An 87-year-old woman presented to her local emergency department with symptoms of acute-onset odynophagia with a sense of fullness in her throat. She was suspected to have an esophageal food impaction. Conservative management was unsuccessful, and she underwent attempted endoscopic removal of the food impaction. The procedure revealed a food impaction at the distal half of the esophagus, with probable tissue erosion, stenosis, and bleeding into the esophageal lumen. Attempts at removal of the food bolus were unsuccessful. During the procedure, she was transiently hypertensive with systolic blood pressures greater than 230 mm Hg and had a self-limited episode of supraventricular tachycardia. She was urgently transferred to Mayo Clinic in Rochester, MN, for further evaluation and management.
The patient's medical history was remarkable for Sjögren syndrome, Raynaud phenomenon, hypertension, and hypothyroidism. Her home medications included atenolol, furosemide, and levothyroxine. She specifically denied taking any anticoagulation or antiplatelet medications. She had no history of tobacco or alcohol use, no history of malignancy, and no evidence of coagulopathy. Of note, 2 months before, she was treated successfully for a food bolus via noninvasive measures (carbonated beverage and intravenous glucagon) at the transferring institution.
-
Which one of the following is the most appropriate management of suspected food bolus in this patient transferred for hemodynamic instability?
Effervescent agent (simethicone, carbonated beverage)
Glucagon monotherapy
Glucagon, an effervescent agent, and water
Proteolytic enzymes (papain, chymotrypsin)
A second endoscopy
Effervescent agents increase intraluminal carbon dioxide, thereby increasing proximal pressure to force the food bolus toward the stomach. These agents are not routinely used as monotherapy for first-line treatment of esophageal food impaction but more often in combination with other agents such as intravenous glucagon. Glucagon, a pancreatic polypeptide that acts by relaxing the smooth muscle of the esophagus and lower esophageal sphincter, is the first-line pharmacologic agent for food impaction. Glucagon monotherapy would be correct if this were the patient's initial presentation; however, given the previous failed attempt, repeating this therapy is unlikely to be successful at this juncture. Either alone or in combination with effervescent agents, these medications only have a success rate of 12% to 50% and should be used with caution due to increased risk of perforation and aspiration.1,2 Proteolytic enzymes are no longer used in emergent treatment of food impaction given the serious adverse effects, including erosion, necrosis, and perforation of the esophageal tissue. For these reasons, flexible endoscopy is the preferred treatment in most cases of esophageal impaction with food bolus. In addition to relieving the obstruction, endoscopy allows direct visualization of any esophageal abnormalities. This is especially true for our patient, who already had evidence of erosion, stenosis, and esophageal bleeding.
On arrival, the patient was given a small amount of water to confirm that the food bolus did not spontaneously pass during transport. Within 3 to 4 minutes, she failed this swallowing trial with spontaneous regurgitation of the ingested fluid. Initial vital signs were as follows: temperature, 36.8°C; blood pressure, 245/92 mm Hg; pulse, 97 beats/min; respiratory rate, 25 breaths/min; oxygen saturation, 100% while breathing room air. She was hemodynamically stabilized with administration of intravenous antihypertensive metoprolol. Because difficulties with endoscopy were likely given the recent failed attempts, she was endotracheally intubated for a second endoscopy. Endoscopy demonstrated impacted food particles that were successfully removed, revealing an acute esophageal dissection (Figure 1).
-
Which one of the following is the most appropriate next step in this patient's evaluation and management?
