Abstract
Women with a germline BRCA1 or BRCA2 mutation or a hereditary predisposition for breast and ovarian cancer have substantial risk of breast or ovarian cancer relative to the general US population. Health care professionals can be instrumental in identifying women at increased risk through obtaining a comprehensive family history and becoming familiar with family history characteristics associated with hereditary predisposition for breast and ovarian cancer. BRCA carriers and women at very high risk benefit from multidisciplinary, individualized medical evaluation and risk management. We conducted a search of MEDLINE from 1989 through 2010 for the terms BRCA1, BRCA2, breast cancer, ovarian cancer, risk assessment, and genetic testing. We reviewed abstracts and relevant randomized and prospective studies that included very high-risk patient groups and BRCA mutation carriers. Herein, we review the role of genetic consultation and BRCA testing and the comprehensive, multisystem recommendations for risk management. A multidisciplinary approach offers the ability to educate those at very high risk about cancer prevention, reduce cancer risk, maximize early detection of breast and ovarian cancer, and improve survival.
CBE = clinical breast examination; MRI = magnetic resonance imaging; OC = oral contraceptive; RRSO = risk-reducing salpingo-oophorectomy
Since genetic testing was introduced, its use for risk assessment by health care professionals has been escalating. Hereditary BRCA1 and BRCA2 mutations account for about 60% of inherited breast cancer and are the only known causes of hereditary breast and ovarian cancer syndrome. Women with a germline mutation in BRCA1 or BRCA2 or a hereditary predisposition for breast cancer have markedly increased risk of early-onset breast cancer and ovarian cancer. Other, rarer hereditary gene mutations are associated with increased risk of breast cancer, such as Li-Fraumeni syndrome, Cowden syndrome, Peutz-Jeghers syndrome, and hereditary diffuse gastric cancer syndrome, but these syndromes are not discussed herein.1
In the United States in 2009, there were approximately 192,370 new cases of breast cancer and 21,550 new cases of ovarian cancer.2 That year, breast cancer deaths were estimated at 40,170 and ovarian cancer deaths at 14,600. Approximately 80% of breast and 90% of ovarian cancer cases are thought to be sporadic with no associated family history. Multifactorial familial risk accounts for approximately 10% to 15% of breast cancer. In the future, testable panels of genetic variants likely will combine to subtly alter risk. Hereditary breast cancer—cancer attributable to a single hereditary gene mutation in either BRCA1 or BRCA2—accounts for approximately 5% of breast cancer cases, characteristically occurring before age 50 years. Approximately 4% to 11% of ovarian cancer is attributable to a germline mutation, with the greatest proportions in cancers diagnosed before age 50 years.3 An estimated 1 in 300 to 1 in 800 US individuals are BRCA carriers (1 in 50 individuals with Ashkenazi Jewish heritage).4,5
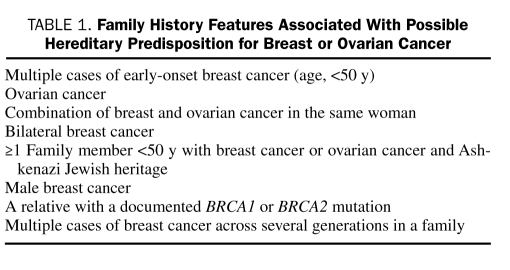
Hereditary breast and ovarian cancer attributed to a mutation in a particular gene (ie, BRCA1 or BRCA2) can be passed on to the next generation, transmitted in an autosomal dominant pattern. The gene mutation may originate from the maternal or the paternal side, and each offspring of a BRCA carrier has a 50% chance of inheriting the mutation.6 Specific characteristics that indicate increased likelihood of a BRCA gene mutation are listed in Table 1.
TABLE 1.
Family History Features Associated With Possible Hereditary Predisposition for Breast or Ovarian Cancer
RISK FACTORS
Advancing age is the strongest individually identified risk factor, but family history provides one of the strongest clues to the possibility of hereditary breast cancer. Family history of breast cancer or ovarian cancer diagnosed at a young age or involving multiple family members may be clues to a hereditary cancer syndrome.
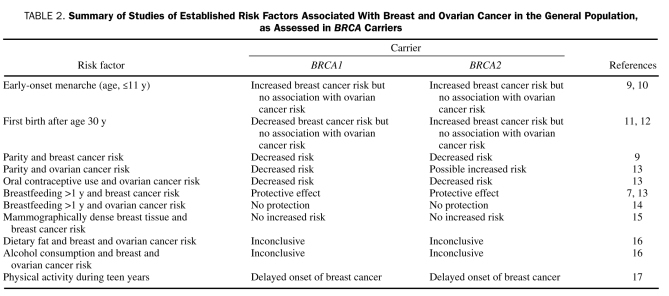
Some reproductive risk factors that are more commonly associated with sporadic breast cancer may differ in how they influence breast cancer risk in BRCA carriers compared with the general population. When stimulated by endogenous or exogenous estrogen during pregnancy or puberty, breast cells that harbor a BRCA1 or BRCA2 mutation react differently to common processes, such as DNA damage repair, cell proliferation, and cell differentiation.7-9 Table 2 lists the results of studies that assessed whether established risk factors for breast cancers in the general population are also risk factors in carriers of BRCA mutations. The data and evidence for these associations are limited and preliminary for women with BRCA mutations, and further studies are necessary to establish true associations.
TABLE 2.
