Abstract
This review provides a practical overview of the excess cancer risks related to radiation from medical imaging. Primary care physicians should have a basic understanding of these risks. Because of recent attention to this issue, patients are more likely to express concerns over radiation risk. In addition, physicians can play a role in reducing radiation risk to their patients by considering these risks when making imaging referrals. This review provides a brief overview of the evidence pertaining to low-level radiation and excess cancer risks and addresses the radiation doses and risks from common medical imaging studies. Specific subsets of patients may be at greater risk from radiation exposure, and radiation risk should be considered carefully in these patients. Recent technical innovations have contributed to lowering the radiation dose from computed tomography, and the referring physician should be aware of these innovations in making imaging referrals.
ASIR = adaptive statistical iterative reconstruction; BMI = body mass index; CT = computed tomography; MRI = magnetic resonance imaging
Radiation dose from medical imaging has come under recent scrutiny in the medical and lay press. This is the result of recent articles on the increased cancer risks associated with computed tomography (CT),1-3 as well as recent cases of excess radiation exposure from CT brain perfusion scans.4 Berrington de Gonzalez et al3 estimated that 29,000 future cancers (approximately 2% of the cancers diagnosed annually in the United States) could be related to CT performed in the United States in 2007. This is comparable to recent estimates of 1.5% to 2.0% by Brenner and Hall.1 This review provides a practical overview of the excess cancer risks related to radiation from medical imaging and suggests how clinicians can play a part in reducing these risks for their patients.
Radiation Dose
Absorbed dose, measured in grays (Gy), quantifies the energy deposited per unit mass. The energy deposition of 1 J/kg of tissue is the equivalent of 1 Gy. Because not all types of radiation produce the same biological effect, the dose equivalent is often used instead of the absorbed dose. The dose equivalent is the product of the absorbed dose and a radiation weighting factor and is expressed in sieverts (Sv). Because the radiation weighting factor for x-rays and gamma rays is 1.0, 1 Gy is equivalent to 1 Sv in medical imaging.5 Radiation doses in medical imaging are typically expressed as millisieverts (mSv). For reference, the average yearly background radiation dose (primarily from radon gas in the home) is around 3 mSv.6
Excess Cancer Risk From Radiation: The Evidence
The relevant biological effect of x-rays and gamma rays is secondary to ionization. Ionization of water molecules can create hydroxyl radicals that may interact with DNA to cause strand breaks or base damage; DNA can also be ionized directly. Although most radiation-induced damage is rapidly repaired, misrepair can lead to point mutations, chromosome translocations, and gene fusions that are linked to cancer induction.1 This effect is typically thought to be stochastic, ie, it can occur at any level of radiation exposure, with the likelihood increasing as the dose increases. The typical lag period between radiation exposure and cancer diagnosis is at least 5 years,3 and in most cases, the lag period may be 1 or 2 decades or longer.7
Most of the evidence on radiation-induced cancer risk comes from 4 groups: Japanese atomic bomb survivors, medically exposed populations, occupationally exposed groups, and environmentally exposed groups.8 Of these groups, the Japanese atomic bomb survivors provide by far the most robust data.9 These data provide clear evidence of radiation-induced cancer risk at doses above 100 mSv,10 but this is of little relevance to medical imaging, except in cases of multiple high-dose examinations (CT, nuclear cardiology, and complex interventional radiology and cardiology procedures using fluoroscopy) in a short time period.
Radiation-induced risk is more controversial at doses between 10 and 100 mSv, the dose range relevant to medical imaging and in particular CT. A single CT of the abdomen may have a dose of around 10 mSv, and patients who undergo multiple CTs or a single multiphasic CT fall into this dose range. Nuclear cardiology examinations also typically fall in this dose range. Some investigators suggest that direct epidemiological data from atomic bomb survivors and nuclear industry workers indicate increased cancer risk in this dose range,9,11,12 whereas others contend that no data support an increased cancer risk below 100 mSv and that neutron irradiation and other confounding factors may explain the putative carcinogenic effect at low doses seen in atomic bomb survivors.13,14
Below 10 mSv, which is a dose range relevant to radiography and some nuclear medicine and CT studies, no direct epidemiological data support increased cancer risk. However, this does not mean that this risk is not present, as even large epidemiological studies would not have the statistical power to detect increased risk, if present, at a low radiation dose.5
Given the paucity of direct epidemiological data, the cancer risks from low-dose radiation have been assessed using models based on the linear, no-threshold theory. This theory holds that excess cancer risks related to low-dose radiation are directly proportional to the dose. This model is used to extrapolate excess cancer risk at low doses from the known risk at higher doses. However, some question the validity of the linear no-threshold theory14 and think that below a certain threshold carcinogenesis ceases to be a concern.
