Abstract
OBJECTIVE: To determine if the attainment of at least 85% of age-predicted maximal heart rate (APMHR), using the equation 220 – age, and/or at least 25,000 as the product of maximal heart rate and systolic blood pressure (rate pressure product, RPP) is an accurate indicator of exertion level during exercise stress testing.
PATIENTS AND METHODS: From May 1, 2009, to February 15, 2010, 238 patients (mean ± SD age, 49.3±11.9 years; 50% male) with symptoms suggestive of myocardial ischemia underwent an exercise stress test with the addition of ventilatory expired gas analysis and a myocardial perfusion study. Ventilatory expired gas analysis determined the peak respiratory exchange ratio (RER), which is considered a valid and reliable variable for quantifying a patient's exertion during exercise.
RESULTS: Of the patients, 207 (87%) attained a peak RER of 1.00 or more, and 123 (52%) attained a peak RER of 1.10 or more. An APMHR of 85% or more and peak RPP of 25,000 or more were both ineffective in identifying patients who put forth a maximal exercise effort (ie, peak RER, ≥1.10). Perceived exertion was a significant indicator (P=.04) of patient exertion, with a threshold of 15 (6-20 scale) being an optimal cut point. The percentage of equivocal myocardial perfusion study results was significantly higher in patients who demonstrated a submaximal exercise effort by peak RER (P≤.007).
CONCLUSION: Aerobic exercise testing is an integral component in the assessment of patients with suspected myocardial ischemia. Our findings indicate that the currently used percentage of APMHR and peak RPP thresholds are ineffective in quantifying a patient's level of exertion during exercise stress testing.
Aerobic exercise testing is an integral component in the assessment of patients with symptoms that are suggestive of myocardial ischemia. The authors found that the currently used age-predicted maximal heart rate of 85% or more and peak rate pressure product of 25,000 or more are both ineffective in identifying patients who put forth a maximal exercise effort, that is, peak respiratory exchange ratio of 1.10 or greater.
APMHR = age-predicted maximal heart rate; BP = blood pressure; CAD = coronary artery disease; ECG = electrocardiography; HR = heart rate; MPS = myocardial perfusion study; RER = respiratory exchange ratio; RPE = rating of perceived exertion; RPP = rate pressure product
The traditional exercise stress test incorporates heart rate (HR), blood pressure (BP), electrocardiography (ECG), and subjective symptom assessment. Ideally, patients who do not prematurely terminate exercise because of an abnormal exercise response exert themselves to a near-maximal or maximal level, ensuring that myocardial tissue is assessed across the entire spectrum of aerobic capacity. In fact, submaximal exertion is recognized as one mechanism for a false-negative exercise stress test.1 Clinicians currently use percentage of age-predicted maximal heart rate (APMHR) achieved (target threshold, ≥85%) and/or maximal rate pressure product (RPP), the product of maximal HR and maximal systolic BP (target threshold, ≥25,000), to quantify exertional level during exercise.2 However, the large degree of natural variability of both HR and BP makes it plausible that neither variable effectively reflects a given individual's level of exercise exertion.2
The peak respiratory exchange ratio (RER), defined as carbon dioxide production divided by oxygen consumption and obtained from ventilatory expired gas analysis, is considered a criterion standard in quantification of exercise exertion level. A peak RER of 1.10 or more is considered a universal indicator of maximal exercise effort independent of patient characteristics such as age, sex, fitness, and disease state.3
The current study aims to assess the ability of percentage of APMHR achieved, maximal RPP, and maximal perceived exercise effort to identify level of exertion as defined by peak RER in patients undergoing stress testing for suspected myocardial ischemia.