Summary of Studies of Established Risk Factors Associated With Breast and Ovarian Cancer in the General Population, as Assessed in BRCA Carriers
Epidemiological studies have shown that, in general, lifestyle modification decreases breast cancer in high-risk women. Risk modification options include regular exercise (30 minutes 3 times per week), avoidance of postmenopausal obesity, reduced alcohol intake (≤1 serving [eg, 12 oz of beer] per day), and avoidance of long-term hormone therapy. One study showed that physical activity during the teen years delayed onset of breast cancer in BRCA mutation carriers.17 Furthermore, normal weight at menarche (P=.02) and low weight at age 21 years (P=.02) also delayed breast cancer onset in carriers. Among BRCA carriers, data are limited on the role of lifestyle modification in reducing breast cancer risk.18
BREAST AND OVARIAN CANCER RISK ESTIMATES AND DIFFERENTIATING FEATURES
Among BRCA1 mutation carriers, the estimated lifetime risk of breast cancer is 40% to 85%, and lifetime risk of ovarian cancer is estimated at 25% to 65%. BRCA2 mutation carriers have about the same risk of breast cancer, with an ovarian cancer risk estimated to be 15% to 20%. The range of cancer risk estimates varies with the population in different studies and in accordance with the mode of ascertainment.19 Lifetime risk is increased dramatically compared with the general population, in which the risk of breast cancer is approximately 13% and the risk of ovarian cancer is 1.5%.20 Yet, given these increased risks relative to the general population, approximately 20% to 30% of BRCA carriers never have breast cancer, and 35% to 85% do not have ovarian cancer.19
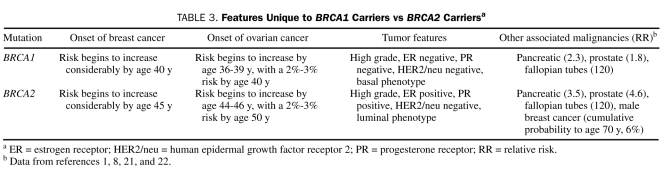
Several unique features differentiate BRCA1 carriers from BRCA2 carriers and may influence surveillance and management options subsequently. In general, risks and age at onset of breast cancer are similar, but molecular and biological features are different. Regarding ovarian cancer, the risk is considerably greater in BRCA1 mutation carriers and age at diagnosis is younger on average than in BRCA2 mutation carriers (Table 3).
TABLE 3.
Features Unique to BRCA1 Carriers vs BRCA2 Carriersa
Assessing Breast Cancer Risk
Breast cancer risk assessment is complicated and can be challenging. Furthermore, no consistent and well-defined definition or threshold for “high-risk” has been established.
The Gail model, a computerized method validated in large populations (available at http://www.cancer.gov/bcrisktool), is not ideal for predicting individual risk. This model incorporates age, reproductive history, breast biopsy history, and breast cancer occurrence in first-degree relatives. The model is valid only for women aged 35 years or older and does not include breast cancer occurrence in second-degree relatives, paternal history, or age at breast cancer diagnosis in affected relatives. It is not appropriate for use in individuals with multiple young affected relatives across several generations or for determining whether to order genetic testing. In general, a 5-year predicted risk of breast cancer that exceeds 1.66% as calculated by the Gail model is considered high risk. This model has been used in determining eligibility for chemoprevention with tamoxifen or raloxifene.23,24
The Claus model is a statistical model that calculates cumulative (lifetime) breast cancer risk specifically on the basis of family history and includes maternal and paternal second-degree relatives and the ages at diagnosis of breast cancer. It does not incorporate ovarian cancer or nonhereditary or reproductive risk factors. Risk estimates are derived from the family history of 5000 breast cancer cases (age, 20-54 years) and age-matched controls in the United States. Both published tables and computerized versions of this model are useful in clinical practice.25
The IBIS model (IBIS Breast Cancer Risk Evaluation Tool, RiskFileCalc version 1.0, copyright 2004, available by contacting ibis@cancer.org.uk), also known as the Tyrer-Cuzick model, is another risk prediction algorithm for assessing breast cancer risk and the probability of having a BRCA mutation found. It incorporates a more extensive family history and includes reproductive risk factors and benign breast disease history.26,27
The BRCAPRO model, version 4.3 (available at http://www.4.utsouthwestern.edu/breasthealth/cagene/default.asp), includes information on affected (both breast and ovarian cancer) and unaffected relatives and, using a Bayesian approach to risk assessment, estimates the likelihood of finding BRCA1 or BRCA2 mutations in a family. This model incorporates family history, age at diagnosis of cancers in the family, presence of bilateral breast cancer, male breast cancer, and Ashkenazi Jewish heritage.4,5,28,29
Numerous other models are designed specifically to estimate the likelihood of a genetic alteration or deleterious mutation. Their results should be interpreted cautiously because they do not predict true breast cancer risk and may give significantly different results for the same patient because of differences in data input and assumptions in output. Genetic counselors use these likelihood models to estimate the probability of carrying a BRCA mutation in order to help inform subsequent discussion regarding the role of genetic testing. No specific risk level has been defined as the right level at which to offer BRCA testing.
Health care professionals who use breast cancer risk calculation models need to be familiar with the limitations, strengths, and weaknesses of each model. Risk assessment is not a static number and patient risk changes over time; therefore, risk needs to be reassessed periodically.30
Assessing Ovarian Cancer Risk
Compared with breast cancer risk modeling, tools for predicting ovarian cancer risk are substantially more limited. The algorithm for evaluating risk of ovarian cancer combines an individual's age and trends in CA-125 levels to assess the likelihood that she has ovarian cancer at the point of testing, but it does not predict future risk or the likelihood of being a carrier of a BRCA mutation.31 Other models, including ultrasonographic scoring systems and tumor marker panels, are designed to assess malignancy risk in a woman with a pelvic mass. Beyond population statistics that describe the occurrence of ovarian cancer in BRCA mutation carriers, no prospective modeling based on biomarkers is available currently to provide individualized assessment of short- or long-term risk of ovarian cancer in high-risk women. The output of the BRCAPRO model provides ovarian cancer risk estimates, subject to all the limitations of computerized modeling.
Genetic Consultation
Genetic consultation and testing are currently mainstream components of a multidisciplinary, individualized medical evaluation aimed at identification of individuals at risk for hereditary breast cancer syndromes. The US Preventive Services Task Force guideline on genetic risk assessment and BRCA mutation testing strongly recommends referral of high-risk individuals for genetic counseling. Programs that provide expertise in clinical genetics are important because BRCA testing has major medical, psychological, ethical, legal, and social implications,32 in addition to the consideration of its relevance to potentially numerous family members.