Despite some controversy over the excess cancer risk of low-dose radiation, the linear no-threshold theory is widely used because an alternative method for assessing the potential risks of low-dose radiation is lacking. In addition, it is this author's opinion that the epidemiological data directly suggest increased cancer risk in the 10 mSv to 100 mSv range, which is relevant to nuclear cardiac and many CT studies. A widely used figure is a 5% excess risk of death from cancer with a 1 Sv (1000 mSv) dose.15,16 This is extrapolated linearly for lower doses. Comparison of this number with the doses from the Table shows that the absolute risk of excess cancer mortality from any individual medical imaging examination is very low, particularly relative to the natural incidence of cancer mortality of approximately 25%.5
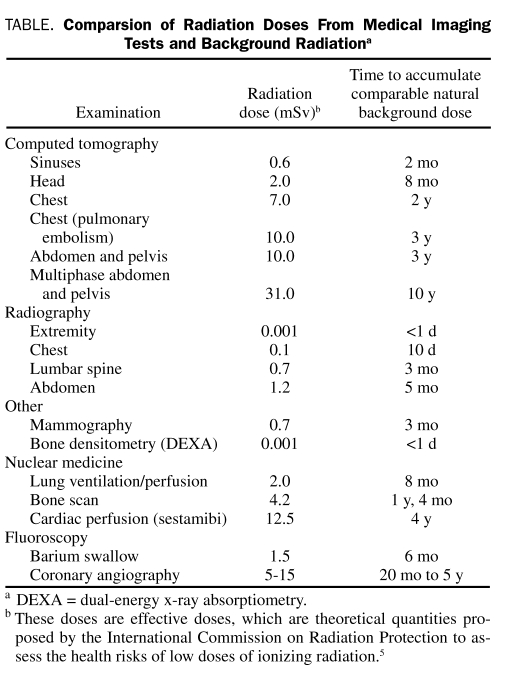
TABLE.
Comparsion of Radiation Doses From Medical Imaging Tests and Background Radiationa
Radiation Dose From Imaging Examinations
A useful way to understand radiation doses from diagnostic examinations is to compare them to average natural background radiation (3 mSv per year) (Table).2,6,17
Radiation doses are sometimes expressed as entrance skin doses. Entrance skin doses are used in conventional radiography: a dose estimate at 1 point in the beam allows estimates of organ doses and effective dose. To assess the health risks of low doses of ionizing radiation, the International Commission on Radiation Protection uses the concept of effective dose.5 The effective dose is not measured but is a theoretical calculated dose based on the organs exposed by the applied radiation multiplied by tissue-weighting factors. Because the tissue-weighting factors can change with new data and continuing analysis of existing data, the effective dose estimates can change over time. It should be noted that dose estimates are generally given for an adult of typical size and may vary substantially depending on patient size and imaging technique. Effective dose estimates are best used to assess the general level of radiation risk and not to determine the exact radiation dose from an imaging study. Effective dose estimates for individual patients are subject to a substantial level of uncertainty.
Several interesting observations can be made on the basis of the data provided in the Table. Computed tomography and some nuclear medicine studies are associated with far higher radiation doses than radiography. In particular, the radiation doses of some CT and nuclear medicine studies fall in the range shown by direct epidemiological evidence to be associated with increased cancer risk. It should also be noted that recent evidence suggests that radiation doses from CT may be highly variable between institutions.2 Radiography doses fall in the range for which no epidemiological evidence exists of increased cancer risk (but a very small increased cancer risk may be present if the linear no-threshold hypothesis is correct). Radiography of the spine and abdomen has substantially higher radiation doses than radiography of the chest and extremities.
Another useful way to express radiation risk is to compare it to common activities of daily life. For example, radiation doses from 0.1 to 1.0 mSv carry an additional risk of death from cancer comparable to the risk of death associated with a flight of 4500 miles, whereas doses in the range of 1 to 10 mSv have a higher risk, comparable to driving 2000 miles.5
Reducing Patient Radiation Dose
Radiation dose from an imaging study can be reduced by 3 methods. First, one can decide not to perform the study at all. Such a decision should be based on proper understanding of the indications of the study, review of any previous imaging that might have already reasonably answered a clinical question, and an assessment of any special patient considerations that increase or decrease risk. Second, an alternative study that does not use ionizing radiation can be selected. Third, less radiation can be used to create the images.