PATIENTS AND METHODS
The current investigation prospectively assessed 278 patients presenting to the Noninvasive Cardiology Clinic of Virginia Commonwealth University Medical Center from May 1, 2009, to February 15, 2010. Patients presented with symptoms suggestive of myocardial ischemia and/or a history of coronary artery disease (CAD). Common indications included chest pain (n=186; 67%), dyspnea (n=29; 10%), and history of CAD (n=14; 5%). Only 19 (8%) of the 238 patients in the cohort included in the final analysis had a history of CAD. Exclusion criteria for the current investigation were myocardial infarction or percutaneous coronary intervention within 3 months of testing, diagnosis of heart failure, left ventricular ejection fraction of less than 35%, moderate lung disease by pulmonary function testing, moderate or severe aortic or mitral valve stenosis, unstable angina or uncontrolled hypertension, previous pacemaker implantation or coronary artery bypass grafting, use of a β-adrenergic blocking agent, and/or inability to collect interpretable ECG and nuclear imaging data. The traditional modifiable and nonmodifiable cardiac risk factors (hypertension, low-density lipoprotein cholesterol profile, tobacco use, obesity, physical activity, diabetes, and family history of early CAD) were recorded on the day of testing. Physical activity frequency, duration, and intensity were measured by patient self-report. Premature termination of the exercise test because of an abnormal hemodynamic or ECG response, as well as angina warranting cessation of exercise, served as postassessment exclusion criteria. The protocol was approved by the Institutional Review Board of Virginia Commonwealth University. All patients signed an informed consent form before inclusion in the study.
Nuclear Imaging Procedures
All patients had the stress portion of a single-photon emission computed tomographic myocardial perfusion study (MPS) on the same day as the exercise stress test with ventilatory expired gas analysis. All single-photon emission computed tomographic MPSs were done with ECG gating (16 time frames in the heart cycle) to allow for determination of global and segmental left ventricular function as well as for detection of perfusion abnormalities. Each result was categorized as the presence and extent of reversibility as follows: 0/normal = no evidence of attenuation or reversibility or fixed defect likely due to soft tissue attenuation; 1/equivocal = small and low-grade reversible defect; 2/mildly abnormal = small reversible defect of moderate grade or reversible defect of moderate size and low grade; 3/moderately abnormal = reversible defect of moderate size and grade; 4/severely abnormal = moderate or large reversible defect of high grade.
Exercise Testing Procedures
All exercise tests were done on a motorized treadmill using the Bruce protocol.4 Exercise testing procedures outlined by the American Heart Association were followed for all assessments.5,6 All patients were continuously monitored with 12-lead ECG (GE Marquette 12SL; GE Healthcare, Waukesha, WI), and hemodynamic measurements were made during each stage of the protocol. Blood pressure was measured with an automated sphygmomanometer (SunTech Tango+; SunTech Medical, Morrisville, NC) with auditory confirmation. Patients were encouraged to exercise to their maximum tolerance. Percentage of APMHR was determined by dividing maximal HR achieved during testing using several established maximal HR prediction equations (listed in Table 1).7,8 Maximal RPP was defined as the product of the highest HR and BP obtained during the last stage of exercise. For level of exertion, the Borg 6-20 Rating of Perceived Exertion (RPE) scale was used in a standard fashion.9
TABLE 1.
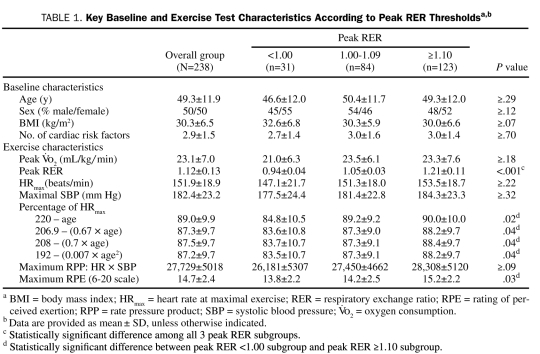
Key Baseline and Exercise Test Characteristics According to Peak RER Thresholdsa,b
Ventilatory expired gas was collected for each test using a metabolic cart (Vmax Encore; SensorMedics, Yorba Linda, CA), which was calibrated before each test. All patients were fitted with a neoprene face mask, and a tight seal around the nose and mouth was confirmed. Peak 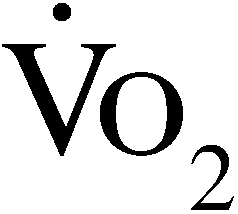 is expressed as the highest 30-second averaged sample obtained during the exercise test in milliliters of oxygen per kilogram per minute. Peak RER is expressed as the highest 10-second averaged value obtained during the exercise test.
is expressed as the highest 30-second averaged sample obtained during the exercise test in milliliters of oxygen per kilogram per minute. Peak RER is expressed as the highest 10-second averaged value obtained during the exercise test.