Genetic counselors are trained in the collection of family history and the use of models that quantify an individual's risk of a BRCA mutation. They provide pretesting education about possible outcomes of testing, the implications of both positive and negative test results for the person's health care and for her relatives, and the risks and limitations of testing. Genetic counselors can determine whether a person is predisposed to other hereditary gene syndromes and provide counseling on appropriate genetic testing associated with specific syndromes. They aid women with the insurance approval process, if necessary, and arrange for collection and submission of the blood sample to Myriad Genetics, Salt Lake City, UT (the only laboratory that provides clinical BRCA testing in the United States at this time). Complete testing (sequencing plus testing for large gene rearrangements in BRCA1 and BRCA2) currently costs around $4000. A list of specialized genetic counselors is available through the National Society of Genetic Counselors (http://www.nsgc.org/resourcelink.cfm).33
Referral to a genetic counselor is recommended for individuals in whom breast cancer developed before age 40 years and those with a strong family history of breast or ovarian cancer30 (Table 1). Genetic counselors can help identify the individual in a family for whom genetic testing is most likely to be informative for family members. Generally, this person is the youngest living, affected, and willing family member. It is paramount that women are counseled that a BRCA test is a predictive test and, although it provides information regarding the likelihood of breast or ovarian cancer, it is not a diagnostic test and does not confirm that a woman will have breast or ovarian cancer. Similarly, a negative result does not guarantee that an individual will not have breast cancer, either due to sporadic causes or other, as yet undefined, genetic factors.
Beyond the associated health care fees, the risks of genetic counseling are minimal. In contrast, genetic testing has known risks, as well as benefits. Genetic testing offers the following benefits: identification of high-risk individuals who will benefit from the initiation of recommended cancer risk management; identification of noncarriers in families with a known mutation, who do not need to have rigorous cancer screening and would be considered at “general population risk”; education in early detection and prevention strategies; and perhaps relief of anxiety through increasing the understanding of medical options. However, it also has its risks and limitations, including its inability to detect all mutations, the continued risk of sporadic cancer, the unproven efficacy of some interventions, and the possibility of psychosocial or economic harm.
In 2008, the Genetic Information Nondiscrimination Act was signed into law by President George W. Bush.34 Genetic counselors can inform patients about this law, which provides protection against discrimination in health insurance underwriting decisions based on genetic information. This protection includes group and individual health insurance and employment practices but does not cover life, disability, or long-term–care insurance and other forms of insurance. The act appears to exclude individuals with health care coverage through the US military, Veterans Affairs, and Indian Health Service because the laws amended by the Genetic Information Nondiscrimination Act do not apply to these groups. Also, persons enrolled in health insurance plans with fewer than 15 members may not be covered. Some states have even stronger protections, which can be addressed with the genetic specialist when indicated.
MANAGEMENT OPTIONS
Surveillance
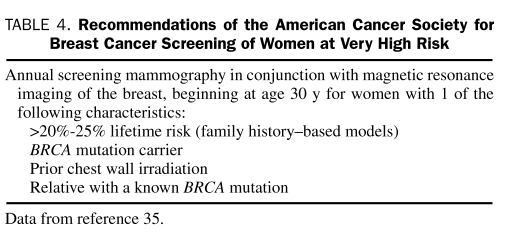
Breast Cancer. Early detection of breast cancer includes the combination of monthly breast self-examination beginning at age 18 years; annual or semiannual clinical breast examination (CBE) by a health care professional; annual mammography beginning at ages 25 and 30 years for BRCA mutation carriers, when breast cancer risk begins to increase; and annual breast magnetic resonance imaging (MRI) beginning at age 30 years.21,35 It is generally recommended that annual screening mammography begin 5 to 10 years earlier than the youngest age at which breast cancer was diagnosed in a first-degree relative or at age 25 years, whichever comes first. The benefit of CBE performed by a health care professional has recently been debated for BRCA carriers; few data show that it adds substantially to cancer detection rates.36 However, CBE was reassuring to women and provided an opportunity for them to connect with their health care professional.36 BRCA carriers also were more likely to conduct breast self-examination, and CBE gave women an increased sense of control.
Digital mammography has been shown to be more accurate than film screen mammography in younger women with dense breast tissue for the detection of breast cancer and is recommended for this population at very high risk.37 Although mammography is the only imaging study to date that has shown a decrease in breast cancer mortality rate of 16%, concerns have been raised about the safety and efficacy of exposure to ionizing radiation when initiating mammographic screening as young as 25 years.38,39 A study assessing the estimated risk of radiation-induced breast cancer from mammographic screening for young BRCA mutation carriers vs the reduction in breast cancer mortality rate showed no net benefit from annual mammographic screening of BRCA mutation carriers aged 25 to 29 years. The net benefit improved slightly at age 30 to 34 years.40
Breast MRI has been shown to increase the sensitivity of malignancy detection to about 80%, whereas mammography sensitivity is estimated to be about 33% in women with a familial or genetic predisposition.41-43 A prospective cohort study evaluating multimodality screening of high-risk women reported that the addition of MRI to mammography had greater potential to detect additional mammographically occult cancers than whole-breast ultrasonography and digital mammography.44 Screening with whole-breast ultrasonography had the lowest sensitivity and biopsy yield, and digital mammography was not found to be a sensitive alternative to MRI in that study. An additional advantage of MRI is the lack of radiation exposure.
Screening guidelines are available from numerous organizations, including the National Comprehensive Cancer Network, the American Cancer Society (Table 4), and the US Preventive Services Task Force. Experts generally agree that intensive screening for breast cancer and ovarian cancer is necessary for high-risk individuals and BRCA carriers—yet, uncertainty surrounds the efficacy of surveillance—and that most cancers found among BRCA carriers are interval cancers (ie, malignancies detected in an interval between screening examinations).21,35,45,46
TABLE 4.