Modified barium swallow
Barium esophagography
Gastrografin esophagography
Computed tomography of the chest
Magnetic resonance imaging of the chest
A modified barium swallow or a pharyngogram is a videofluoroscopy swallow study, performed to evaluate the anatomy and function of the oropharynx using contrast agents with variable consistencies, from barium-coated crackers to thin liquids. It is often used to evaluate patients at risk for aspiration, such as those who have just had a stroke and failed their bedside swallowing assessment.3 It would not be the most appropriate next step in this patient. Contrast-enhanced esophagography, with either barium or Gastrografin, remains the standard for evaluating a possible esophageal dissection. Because of the high risk for esophageal perforation, Gastrografin esophagography is the preferred choice for diagnostic evaluation, as spillage of barium in the mediastinum may cause local inflammation and fibrosis.3 Computed tomography or magnetic resonance imaging of the chest would not be the appropriate next step because of their frequent inability to locate the exact site of perforation or dissection.3,4 However, these tests could be considered once the diagnosis of esophageal dissection has been made to help identify any underlying cause, such as malignancy or hematoma.
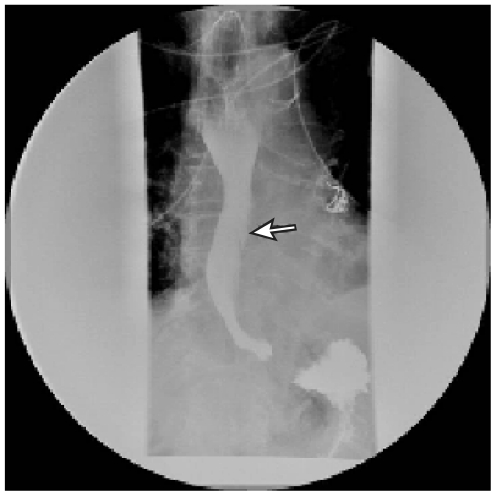
The patient underwent a Gastrografin contrast study to delineate the extent of the esophageal dissection, specifically to rule out a transmural dissection. This study revealed a beak-like cleft at the left lateral aspect of the mid to distal esophagus, representing the entry point of a submucosal dissection. Neither retention (within the defect) nor extravasation of the contrast agent was observed (Figure 2).
-
Given the findings on Gastrografin contrast study, which one of the following steps would not be appropriate in the management of this patient?
Strictly regulate diet (no oral intake)
Admit to the intensive care unit
Initiate intravenous fluids and consider total parenteral nutrition (TPN)
Prepare for emergent surgical repair
Start broad-spectrum intravenous antibiotics with anaerobic coverage (eg, piperacillin-tazobactam)
The treatment for intramucosal esophageal dissection is typically conservative. Restricting oral intake is optimal because any further insult to the esophageal lining could worsen the esophageal dissection. Admission to the intensive care unit for monitoring would be appropriate if there was any indication of hemodynamic instability. Most patients treated conservatively ingest nothing for days. In this patient, it would be appropriate to initiate intravenous maintenance fluids and consider TPN for nutritional support after 5 to 7 days. The current standard of practice is to initiate TPN when the gastrointestinal tract is not functional or cannot be accessed or when the patient's nutritional needs are greater than those that can be met through the gastrointestinal tract.5 Although the appropriate time to initiate TPN remains controversial in healthy patients with no other evidence of protein-calorie malnutrition or severe illness, it is reasonable to initiate TPN after they have ingested nothing for 5 to 7 days.6 In a patient without esophageal perforation as evidenced by contrast extravasation and/or pneumomediastinum, immediate surgery is not indicated. However, surgery can be reconsidered if the esophageal dissection does not heal after a protracted course or if it progresses to an esophageal perforation. Empiric intravenous antibiotics with adequate coverage for enteric pathogens should be initiated.
Our patient was admitted to the medical intensive care unit; she was monitored for 48 hours until her vital signs normalized before being transferred to a general medicine service. Her diet continued to be strictly regulated (nothing by mouth), and she was given intravenous fluids. Total parenteral nutrition was initiated 5 days after the initial onset of symptoms. Intravenous antibiotic therapy (piperacillin-tazobactam) was initiated.
-
Which one of the following is the most appropriate follow-up for this patient to ensure appropriate clinical improvement?