The following exercise test termination criteria were used: onset of severe typical angina, arrhythmias (frequent premature ventricular contractions; 3 or more beats of nonsustained ventricular tachycardia; new-onset atrial fibrillation, atrial flutter, or atrial tachycardia with rapid response; second-degree or third-degree heart block), hypotension, bradycardia or decrease in HR with same or greater workload, dyspnea, intermittent claudication, central nervous system symptoms (ie, ataxia or vertigo), marked hypertension, more than 2 mm of horizontal or down-sloping ST-segment depression or 1 mm or more of ST-segment elevation, and patient's request to stop or inability to keep up with the treadmill.
Statistical Analyses
One-way analysis of variance assessed the difference in key continuous variables according to peak RER–defined subgroups (<1.00, 1.00-1.09, ≥1.10). When a significant difference was detected, post hoc analysis was performed by the Tukey honestly significant difference test. The percentage of patients who achieved an APMHR of at least 85% was assessed with χ2 analysis using the 220 – age equation and a peak RPP of at least 25,000 according to peak RER subgroup. Differences in sex distribution and MPS results were also evaluated using χ2 analysis, and the Kruskal-Wallis test assessed differences in maximal RPE and number of cardiac risk factors among peak RER subgroups. Receiver operating characteristic curve analysis determined the ability of the percentage of APMHR achieved, maximal RPE, and maximal RPP to identify patients surpassing peak RER thresholds of 1.00, 1.05, and 1.10. P<.05 was considered significant.
RESULTS
Of the 278 patients initially included in this analysis, 40 (14%) were excluded because of premature termination of the exercise test for the following reasons: 14 (5%), hemodynamic abnormalities; 12 (4%), severe angina; 6 (2%), ECG changes suggestive of severe ischemia; and 8 (3%), development of arrhythmias (ie, ventricular ectopy). The remaining 238 (86%) were included in the final analysis described in subsequent sections.
Of the 238 patients included in the study, 207 (87%) reached or surpassed a peak RER of 1.00, and 123 (52%) achieved an RER of 1.10 or more. The remaining 31 patients (13%) failed to reach a peak RER of 1.00. Baseline and patient characteristics for the overall group and peak RER subgroups are listed in Table 1. No differences in baseline characteristics were observed among peak RER sub groups. With respect to exercise test variables, peak RER was significantly different among all 3 subgroups. Regardless of the equation used to predict maximal HR, the percentage of predicted value was significantly lower in patients with a peak RER of less than 1.00 compared with those with a peak RER of 1.10 or more. Finally, peak RPE was significantly lower in patients with a peak RER of less than 1.00 compared with those with a peak RER of 1.10 or more.
In the overall cohort, 174 patients (73%) achieved an APMHR of at least 85% using the 220 – age equation, and 171 (72%) achieved a peak RPP of at least 25,000. Moreover, 202 patients (85%) achieved 1 or both of the aforementioned traditional criteria to gauge level of exercise exertion. The numbers of patients in the peak RER subgroups who achieved an APMHR of at least 85% using the 220 – age equation were as follows: <1.00, 21 (68%); 1.00-1.09, 58 (69%); ≥1.10, and 92 (75%). The difference in the percentage of patients achieving an APMHR of at least 85% according to peak RER subgroup was not statistically significant (P≥.09). The numbers of patients achieving a peak RPP of at least 25,000 in the peak RER subgroups were as follows: <1.00, 21 (68%); 1.00-1.09, 61 (74%); and ≥1.10, 89 (72%). The difference in percentage of patients achieving this peak RPP threshold was likewise not statistically significant according to peak RER subgroup (P≥.23).
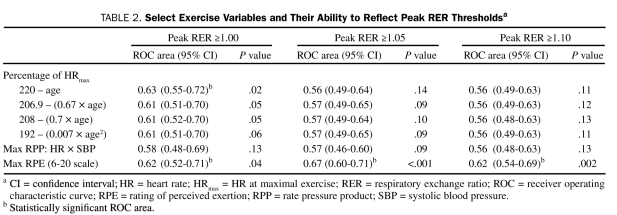
Receiver operating characteristic curve analysis results are provided in Table 2. Percentage of APMHR determined using the 220 – age equation was significant only for identifying patients who achieved a peak RER of at least 1.00. The optimal dichotomous threshold for this HR-derived variable was 88% (62% sensitivity/65% specificity). The peak RPE classification scheme was significant for identifying all 3 peak RER thresholds. The optimal dichotomous threshold for peak RPE was 15 for the peak RER ≥1.00 (59% sensitivity/62% specificity), ≥1.05 (64% sensitivity/62% specificity), and ≥1.10 (66% sensitivity/56% specificity) thresholds.