Recommendations of the American Cancer Society for Breast Cancer Screening of Women at Very High Risk
Breast cancer surveillance offers a number of advantages: it is noninvasive, has no long-term adverse effects, does not interfere with childbearing, and leaves other options open. In addition, MRI of the breast has about 80% sensitivity for malignancy detection. However, MRI surveillance also has its limitations: it does not prevent breast cancer and has not been proven to reduce breast cancer–related death in BRCA mutation carriers; its efficacy in BRCA carriers is not as clearly documented as in the general population; the risk of false-positive results with breast MRI screening can lead to additional imaging or biopsies, or both, and associated anxiety may result from the additional follow-up; and testing is costly.
Ovarian Cancer. Ovarian cancer surveillance for BRCA mutation carriers includes screening with annual or semiannual transvaginal pelvic ultrasonography with Doppler imaging beginning at age 35 years or at 5 to 10 years younger than the age at earliest ovarian cancer diagnosis in the family, along with CA-125 testing.47 Unfortunately, no evidence shows reduction in ovarian cancer–related death with pelvic ultrasonography and CA-125 screening in these high-risk women. However, these surveillance strategies continue to be recommended for early cancer detection in BRCA mutation carriers who have not yet had their ovaries removed. A prospective nonrandomized investigation, sponsored by the Gynecologic Oncology Group, aims to document the net health effects of risk-reducing salpingo-oophorectomy (RRSO) vs twice-yearly screening in high-risk women.48 Importantly, this study includes multisystem outcome measures, such as osteoporosis, cardiac disease, medication use, and psychometric assessments, in addition to cancer-specific outcomes. The screening option uses a stepwise algorithm based on CA-125 levels over time; ultrasonographic screening and a 5-year follow-up period will be completed in November 2011.
Ovarian cancer surveillance with use of ultrasonography and CA-125 testing has the following advantages: it is noninvasive, causes limited disruption of normal activity, and preserves fertility and native hormone physiology. Disadvantages of the surveillance include frequent false-positive results, increased anxiety due to screening in approximately one-third of women,46,49 examination discomfort, cost over time, and lack of evidence that it improves clinical outcomes or saves lives.
Chemoprevention
Tamoxifen and raloxifene, selective estrogen receptor modulators approved for breast cancer risk reduction, are generally prescribed for 5 years, and their role beyond this time frame is unknown. Results from a large North American prevention trial comparing tamoxifen with raloxifene in high-risk postmenopausal women showed a 50% reduction in invasive breast cancer with both treatments.50 Chemoprevention trials and the updated results of the Study of Tamoxifen and Raloxifene have also shown that these 2 medications are good preventive options for women at moderately high risk.51,52 However, in BRCA carriers specifically, the effectiveness is unclear. Results of 1 small study suggested that tamoxifen might reduce breast cancer risk in BRCA2 mutation carriers but not BRCA1 carriers.17,53 In another study of women with BRCA1 and BRCA2 mutations, tamoxifen reduced the risk of contralateral breast cancer, and its protective effect seemed independent of that of RRSO.54 Debate is long standing about the benefit of tamoxifen in BRCA1 carriers, given the predominance of estrogen receptor–negative disease. Furthermore, no data are available on the efficacy of raloxifene for breast cancer risk reduction among BRCA carriers.55 A large prevention study in Italy, the Aromasin Prevention Trial, is under way in BRCA carriers, comparing exemestane vs placebo, and its primary end point is disease-free survival at 7 years.56
Ongoing molecular biology research has led to improved understanding of estrogen-independent signaling pathways, as well as the complexity of mammary tumorigenesis. This knowledge is paramount in the development of novel agents that can target nonendocrine pathways, cellular proliferation, and tumor progression. Other agents under investigation include cyclooxygenase-2 inhibitors, tyrosine kinase inhibitors, poly (adenosine diphosphate–ribose) polymerase inhibitors, DL-α-difluoromethylornithine, and nonsteroidal anti-inflammatory drugs. Clinical trials and development of targeted preventive and therapeutic regimens are needed in the care of BRCA carriers, given their unique molecular biology.57
Benefits of chemoprevention are that it may reduce the risk of estrogen receptor–positive breast cancer and may prevent osteoporosis. Limitations are that it does not eliminate risk of cancer, and data on its use in BRCA carriers are limited. Furthermore, adverse risks include thromboembolic events, interference with childbearing (during the years of use), and endometrial cancer risk (with tamoxifen use).58
Use of oral contraceptives (OCs) is associated with a reduction in ovarian cancer risk of 40% to 50% after 3 years' cumulative use.13,59 Long-term use of OCs has been associated with a slight increase in risk of breast cancer among BRCA1 mutation carriers; however, there is no measurable increased risk of breast cancer with OC use (in other high-risk women) of 5 or fewer years.60,61
Advantages of chemoprevention of ovarian cancer with OC use are that it is well tolerated and inexpensive, does not affect future fertility, and leaves other options open. Disadvantages include the adverse effects of OCs, including thromboembolic risk; the continued need to screen for ovarian cancer62,63; and the possible increased risk of breast cancer with use exceeding 5 years.
Surgical Risk Reduction Strategies
Bilateral prophylactic mastectomy (also known as risk-reduction mastectomy) removes most, but not all, breast tissue. This procedure has been shown to significantly reduce breast cancer risk in women with a family history of breast cancer. Several studies have shown a 90% to 95% reduction in breast cancer risk among BRCA carriers,64,65 meaning that women with BRCA mutations can achieve a level of breast cancer risk that is the same or lower than that of the general population. The results from a recent prospective cohort of BRCA1 and BRCA2 mutation carriers provided further confirmation of the known benefit of risk-reducing mastectomy in lowering the risk of breast cancer.66 Two options are available: total (simple) mastectomy and subcutaneous mastectomy. Total mastectomy removes slightly more breast tissue than subcutaneous mastectomy, whereas the latter procedure preserves the nipple-areolar complex. The absolute risk reduction after risk-reduction mastectomy has not been clearly defined.