Follow-up esophagography in 2 to 4 weeks
Follow-up endoscopy in 2 to 4 weeks
Surgical exploration
No follow-up is required after discharge
Advance diet as tolerated
Follow-up esophagography in 2 to 4 weeks is recommended because complete resolution of intramural spontaneous dissections typically occurs within 4 weeks. Although there are no formal guidelines for follow-up of an intramural esophageal dissection, most reviews suggest serial esophagography as the test of choice to assess for resolution. Endoscopic intervention is not appropriate and would only be indicated if clinical progression of the dissection was suspected and/or the patient was still exhibiting symptoms.7 Surgical exploration is typically not indicated during the initial presentation and is not recommended to assess for resolution. As noted, most cases resolve with conservative management.3,8,9 Inadequate patient follow-up or premature diet advancement before confirmation of appropriate healing of the dissection site may increase the risk of further esophageal damage.
This patient's clinical presentation was complicated by difficult-to-control blood pressure and development of atrial fibrillation with rapid ventricular response. Given that she was allowed nothing by mouth, she required administration of intravenous medications for blood pressure and heart rate control. For this reason, follow-up esophagography was performed 7 days after initial presentation to assess for interval improvement or progression and showed a questionable marginal increase in the size of the false lumen. Because assessment of the lumen at the dissection point was difficult, CT of the chest was performed, showing no change in the lumen size and no evidence of transmural perforation. Given the need for a stable oral medication regimen, the patient was given a 48-hour trial of a clear liquid diet, which she tolerated without issue. She was advanced to a full liquid diet, and her TPN was discontinued after 5 days because she was able to maintain appropriate caloric intake. She tolerated administration of her oral medications, and an effective blood pressure and heart medication schedule was established. She remained hemodynamically stable with normal blood pressure and heart rate and was discharged from the hospital on day 14. She was instructed to maintain a full liquid diet for 2 weeks until her scheduled thoracic surgery and follow-up esophagography.
-
Which one of the following is not known to be a risk factor for development of this patient's condition?
Sex
Age
Sjögren syndrome
Therapeutic anticoagulation
Food bolus with previous esophagogastroduodenoscopy
Spontaneous intramucosal esophageal dissection is noted to occur more frequently in women in their seventh or eighth decades.10 Although patients with Sjögren syndrome commonly have xerostomia and dysphagia, this syndrome is not known to be associated with esophageal dissection or perforation. Long-term anticoagulation therapy is a known risk factor for esophageal dissection. The mechanism of dissection is an initial mucosal injury from an esophageal hematoma that leads to a full separation of the mucosal or submucosal layers from the deeper muscular layers.10 A food bolus and use of any form of esophageal instrumentation, including placement of a nasogastric feeding tube, have been reported as triggering events for an esophageal dissection.8
When this patient returned 2 weeks later, she was completely asymptomatic and reported good tolerance of her clear liquid diet. Follow-up esophagography showed complete resolution of the esophageal dissection, and thus a general diet was reinstated. Four months later, she continued to do well, with no recurrence of the dissection.
FIGURE 1.
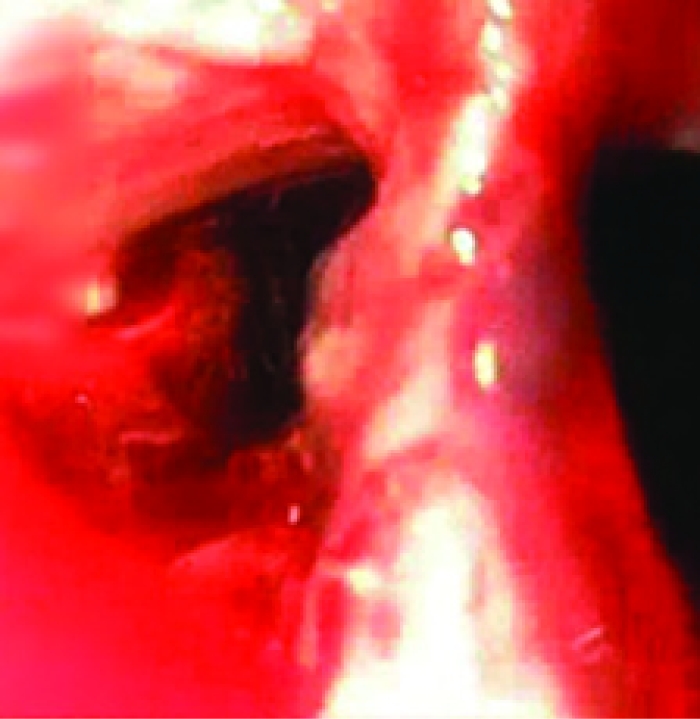
A false lumen (left), revealed during upper endoscopy, led to a noncommunicating pouch.