TABLE 2.
Select Exercise Variables and Their Ability to Reflect Peak RER Thresholdsa
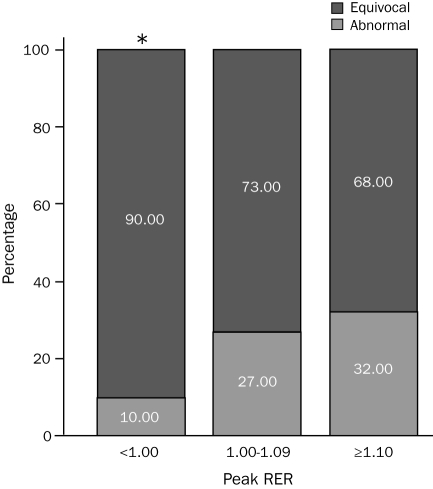
The numbers of patients with either an equivocal or abnormal MPS in the peak RER subgroups were as follows: <1.00, 10 (32%); 1.00-1.09, 22 (26%); and ≥1.10, 41 (33%). The distribution of equivocal and abnormal MPS results according to peak RER subgroup is illustrated in the Figure. The percentage of patients with an equivocal MPS was significantly higher in the peak RER <1.00 subgroup compared with both the peak RER 1.00-1.09 and ≥1.10 subgroups.
FIGURE.
Distribution of equivocal and abnormal myocardial perfusion studies according to peak respiratory exchange ratio (RER).
* P≤.007 for peak RER <1.00 subgroup vs both other subgroups.
DISCUSSION
A peak RER of 1.10 or more is well recognized to reflect maximal exertion irrespective of baseline patient characteristics.10,11 Others have proposed that a peak RER threshold of 1.05 may be an indicator of acceptable exertion level during aerobic exercise testing.12 In the absence of an abnormal physiologic response to aerobic exertion,6 termination of the exercise test at a peak RER of less than 1.00 is generally considered an indicator of poor patient effort.13 The results of the current study indicate that traditional HR and BP parameters are ineffective indicators of achieving a peak RER of 1.05 or 1.10 during exercise stress tests that were not terminated because of an abnormal physiologic response. Although most patients included in the current analysis met the criteria of an APMHR of at least 85% and/or a peak RPP of at least 25,000, only slightly more than 50% met or surpassed a peak RER of 1.10. Other HR equations have been proposed as better predictors of age-related maximal HR with a lower standard error of estimate.7,8 The results of the current study indicate that these alternative HR equations are not effective in predicting a patient's level of exertion and that values are similar to those obtained with the more often used 220 – age equation.