Bilateral prophylactic salpingo-oophorectomy (ie, RRSO) involves removal of the ovaries and fallopian tubes. This outpatient procedure is commonly performed laparoscopically and the choice in favor of or against concomitant hysterectomy must be individualized. It is associated with ovarian cancer risk reduction of approximately 80% to 90%.67 The residual risk is due to development of primary peritoneal carcinomatosis, which is pathologically indistinguishable from ovarian carcinoma. In addition, it is associated with a breast cancer risk reduction estimated at 40% to 50% and breast cancer protection as high as 73% in BRCA2 mutation carriers when it is performed premenopausally for women with no prior diagnosis of breast cancer.67 The benefit diminishes when RRSO is performed closer to the age of natural menopause. Investigators of a recent meta-analysis hypothesize that the benefits of RRSO may differ in accordance with the mutated gene (BRCA1 vs BRCA2) and the estrogen receptor status of subsequent breast cancers.68 Although RRSO has been characterized as the single most therapeutically effective and cost-effective measure for cancer risk reduction in BRCA mutation carriers,69,70 women should be reminded that a small risk of a peritoneal carcinomatosis of about 0.2% per year persists after RRSO.71,72 The recently published prospective cohort of BRCA1 and BRCA2 mutation carriers provided further confirmation of the benefit of bilateral RRSO. Decreased rates of breast cancer and ovarian cancer–specific mortality and all-cause mortality and decreased risk of ovarian cancer and breast cancer were associated with the RRSO procedure.66
No national consensus has been reached regarding the ideal age for RRSO, but observational studies support earlier onset of ovarian cancer in women with BRCA1 mutations than with BRCA2 mutations. We advise that women with BRCA mutations be referred to a gynecologic oncologist to discuss RRSO options. Ideally, initial consultation for a BRCA1 mutation carrier should take place when the woman approaches her mid-thirties. Referral to a gynecologic oncologist can be delayed somewhat for a BRCA2 carrier until about her early forties.73
A disadvantage of RRSO is that it induces surgical menopause and premature estrogen deficiency.74 Studies have reported the possibility that, after RRSO, younger women may safely take hormones to manage menopausal symptoms and derive other estrogen-related benefits through age 50 years, which is about the time of natural menopause.49,75 Some women may choose to have a hysterectomy at the time of the RRSO, which permits the use of estrogen without progestins.
Recent studies raise a cautionary note about the global health effects of premature estrogen deficiency, which may increase the risk of cardiovascular disease, hyperlipidemia, and the metabolic syndrome in women at high risk for hereditary breast cancer,76 as well as exacerbate menopausal symptoms. In 1 study, the investigators observed that this risk increased with younger age at RRSO and that there was no increased risk of dementia when estrogen replacement was delayed until the age of menopause.77 In contrast, increased risk of parkinsonism was documented in the same study group, and the women who received estrogen replacement therapy were not protected against parkinsonism. Osteoporosis and fracture risk are a concern with estrogen deficiency, and we recommend close monitoring with dual-energy x-ray absorptiometry every 2 years. If evidence of osteopenia or osteoporosis risk exists, then bisphosphonate treatment may need to be initiated.
Several studies have assessed the effect of short-term hormone replacement therapy on breast cancer risk after RRSO. It has not been found to negate the protective effect on subsequent breast cancer risk in BRCA carriers, and it may even be associated with a decreased risk in this population.75,78 Women should be informed of the risks associated with premature estrogen deficiency and the risks and benefits of short-term estrogen replacement.79
The advantages of RRSO are as follows: it reduces risk of ovarian cancer and breast cancer, decreases cancer-related anxiety, offers a 1-time action for long-term protection, and may detect occult carcinoma (early stage). Disadvantages of RRSO include surgical risk, residual risk of peritoneal carcinoma, menopause induction with estrogen deficiency–related health effects, impact on childbearing, and a potential effect on body image and sexuality.
Many women are interested in cancer risk–reduction surgery before the recommended ages for RRSO. Bilateral tubal ligation offers an interesting intermediate option for BRCA1 mutation carriers. Numerous retrospective studies document ovarian cancer risk reduction in women who have undergone tubal ligation.80 A 2001 case-control study of women with documented BRCA mutations showed that bilateral tubal ligation in BRCA1 carriers decreased the risk of ovarian cancer (odds ratio, 0.37; P=.0003) and reported a trend toward better protection with earlier ligation.80 No protection was evident in BRCA2 mutation carriers. Furthermore, in BRCA1 mutation carriers, the risk-reducing effects of OC use and bilateral tubal ligation appear to be additive, with an observed reduction of 72% in the occurrence of ovarian cancer. Although the mechanism by which tubal ligation protects against ovarian cancer is unknown, the procedure may be a surrogate for or somehow correlated with the fertility reduction that has been observed in a subset of BRCA1 carriers.81
Cost-Effectiveness of Preventive Strategies
In 2006, investigators used Markov modeling to simulate the cost-effectiveness of the preventive strategies available to BRCA1 and BRCA2 mutation carriers.70 The women included in this decision analysis were aged between 35 and 50 years. The model showed that the most cost-effective strategies were RRSO alone or with mastectomy compared with surveillance in the clinical setting of genetic risk. This model also showed that mastectomy had a more favorable cost-effectiveness profile as women became older and for BRCA2 carriers. Study limitations included the accuracy of a computer model in predicting real-life situations and personal values shared by women having to make decisions about surgical vs nonsurgical options. The surveillance option used in this model included annual mammography, CBE, semiannual pelvic examinations, ultrasonography, and CA-125 studies. However, since 2006, the surveillance option has included annual breast MRI in conjunction with annual mammography in accordance with the American Cancer Society recommendations (Table 4).70
Psychological Considerations of Surgical Interventions
Although RRSO is associated with temporary surgical adverse effects, most women recover baseline functioning within 1 year.74 Time provided for counseling regarding menopausal health risks and use of hormone therapy is critical. Health care professionals may find it helpful to refer women considering RRSO to a breast specialist or gynecologic oncologist, or both, for a multidisciplinary assessment, given the health care movement toward personalized medicine.