FIGURE 2.
Gastrografin esophagram revealing a beak-like cleft (arrow), which represents the entry point of the dissection and false lumen.
DISCUSSION
Intramural esophageal dissection occurs rarely and was first reported in 1968 by Marks and Keet.11 It is noted to occur more commonly in women in their seventh or eighth decades and more frequently in people receiving long-term anticoagulation therapy.10 The mechanism of dissection appears to be an initial inciting traumatic break in the mucosa, which subsequently leads to a full separation of the mucosal or submucosal layers from the deeper muscular layers as a result of increased intraesophageal pressure.10 Dissections have been reported to occur as a result of foreign body ingestion7 or a food bolus or soon after esophageal instrumentation, including endoscopy,12 nasogastric tube placements,8 and transesophageal echocardiography.13 Esophageal hematoma is another possible triggering event, and this may be why patients receiving anticoagulation therapy are at higher risk of developing spontaneous intramural bleeding.10
The most common presenting symptoms of intramural esophageal dissection are sudden-onset severe retrosternal pain (83%), hematemesis (71%), odynophagia (41%), and dysphagia (32%), usually 4 to 5 hours after the inciting event.9 Signs indicative of esophageal perforation include pain, fever, tachycardia, subcutaneous crepitus, and neck or chest swelling.
Contrast-enhanced esophagography is the standard for evaluating a possible esophageal dissection. Two common contrast agents used in esophagography are barium sulfate preparations and water-soluble organic iodine compounds, such as diatrizoate meglumine (Gastrografin). When acute perforation is suspected, Gastrografin is preferred over barium because spillage of barium into the mediastinum will result in local inflammation, with subsequent granuloma formation and fibrosis. However, with Gastrografin, a small perforation or any that is walled off or in an area of spasm may be missed. If no extravasation is seen on the Gastrografin study, barium esophagography should be performed to evaluate for any small leaks that were previously undetected. However, in cases of lung pathologies (eg, possible aspiration or a tracheoesophageal fistula), barium is preferred over Gastrografin because the latter is extremely hypertonic and can induce pulmonary edema, pneumonia, or death, whereas aspiration of small amounts of barium seems to have little clinical relevance. Most of the barium is cleared from the major bronchi and trachea within hours, although some remains in the interstitium and in macrophages.3
On contrast studies, 2 signs indicate intramural dissection: the mucosal stripe sign (the so-called linear stripe) and the double-barreled sign, which is the result of contrast media contained within the true and false lumens.4,14 Esophageal lumen narrowing or obstruction may also be seen. Any extravasation of contrast media is highly suggestive of complete perforation of the esophagus wall and signals the need for prompt surgical management.
Most esophageal dissections resolve with conservative treatment within 4 weeks. Patients with no oral intake for more than 7 days should begin receiving TPN for enteral support.6 To our knowledge, no data are available on the optimal duration of TPN therapy, and this could be an avenue for future studies.
There have been case reports of treatment with an endoscopically placed self-expanding metal stent in protracted esophageal dissection.15 The stent enables compression at the dissection site while allowing for passage of orally ingested material and prevents food and liquids from leaking. This results in an earlier enteral feeding, as early as 24 hours after stent placement. Surgical intervention is typically limited to cases of intramural esophageal dissection with suspected progression to esophageal perforation.