Our findings indicate that peak RPE may provide insight into the level of patient effort, perhaps at a level superior to the percentage of APMHR and/or peak RPP achieved. This may suggest that the variability of peak RPE is less than that found in both the HR and systolic BP response to maximal aerobic exercise.14 On the Borg 6-20 RPE scale,9 a value of 15 (verbal anchor = hard) was the optimal dichotomous threshold, a value proposed to indicate a higher likelihood of surpassing anaerobic threshold during aerobic exercise testing.2
Although percentage of APMHR and peak RPE provide some value in gauging a patient's level of exertion, sensitivity and specificity levels appear to be on the lower end of clinical acceptability (ie, within the 60% range). Thus, our findings support considering the implementation of ventilatory expired gas analysis during the assessment of patients with suspected myocardial ischemia. Its use to ensure adequate patient exertion via peak RER may be enough justification to support a paradigm shift. However, it has other potential advantages. The current body of evidence clearly supports the robust prognostic value of aerobic capacity in patients with suspected or confirmed cardiovascular disease.15,16 Currently, aerobic capacity is estimated from treadmill speed and grade or ergometry workload during the exercise stress test. This approach is problematic in that the error in aerobic capacity estimation may be rather large, creating the potential for a less than optimal prognostic assessment. Moreover, recent data suggest that abnormal ventilatory expired gas responses to exercise may be an accurate indicator of myocardial ischemia, substantially outperforming ECG assessment.17,18
Admittedly, the use of ventilatory expired gas analysis technology is not common in current exercise stress testing laboratories owing to several perceived barriers, including patient comfort, technical expertise, space requirements, and fiscal constraints. The use of a neoprene face mask, which does not impede ventilation through the nose or mouth, minimizes patient discomfort and concerns about the collection procedures for ventilatory expired gas potentially impeding exercise test performance. Ventilatory expired gas analysis systems have also become (1) dramatically more user-friendly, with decreased calibration time (<5 minutes); (2) a good deal smaller, reducing space requirements; and (3) substantially less expensive. Moreover, the inclusion of ventilatory expired gas analysis may increase revenue at the current reimbursement rates and the volume of tests conducted in a typical exercise stress testing laboratory on a yearly basis.19 These changes, in conjunction with current scientific evidence strongly supporting its use,13 reduce perceived and actual barriers to adding ventilatory expired gas analysis to the exercise stress testing laboratory.
Perhaps the finding of the current investigation with most impact is the significant difference in MPS distribution according to peak RER level. The percentage of equivocal MPS studies was significantly higher, whereas the percentage of abnormal MPS results was significantly lower, in the peak RER <1.00 subgroup compared with both other subgroups. Although not statistically significant, a trend toward a lower percentage of equivocal and higher percentage of abnormal MPS results was found in the peak RER ≥1.10 subgroup compared with the peak RER 1.00-1.09 subgroup. From these findings, one may posit that a portion of the equivocal MPS results in the peak RER <1.00 subgroup, and potentially the peak RER 1.00-1.09 subgroup, would be abnormal if the patient achieved a higher level of exertion. One may argue that only 31 patients, making up 13% of the sample, were found to have a peak RER of less than 1.00 in the current study and that this number does not justify the inclusion of ventilatory expired gas analysis during exercise stress testing. Moreover, only 9 MPS results were equivocal in this group and, if trends were consistent with the 2 higher peak RER subgroups, only 2 of the 9 equivocal results would shift to an abnormal level with maximal exercise exertion. Consider, however, the extrapolation of this small percentage of patients over a year in a high-volume exercise stress testing center. The Noninvasive Cardiology Center at Virginia Commonwealth University conducts about 3000 nuclear stress tests each year. If trends in the current study were extrapolated to the entire population undergoing testing at this center over a given year, 390 tests would present with a peak RER of less than 1.00, and about 112 would have an equivocal MPS. Conservatively hypothesizing that 20% of the equivocal MPS results would be reclassified as abnormal with a higher level of exercise exertion, about 20 patients a year with a submaximal exercise effort may have a false-negative MPS. This number may still not seem significant until extrapolated out to the number of exercise stress tests conducted yearly on a national and international level.20-22 To address this hypothesis, future research is needed to identify a peak RER threshold that captures the vast majority of patients who develop myocardial dysfunction due to ischemia if exerted to a sufficient level. This type of study would benefit from using a criterion standard comparative assessment that could quantify real-time abnormal shifts in myocardial function, such as stress echocardiography,23 with simultaneous assessment of RER.
One obvious limitation of the current study was its inability to determine if the higher percentage of equivocal studies in the low peak RER subgroup was truly indicative of patients who would have had an abnormal MPS had they been exerted to a higher level. This finding should therefore be viewed as compelling but in need of additional research to determine clinical importance. However, our results convincingly indicate that the currently used percentage of APMHR and peak RPP thresholds are ineffective in gauging the level of exercise intensity achieved. The results of the current study are highly relevant to primary care physicians because ensuring an appropriate level of exertion during exercise stress testing is recognized as a clinically important goal.24
CONCLUSION
Exercise stress testing will certainly continue to be a key clinical assessment in patients with suspected myocardial ischemia.25 Our results indicate that current practices used to determine the level of exercise exertion during this assessment are inaccurate. Perceived exertion adds some value to determining a patient's level of exertion but still seems to produce a large degree of predictive error that may prove clinically unacceptable. The findings presented here further indicate that unidentified submaximal exertion may lead to a misclassification of MPS results. As such, consideration of adding ventilatory expired gas analysis technology to improve test quality in all patients undergoing an exercise stress test may be warranted.