It is imperative that all imaging tests, particularly those with potential patient harm, be performed only when indicated. Although the absolute radiation risk of any individual medical imaging study is small, these risks could be clinically relevant when compared with benefits that are very low or not established. For example, the benefit of whole-body CT screening in asymptomatic individuals has not been defined. The radiation risk of these studies (and possible follow-up studies generated by the initial screening) may be clinically relevant if compared with the uncertain benefit, particularly if taking into account the additional risks of false-positive results and overdiagnosis. The use of published appropriateness criteria for various patient conditions (perhaps as clinical decision support integrated into electronic order systems) can be helpful in this risk-benefit evaluation.18 In a recent study, 26% of outpatient CT and magnetic resonance imaging (MRI) studies at a single academic medical center were not considered appropriate on the basis of evidence-based appropriateness criteria.19 Of these studies, 24% had positive results, compared with 58% of studies considered appropriate. A radiologist should be consulted if uncertainty exists as to the most suitable imaging study.
Review of imaging history is essential when an imaging study for any patient is considered because it can sometimes obviate the need for additional imaging or allow for a more focused, lower-dose current examination. Review of imaging history should also reveal high cumulative radiation exposures, which may alter future imaging decisions. In a recent study,20 many patients with chronic and recurrent conditions such as renal colic had total effective doses of greater than 50 mSv from imaging in a 3-year period.
Even more hesitation is merited in the use of moderately high doses of radiation in pregnant or younger patients, in women undergoing chest CT, in patients who have a high body mass index (BMI), or in those who are undergoing multiphasic CT. Conversely, given the typical lag period of 1 to 2 decades or more7 between radiation exposure and cancer diagnosis, radiation dose may not be of concern in some very ill or very elderly patients.
A full discussion of radiation exposure in pregnancy is beyond the scope of this review, but the potential biological effects of in utero radiation include childhood cancer, prenatal death, intrauterine growth restriction, small head size, mental retardation, and organ malformation.21 Imaging examinations of the maternal head, neck, chest, and peripheral extremities can be performed with negligible risks to the conceptus. Although the absolute risk to the conceptus from imaging studies of the maternal abdomen and pelvis is small, these studies should be avoided unless no other option is available.
Younger patients are at a substantially higher risk from radiation because they have more remaining years of life during which a radiation-induced cancer might develop. For example, Smith-Bindman et al2 estimate that, compared with a patient aged 40 years, the risk of cancer from a radiation imaging test is doubled for a patient aged 20 years and 50% lower for a patient aged 60 years. This review will not discuss pediatric patients, but very young children are at additional risk because they are also inherently more radiosensitive,1 perhaps 3 to 4 times more sensitive than adults.5
The projected risk for women undergoing studies that expose the chest are higher than in men because of the additional risk of breast cancer and high lung cancer risk coefficients.3 For example, Smith-Bindman et al2 estimate that 1 in 270 women who undergo CT coronary angiography at age 40 years will develop cancer from that scan, compared with 1 in 600 men.
Patients with a high BMI will often receive a greater radiation dose. As the thickness of the area being imaged increases, greater x-ray penetration is needed to create acceptable images, which increases radiation dose. The effective radiation dose from radiographic and fluoroscopic examinations for patients with a high BMI can be much higher.22,23 For patients undergoing CT, a high BMI often limits which radiation dose reduction techniques can be used. If patients with a high BMI are scanned with the same technique as patients with a lower BMI, the amount of incident radiation will be suboptimal and the resultant images will typically appear grainy or “noisy.” Even if the incident radiation is increased, image noise may still compromise the scan quality in patients with a very high BMI. Many techniques for reducing radiation dose in CT result in greater image noise. In patients with a low or average BMI, these techniques can often be used without substantially affecting image quality, but this is often not the case in patients with a high BMI whose studies are already more noisy. Unfortunately, patients with a high BMI are typically not good candidates for ultrasonography. Magnetic resonance imaging (MRI) is a possibility, but patients with a very high BMI might require open MRI that often has lesser image quality. Note that a high BMI does not substantially affect radiation dose for nuclear medicine studies.24
In multiphasic CT, the same organ is scanned multiple times in different phases of contrast enhancement. For example, in a multiphasic liver CT, the liver might be scanned up to 4 times. Compared with a standard CT, a multiphasic liver CT might improve the detection and characterization of liver lesions. However, in the study of Smith-Bindman et al,2 the radiation dose of multiphasic CT studies was almost 4-fold higher than single-phase CT studies. Magnetic resonance imaging can often be substituted for multiphasic studies, with comparable if not greater diagnostic accuracy.25,26 The radiologist is a good resource for questions as to when MRI might be substituted for CT.