In summary, esophageal dissection is a rare but potentially complicated condition. It is imperative for clinicians to recognize the presentation of an esophageal dissection, effectively diagnose the condition using contrast-enhanced esophagography as a first-line tool, and incorporate appropriate management strategies. These include conservative measures, such as ensuring that the patient ingests nothing by mouth; initiation of intravenous maintenance fluids; and initiation of a broad-spectrum antibiotic. Total parenteral nutrition should be started in patients with no oral intake for more than 7 days. If any evidence of esophageal perforation exists, early surgical intervention is indicated.
Acknowledgments
We thank Dr. Jeffrey Geske, MD, for his guidance and advice in the preparation of this article, Dr. Elizabeth Rajan, MD, for providing the esophagogastroduodenoscopic images, and Ms. Gretchen Schiesser for editing the image.
See end of article for correct answers to questions.
Correct answers: 1. e, 2. c, 3. d, 4. a, 5. c
REFERENCES
- 1.Blair SR, Graeber GM, Cruzzavala JL, et al. Current management of esophageal impactions. Chest. 1993;104:1205-1209 [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Anderson R. Best evidence topic report. Effervescent agents for oesophageal food bolus impaction. Emerg Med J. 2005;22:123-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore RM, Levine M. Textbook of Gastrointestinal Radiology. 2nd ed.Philadelphia, PA: W. B. Saunders Company; 2000. [Google Scholar]
- 4.Hsu CC, Changchien CS. Endoscopic and radiological features of intramural esophageal dissection. Endoscopy. 2001;33:379-381 [DOI] [PubMed] [Google Scholar]
- 5.Russell MK, Andrews MR, Brewer CK, Rogers JZ, Seidner DL. Standards for specialized nutrition support: adult hospitalized patients. Nutr Clin Pract. 2002;17:384-391 [DOI] [PubMed] [Google Scholar]
- 6.McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33:277-316 [DOI] [PubMed] [Google Scholar]
- 7.Wu HC, Hsia JY, Hsu CP. Esophageal laceration with intramural dissection mimics esophageal perforation. Interact Cardiovasc Thorac Surg. 2008;7:864-865 [DOI] [PubMed] [Google Scholar]
- 8.Kim MK, Kim BW, Jang JW, et al. Long-distance esophagogastric submucosal dissection after minimal esophageal trauma of a gastric tube. Gastrointest Endosc. 2008;68:605-607 [DOI] [PubMed] [Google Scholar]
- 9.Hanson JM, Neilson D, Pettit SH. Intramural oesophageal dissection. Thorax. 1991;46:524-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan GQ, Heitmiller RF. Intramural esophageal dissection. Ann Thorac Surg. 1997;63:1785-1786 [DOI] [PubMed] [Google Scholar]
- 11.Marks IN, Keet AD. Intramural rupture of the oesophagus. BMJ. 1968;3:536-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang SJ, Tang L, Jazrawi SF, Meyer D, Wait MA, Myers LL. Iatrogenic esophageal submucosal dissection after attempted diagnostic gastroscopy (with videos). Laryngoscope. 2009;119:36-38 [DOI] [PubMed] [Google Scholar]
- 13.El-Chami MF, Martin RP, Lerakis S. Esophageal dissection complicating transesophageal echocardiogram–the lesson to be learned: do not force the issue. J Am Soc Echocardiogr. 2006;19(5):579e5-579.e7 [DOI] [PubMed] [Google Scholar]
- 14.Steadman C, Kerlin P, Crimmins F, et al. Spontaneous intramural rupture of the oesophagus. Gut. 1990;31:845-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Lee SO. Circumferential intramural esophageal dissection successfully treated by endoscopic procedure and metal stent insertion. J Gastroenterol. 2005;40:1065-1069 [DOI] [PubMed] [Google Scholar]