A study by Frost et al82 reported that 74% of women had decreased emotional concern after risk-reduction surgery and favorable psychological and social outcome. Further, long-term satisfaction was greater when the patient, not the physician, initiated discussion of risk-reduction breast surgery.83 Another study reported no anxiety or depression and no negative effects on quality of life after surgery, but 48% of women reported feeling less sexually attractive.84
The most frequent reason for reoperation rates after risk-reduction mastectomy was concern over implants (eg, failure, aesthetic issues, silicone anxiety). Not surprisingly, reoperation was associated with less satisfaction about the decision to have risk-reduction mastectomy.83 The reoperation rate was greater after subcutaneous mastectomy and reconstruction than after simple mastectomy and reconstruction (43% vs 15%; P<.001).
Decision Tool for Assessing Surgical Strategies vs Surveillance
A computer simulation model to estimate survival probability and causes of death in women with BRCA mutations can be used to assist patients and health care professionals regarding management options.85 The model was designed by compiling available data from the medical literature for a 25-year-old woman with a BRCA1 or BRCA2 mutation and calculating survival probability and cause of death to age 70 and 80 years. The model assumes the following clinically relevant situations: (1) no intervention, (2) annual mammography plus MRI from ages 25 to 69 years without surgery, (3) screening plus prophylactic mastectomy at age 40 years, and (4) screening plus prophylactic RRSO at age 40 years. This decision analysis includes illustrative bar graphs and survival probability curves and tables depicting advantages associated with surgical vs nonsurgical options. Overall, the decision tool represents an important advance in guiding treatment choices, given that a randomized trial is unlikely to be designed that could compare survival benefit in this very-high-risk population. In summary, prophylactic RRSO at age 40 years, plus prophylactic mastectomy at age 25 years, was the most effective management strategy for BRCA1 carriers, resulting in substantial improvement in survival compared with no intervention (79% vs 53%). This improvement differed for BRCA2 carriers, in whom the absolute increase in survival was smaller (83% vs 71%). Interestingly, the option of mammography plus MRI screening offered a survival probability similar to that of prophylactic mastectomy in the presence of prophylactic RRSO at age 40 years.
CONCLUSION
Breast health specialists, genetic counselors, gynecologic oncologists, and primary health care physicians all have an important role in discussing risk-reduction strategies with women at very high risk of breast and ovarian cancer. Evaluating patient risk factors and obtaining a comprehensive family history are important steps in assessing breast and ovarian cancer risks. Genetic testing can identify individuals at very high risk for hereditary breast and ovarian cancer. Evidence is accumulating, and efficacy data are currently available for some, but not all, medical interventions for BRCA1 and BRCA2 mutation carriers. A coordinated team effort can provide a supportive environment and personalized approach for patients facing difficult surgical vs nonsurgical decisions related to management of hereditary breast and ovarian cancer. However, newer information on the risks associated with early RRSO may provide the impetus for development of models that predict individual risk on the basis of the assessment of multiple traits.
REFERENCES
- 1.Lindor NM, McMaster ML, Lindor CJ, Greene MH, National Cancer Institute. Division of Cancer Prevention. Community Oncology and Prevention Trials Research Group Concise handbook of familial cancer susceptibility syndromes: second edition. J Natl Cancer Inst Monogr. 2008;2008(38):1-93 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-249 [DOI] [PubMed] [Google Scholar]
- 3.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG The American Congress of Obstetricians and Gynecologists Web site. Washington, DC: American Congress of Obstetricians and Gynecologists ACOG; http://www.acog.org/from_home/proxy/ Accessed September 20, 2010 [Google Scholar]
- 5.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665-676 [DOI] [PubMed] [Google Scholar]
- 6.Jatoi I, Anderson WF. Management of women who have a genetic predisposition for breast cancer. Surg Clin North Am. 2008;88(4):845-861 [DOI] [PubMed] [Google Scholar]
- 7.Andrieu N, Goldgar DE, Easton DF, et al. EMBRACE. GENEPSO. GEO-HEBON. IBCCS Collaborators Group Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS). J Natl Cancer Inst. 2006;98(8):535-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies [published correction appears in Am J Hum Genet. 2003;73(3):709] Am J Hum Genet. 2003;72(5):1117-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou AC, Rookus M, Andrieu N, et al. EMBRACE. GENEPSO. GEOHEBON Reproductive and hormonal factors, and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: results from the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2009;18(2):601-610 [DOI] [PubMed] [Google Scholar]
- 10.Kotsopoulos J, Olopado OI, Ghadirian P, et al. Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005;7(5):R833-R843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebbeck TR, Wang Y, Kantoff PW, et al. Modification of BRCA1- and BRCA2-associated breast cancer risk by AIB1 genotype and reproductive history. Cancer Res. 2001;61(14):5420-5424 [PubMed] [Google Scholar]
- 12.Andrieu N, Smith T, Duffy S, et al. The effects of interaction between familial and reproductive factors on breast cancer risk: a combined analysis of seven case-control studies. Br J Cancer. 1998;77(9):1525-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin JR, Risch HA, Lubinski J, et al. Hereditary Ovarian Cancer Clinical Study Group Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet Oncol. 2007;8(1):26-34 [DOI] [PubMed] [Google Scholar]
- 14.Modugno F, Moslehi R, Ness RB, et al. Reproductive factors and ovarian cancer risk in Jewish BRCA1 and BRCA2 mutation carriers (United States). Cancer Causes Control. 2003;14(5):439-446 [DOI] [PubMed] [Google Scholar]