Referring physicians can play a role in assuring that radiation dose is minimized for all their patients by considering radiation dose reduction when choosing where to refer their patients for imaging. Smith-Bindman et al2 found a mean 13-fold variation within and across institutions between the highest and lowest dosages for specific CT studies. It is difficult for referring physicians to determine which radiology facilities operate at the lower end of the radiation dose spectrum. It is important to inquire whether the facility is accredited for CT by the American College of Radiology because accredited facilities are required to undergo periodic assessments of radiation dose27 and are more likely to have considered protocol modifications to reduce dose. Another important factor to consider is whether the facility uses the adaptive statistical iterative reconstruction (ASIR) technique28 to enable low-dose CT. This new image reconstruction technique creates less noisy images, which allows radiation dose to be substantially decreased for a wide range of CT studies. These dose-reduction techniques can substantially reduce radiation risk. For example, a 40-year-old woman undergoing CT coronary angiography is estimated to have a 1 in 270 chance of developing cancer at a radiation dose of approximately 20 mSv2; using ASIR (in conjunction with other dose-reduction techniques), the same examination could be performed with a dose of less than 1 mSv.29 Currently, only some facilities have invested in ASIR and other dose-reduction technologies, but the incentive for that investment increases when referring physicians factor this into their referral patterns.
CONCLUSION
A basic knowledge of radiation risk is useful in counseling patients who express concern about this issue. In most cases, the benefits of indicated medical imaging will outweigh the relatively small excess cancer risk, and patient management should not be altered on the basis of radiation risk. However, for certain subsets of patients, radiation risk should be of greater concern to the clinician. In addition, clinicians can play a role in minimizing radiation risk to their patients by referring their patients to centers with a commitment to minimizing radiation dose.
On completion of this article, you should be able to (1) recognize the doses of common medical imaging studies, (2) recognize which patients may be at increased risk from radiation, and (3) be familiar with the evidentiary base for determining excess cancer risks from low-dose radiation.
CME Questions About Radiation Risk
-
Which one of the following is the average annual background radiation dose?
0.001 mSv
0.7 mSv
3 mSv
10 mSv
50 mSv
-
Which one of the following is an average effective radiation dose from single-phase computed tomography (CT) of the abdomen and pelvis?
0.001 mSv
0.7 mSv
3 mSv
10 mSv
50 mSv
-
Which one of the following is the average effective radiation dose from screening mammography (2 views)?
0.001 mSv
0.7 mSv
3 mSv
10 mSv
50 mSv
-
Which one of the following statements is false?
The linear no-threshold theory is typically used to estimate radiation risks from low-dose radiation
Direct epidemiological evidence supports increased cancer risk from exposure to radiation at doses be low 10 mSv
A 5% risk of excess cancer death from a 1 Sv (1000 mSv) dose is a commonly used index of radiation risk
Most direct epidemiological evidence on radiation risk comes from Japanese atomic bomb survivors
A stochastic effect is possible at any level of exposure, with the likelihood of the effect increasing as the dose increases
-
Which one of the following patient groups would not be at increased relative risk from radiation exposure?