REFERENCES
- 1.Higgins JP, Higgins JA. Electrocardiographic exercise stress testing: an update beyond the ST segment. Int J Cardiol. 2007;116(3):285-299 [DOI] [PubMed] [Google Scholar]
- 2.Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694-1740 [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Williams MA, et al. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation. 2007;116(3):329-343 [DOI] [PubMed] [Google Scholar]
- 4.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85(4):546-562 [DOI] [PubMed] [Google Scholar]
- 5.Myers J, Arena R, Franklin B, et al. Recommendations for clinical exercise laboratories: a scientific statement from the American Heart Association. Circulation. 2009;119(24):3144-3161 [DOI] [PubMed] [Google Scholar]
- 6.Gibbons RJ, Balady GJ, Timothy BJ, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40(8):1531-1540 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153-156 [DOI] [PubMed] [Google Scholar]
- 8.Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc. 2007;39(5):822-829 [DOI] [PubMed] [Google Scholar]
- 9.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381 [PubMed] [Google Scholar]
- 10.Myers JN. Information from ventilatory gas exchange data. in: Essentials of Cardiopulmonary Exercise Testing, Champaign, IL: Human Kinetics; 1996:83-108 [Google Scholar]
- 11.Arena R, Myers J, Guazzi M. The clinical significance of aerobic exercise testing and prescription: from apparently healthy to confirmed cardiovascular disease. Am J Lifestyle Med. 2008;2(6):519-536 [Google Scholar]
- 12.Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates–2006. J Heart Lung Transplant. 2006;25(9):1024-1042 [DOI] [PubMed] [Google Scholar]
- 13.Balady GJ, Arena R, Sietsema K, et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement From the American Heart Association. Circulation. 2010;122(2):191-225 [DOI] [PubMed] [Google Scholar]
- 14.Armstrong L, Balady G, Berry M, et al. Interpretation of clinical exercise test data. in: Whaley MH, Brubaker PH, Otto R, eds. ACSM's Guidelines for Exercise Testing and Prescription. 7th ed.Philadelphia, PA: Lippincott Williams and Wilkins; 2007:115-129 [Google Scholar]
- 15.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024-2035 [DOI] [PubMed] [Google Scholar]
- 16.Arena R, Myers J, Guazzi M. The future of aerobic exercise testing in clinical practice: is it the ultimate vital sign? Future Cardiol. 2010;6(3):325-342 [DOI] [PubMed] [Google Scholar]
- 17.Belardinelli R, Lacalaprice F, Carle F, et al. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J. 2003;24(14):1304-1313 [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry S, Arena R, Wasserman K, et al. Exercise-induced myocardial ischemia detected by cardiopulmonary exercise testing. Am J Cardiol. 2009;103(5):615-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond E. Developing a cardiopulmonary exercise testing laboratory. Chest. 2007;132(6):2000-2007 [DOI] [PubMed] [Google Scholar]
- 20.Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300(15):1765-1773 [DOI] [PubMed] [Google Scholar]
- 21.Cohen MC, Stafford RS, Misra B. Stress testing: national patterns and predictors of test ordering. Am Heart J. 1999;138(6 Pt 1):1019-1024 [DOI] [PubMed] [Google Scholar]
- 22.Myers J, Voodi L, Umann T, Froelicher VF. A survey of exercise testing: methods, utilization, interpretation, and safety in the VAHCS. J Cardiopulm Rehabil. 2000;20(4):251-258 [DOI] [PubMed] [Google Scholar]
- 23.Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement. Eur J Echocardiogr. 2008;9(4):415-437 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Higgins JA. Electrocardiographic exercise stress testing: an update beyond the ST segment. Int J Cardiol. 2007;116(3):285-299 [DOI] [PubMed] [Google Scholar]
- 25.Gibbons RJ. Noninvasive diagnosis and prognosis assessment in chronic coronary artery disease: stress testing with and without imaging perspective. Circ Cardiovasc Imaging. 2008;1(3):257-269 [DOI] [PubMed] [Google Scholar]