic Disord. 2007;9:465–466. doi: 10.1684/epd.2007.0132. [DOI] [PubMed] [Google Scholar]
- 39.Crawford P, Feely M, Guberman A, Kramer G. Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure. 2006;15:165–176. doi: 10.1016/j.seizure.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. Am J Epidemiol. 1995;142:91–98. doi: 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- 41.Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, Hennessy S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther. 2008;84:581–8. doi: 10.1038/clpt.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Abadie A. Semiparametric difference-in-differences estimators. Rev Econ Stud. 2005;72:1–19. [Google Scholar]
- 44.Schneeweiss S, Maclure M, Soumerai SB, Walker AM, Glynn RJ. Quasi-experimental longitudinal designs to evaluate drug benefit policy changes with low policy compliance. J Clin Epidemiol. 2002;55:833–841. doi: 10.1016/s0895-4356(02)00437-7. [DOI] [PubMed] [Google Scholar]
- 45.Suissa S. The case-time-control design. Epidemiology. 1995;6:248–253. doi: 10.1097/00001648-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Suissa S. The case-time-control design: further assumptions and conditions. Epidemiology. 1998;9:441–445. [PubMed] [Google Scholar]
- 47.Greenland S. Confounding and exposure trends in case-crossover and case-time-control designs. Epidemiology. 1996;7:231–239. doi: 10.1097/00001648-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49:446–454. doi: 10.1111/j.1528-1167.2007.01414.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.