Young patients
Female patients
Patients with high body mass index
Elderly patients
Patients undergoing multiphasic CT
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
Because the Concise Review for Clinicians contributions are now a CME activity, the answers to the questions will no longer be published in the print journal. For CME credit and the answers, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Brenner DJ, Hall EJ. Computed tomography:an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284 [DOI] [PubMed] [Google Scholar]
- 2.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography exams and the associated lifetime attributed risk of cancer. Arch Intern Med. 2009;169(22):2078-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomography scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration (FDA) Safety Investigation of CT brain perfusion scans: update 12/8/2009. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm185898.htm Accessed August 26, 2010
- 5.Verdun FR, Bochud F, Gudinchet F, et al. Radiation risk: what you should know to tell your patient. Radiographics. 2008;28:1807-1816 [DOI] [PubMed] [Google Scholar]
- 6.Radiation exposure from x-ray examinations. RadiologyInfo.org Web site. http://www.radiologyinfo.org/en/safety/index.cfm?pg=sfty_xray Accessed August 26, 2010
- 7.Amis ES, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4:272-284 [DOI] [PubMed] [Google Scholar]
- 8.Little MP, Wakeford R, Tawn EJ, et al. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 2009;251(1):6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce DA, Preston DL. Radiation-induced cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154:178-186 [DOI] [PubMed] [Google Scholar]
- 10.US National Academy of Sciences. National Research Council. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation Health Risks from Exposure to Low Levels of Ionizing Radiation. BEIR VII Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 11.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors:1598-1998. Radiat Res. 2007;168(1):1-64 [DOI] [PubMed] [Google Scholar]
- 12.Cardis E, Vrijheid M, Blettner M, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396-416 [DOI] [PubMed] [Google Scholar]
- 13.Tubiana M, Aurengo A, Averback D, et al. Dose-Effect Relationships and Estimation of the Carcinogenic Effects of Low Doses of Ionizing Radiation. Paris, France: Academie des Sciences and Academie Nationale de Medecine; 2005. [DOI] [PubMed] [Google Scholar]
- 14.Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251(1):13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Commission on Radiological Protection 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 1991;21(1-3):1-201 [PubMed] [Google Scholar]
- 16.National Council on Radiation Protection and Measurements Limitation of Exposure to Ionizing Radiation. Bethesda, MD: National Council on Radiation Protection and Measurements: 1993. [Google Scholar]
- 17.Radiation exposure from medical diagnostic imaging procedures. Health Physics Society Web site. www.hps.org/documents/meddiagimaging.pdf Accessed August 26, 2010
- 18.Sistrom CL. The ACR appropriateness criteria: translation to practice and research. J Am Coll Radiol. 2005;2(1):61-67 [DOI] [PubMed] [Google Scholar]
- 19.Lehnert BE, Bree RL. Analysis of appropriateness of outpatient CT and MRI referred from primary care clinics at an academic medical center: how critical is the need for improved decision support [published correction appears in J Am Coll Radiol. 2010;7(6):466]?J Am Coll Radiol. 2010;7(3):192-197 [DOI] [PubMed] [Google Scholar]
- 20.Stein EG, Haramati LB, Bellin E, et al. Radiation exposure from medical imaging in patients with chronic and recurrent conditions. J Am Coll Radiol. 2010;7(5):351-359 [DOI] [PubMed] [Google Scholar]
- 21.McCollough CH, Schueler BA, Atwell TD, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27(4):909-917 [DOI] [PubMed] [Google Scholar]
- 22.Yanch JC, Behrman RH, Hendricks MJ, McCall JH. Increased radiation dose to overweight and obese patients from radiographic examinations. Radiology. 2009;252(1):128-139 [DOI] [PubMed] [Google Scholar]
- 23.Ector J, Dragusin O, Adrriaenssens B, et al. Obesity is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. J Am Coll Cardiol. 2007;50(3):234-242 [DOI] [PubMed] [Google Scholar]
- 24.Clark LD, Stabin MG, Fernald MJ, Brill AB. Changes in radiation dose with variations in human anatomy: moderately and severely obese adults. J Nucl Med. 2010;51(6):929-932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitton MB, Kloechner R, Herber S, et al. MRI versus 64-row MDCT for diagnosis of hepatocellular cancer. World J Gastroenterol. 2009;15(48):6044-6051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HS, Lee JM, Choi HK, et al. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009;30(3):586-595 [DOI] [PubMed] [Google Scholar]
- 27.Computed tomography [accreditation program]. American College of Radiology Web site. http://www.acr.org/accreditation/computed.aspx Accessed August 26, 2010
- 28.Silva AC, Lawder HJ, Hara A, et al. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol. 2010;194(1):191-199 [DOI] [PubMed] [Google Scholar]
- 29.Heilbron BG, Leipsic J. Submillisievert coronary computed tomography angiography using adaptive statistical iterative reconstruction: a new reality. Can J Cardiol. 2010;26(1):35-36 [DOI] [PMC free article] [PubMed] [Google Scholar]