- 15.Passaperuma K, Warner E, Hill KA, Gunasekara A, Yaffe MJ. Is mammographic breast density a breast cancer risk factor in women with BRCA mutations? J Clin Oncol. 2010;28(23):3779-3783 [DOI] [PubMed] [Google Scholar]
- 16.Nkondjock A, Ghadirian P. Epidemiology of breast cancer among BRCA mutation carriers: an overview. Cancer Lett. 2004;205(1):1-8 [DOI] [PubMed] [Google Scholar]
- 17.King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRC1 and BRCA2. Science. 2003October24;302(5645):643-646 [DOI] [PubMed] [Google Scholar]
- 18.Nkondjock A, Robidoux A, Paredes Y, Narod SA, Ghadirian P. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res Treat. 2006;98(3):285-294 [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329-1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute Surveillance Research Web site. Where can I find statistics related to the Lifetime Risk of Developing or Dying of Cancer? Updated January22, 2010. http://surveillance.cancer.gov/statistics/types/lifetime_risk.html Accessed September 20, 2010
- 21.Robson M, Offit K. Clinical practice: management of an inherited predisposition to breast cancer. N Engl J Med. 2007;357(2):154-162 [DOI] [PubMed] [Google Scholar]
- 22.Thompson D, Easton DF, Breast Cancer Linkage Consortium Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358-1365 [DOI] [PubMed] [Google Scholar]
- 23.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886 [DOI] [PubMed] [Google Scholar]
- 24.Euhus DM, Leitch AM, Huth JF, Peters GN. Limitations of the Gail model in the specialized breast cancer risk assessment clinic. Breast J. 2002;8(1):23-27 [DOI] [PubMed] [Google Scholar]
- 25.Claus EB, Risch N, Thompson WD. The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat. 1993;28(2):115-120 [DOI] [PubMed] [Google Scholar]
- 26.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors [published correction appears in Stat Med. 2005;24(1):156] Stat Med. 2004;23(7):1111-1130 [DOI] [PubMed] [Google Scholar]
- 27.Cuzick J. IBIS Breast Cancer Risk Evaluation Tool Web site. Description of breast cancer risk program. Updated January31, 2008. http://ems-trials.org/riskevaluator/ Accessed September 20, 2010
- 28.Berry DA, Parmigiani G, Sanchez J, Schildkraut J, Winer E. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J Natl Cancer Inst. 1997;89(3):227-238 [DOI] [PubMed] [Google Scholar]
- 29.UT Southwestern Medical Center Requesting CancerGene. Dallas, TX: The University of Texas Southwestern Medical Center at Dallas; 2010. http://www8.utsouthwestern.edu/utsw/cda/dept47834/files/68221.html Accessed September 20, 2010 [Google Scholar]
- 30.US Preventive Services Task Force Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement [published correction appears in Ann Intern Med. 2005;143(7):547] Ann Intern Med. 2005;143(5):355-361 [DOI] [PubMed] [Google Scholar]
- 31.Menon U, Gentry-Maharaj A, Ryan A, et al. Recruitment to multicentre trials–lessons from UKCTOCS: descriptive study [published correction appears in BMJ. 2008;337:a2976] BMJ. 2008;337:a2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amir E, Evans DG, Shenton A, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003;40(11):807-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Society of Genetic Counselors. (nsgc) Web site National Society of Genetic Counselors, Inc; 1995-2009. http://www.nsgc.org/ Accessed September 20, 2010 [Google Scholar]
- 34.Genetic Information Nondiscrimination Act of 2008. Pub L 110-233, 122 Stat. 881 (May21, 2008).
- 35.Saslow D, Boetes C, Burke W, et al. American Cancer Society Breast Cancer Advisory Group American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography [published correction appears in CA Cancer J Clin. 2007;57(3):185] CA Cancer J Clin. 2007;57(2):75-89 [DOI] [PubMed] [Google Scholar]
- 36.Spiegel TN, Hill KA, Warner E. The attitudes of women with BRCA1 and BRCA2 mutations toward clinical breast examinations and breast self-examinations. J Womens Health (Larchmt). 2009;18(7):1019-1024 [DOI] [PubMed] [Google Scholar]
- 37.Pisano ED, Gatsonis C, Hendrick E, et al. Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group Diagnostic performance of digital versus film mammography for breast-cancer screening [published correction appears in N Engl J Med. 2006;355(17):1840] N Engl J Med. 2005;353(17):1773-1783 [DOI] [PubMed] [Google Scholar]
- 38.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5, part 1):347-360 [DOI] [PubMed] [Google Scholar]
- 39.Ronckers CM, Doody MM, Lonstein JE, Stovall M, Land CE. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(3):605-613 [DOI] [PubMed] [Google Scholar]
- 40.Berrington de Gonzalez A, Berg CD, Visvanathan K, Robson M. Estimated risk of radiation-induced breast cancer from mammographic screening for young BRCA mutation carriers. J Natl Cancer Inst. 2009;101(3):205-209 [DOI] [PubMed] [Google Scholar]
- 41.Warner E, Plewes DB, Shumak RS, et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol. 2001;19(15):3524-3531 [DOI] [PubMed] [Google Scholar]
- 42.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317-1325 [DOI] [PubMed] [Google Scholar]
- 43.Kriege M, Brekelmans CT, Boetes C, et al. Magnetic Resonance Imaging Screening Study Group Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427-437 [DOI] [PubMed] [Google Scholar]
- 44.Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol. 2009;27(36):6124-6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson HD, Huffman LH, Fu R, Harris EL, U.S. Preventive Services Task Force Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force [published correction appears in Ann Intern Med. 2005;143(7):547] Ann Intern Med. 2005;143(5):362-379 [DOI] [PubMed] [Google Scholar]
- 46.Komenaka IK, Ditkoff BA, Joseph KA, et al. The development of interval breast malignancies in patients with BRCA mutations. Cancer. 2004;100(10):2079-2083 [DOI] [PubMed] [Google Scholar]
- 47.National Comprehensive Cancer Network NCCN & Clinical Resources Web site. NCCN clinical practice guidelines in oncology (NCCN Guidelines). Genetic/familial high-risk assessment: breast and ovarian. Hereditary breast and/or ovarian cancer. Version 1.2010. 2010. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed August 21, 2010
- 48.Greene MH, Piedmonte M, Alberts D, et al. A prospective study of risk-reducing salpingo-oophorectomy and longitudinal CA-125 screening among women at increased genetic risk of ovarian cancer: design and baseline characteristics: a Gynecologic Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2008;17(3):594-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kauff ND, Barakat RR. Risk-reducing salpingo-oophorectomy in patients with germline mutations in BRCA1 or BRCA2. J Clin Oncol. 2007;25(20):2921-2927 [DOI] [PubMed] [Google Scholar]
- 50.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project (NSABP) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in 2006;296(24):2926. JAMA. 2007;298(9):973] JAMA. 2006;295(23):2727-2741 [DOI] [PubMed] [Google Scholar]
- 51.Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-715, W-226-35 [DOI] [PubMed] [Google Scholar]
- 52.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila). 2010;3(6):696-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388 [DOI] [PubMed] [Google Scholar]
- 54.Narod SA, Dube MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94(23):1773-1779 [DOI] [PubMed] [Google Scholar]
- 55.Bevers TB, Anderson BO, Bonaccio E, et al. National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060-1096 [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Brown PH. Strategies of hormonal prevention. In: Fuqua SAW, ed. Hormone Receptors in Breast Cancer. New York, NY: Springer; 2009:195-229 [Google Scholar]
- 57.Li Y, Brown PH. Translational approaches for the prevention of estrogen receptor-negative breast cancer. Eur J Cancer Prev. 2007;16(3):203-215 [DOI] [PubMed] [Google Scholar]
- 58.Pichert G, Bolliger B, Buser K, Pagani O, Swiss Institute for Applied Cancer Research Network for Cancer Predisposition Testing and Counseling Evidence-based management options for women at increased breast/ovarian cancer risk. Ann Oncol. 2003;14(1):9-19 [DOI] [PubMed] [Google Scholar]
- 59.Narod SA, Risch H, Moslehi R, et al. Hereditary Ovarian Cancer Clinical Study Group Oral contraceptives and the risk of hereditary ovarian cancer. N Engl J Med. 1998;339(7):424-428 [DOI] [PubMed] [Google Scholar]
- 60.Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14(2):350-356 [DOI] [PubMed] [Google Scholar]
- 61.Haile RW, Thomas DC, McGuire V, et al. kConFab Investigators. Ontario Cancer Genetics Network Investigators BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1863-1870 [DOI] [PubMed] [Google Scholar]
- 62.NIH consensus conference. NIH Consensus Development Panel on Ovarian Cancer Ovarian cancer: screening, treatment, and follow-up. JAMA. 1995;273(6):491-497 [PubMed] [Google Scholar]
- 63.Berliner JL, Fay AM, Practice Issues Subcommittee of the National Society of Genetic Counselors' Familial Cancer Risk Counseling Special Interest Group Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;16(3):241-260 [DOI] [PubMed] [Google Scholar]
- 64.Hollingsworth AB, Singletary SE, Morrow M, et al. Current comprehensive assessment and management of women at increased risk for breast cancer. Am J Surg. 2004;187(3):349-362 [DOI] [PubMed] [Google Scholar]
- 65.Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22(6):1055-1062 [DOI] [PubMed] [Google Scholar]
- 66.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331-1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grann VR, Jacobson JS, Thomason D, Hershman D, Heitjan DF, Neugut AI. Effect of prevention strategies on survival and quality-adjusted survival of women with BRCA1/2 mutations: an updated decision analysis. J Clin Oncol. 2002;20(10):2520-2529 [DOI] [PubMed] [Google Scholar]
- 70.Anderson K, Jacobson JS, Heitjan DF, et al. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann Intern Med. 2006;144(6):397-406 [DOI] [PubMed] [Google Scholar]
- 71.Levine DA, Argenta PA, Yee CJ, et al. Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol. 2003;21(22):4222-4227 [DOI] [PubMed] [Google Scholar]
- 72.Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25(10):1283-1289 [DOI] [PubMed] [Google Scholar]
- 73.Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingo-oophorectomy and prophylactic mastectomy. Am J Obstet Gynecol. 2004;191(4):1113-1123 [DOI] [PubMed] [Google Scholar]
- 74.Fang CY, Cherry C, Devarajan K, Li T, Malick J, Daly MB. A prospective study of quality of life among women undergoing risk-reducing salpingo-oophorectomy versus gynecologic screening for ovarian cancer. Gynecol Oncol. 2009;112(3):594-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rebbeck TR, Friebel T, Wagner T, et al. PROSE Study Group Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804-7810 [DOI] [PubMed] [Google Scholar]
- 76.Michelsen TM, Pripp AH, Tonstad S, Trope CG, Dorum A. Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: a controlled observational study. Eur J Cancer. 2009;45(1):82-89 [DOI] [PubMed] [Google Scholar]
- 77.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074-1083 [DOI] [PubMed] [Google Scholar]
- 78.Eisen A, Lubinski J, Gronwald J, et al. Hereditary Breast Cancer Clinical Study Group Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100(19):1361-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;2005(34):83-86 [DOI] [PubMed] [Google Scholar]
- 80.Narod SA, Sun P, Ghadirian P, et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet. 2001;357(9267):1467-1470 [DOI] [PubMed] [Google Scholar]
- 81.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frost MH, Schaid DJ, Sellers TA, et al. Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA. 2000;284(3):319-324 [DOI] [PubMed] [Google Scholar]
- 83.Frost MH, Slezak JM, Tran NV, et al. Satisfaction after contralateral prophylactic mastectomy: the significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol. 2005;23(31):7849-7856 [DOI] [PubMed] [Google Scholar]
- 84.Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol. 2008;26(24):3943-3949 [DOI] [PubMed] [Google Scholar]
- 85.Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28(2):222-231 [DOI] [PMC free article] [PubMed] [Google Scholar]