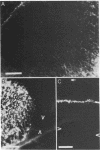
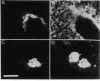
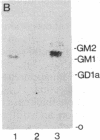
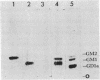
Abstract
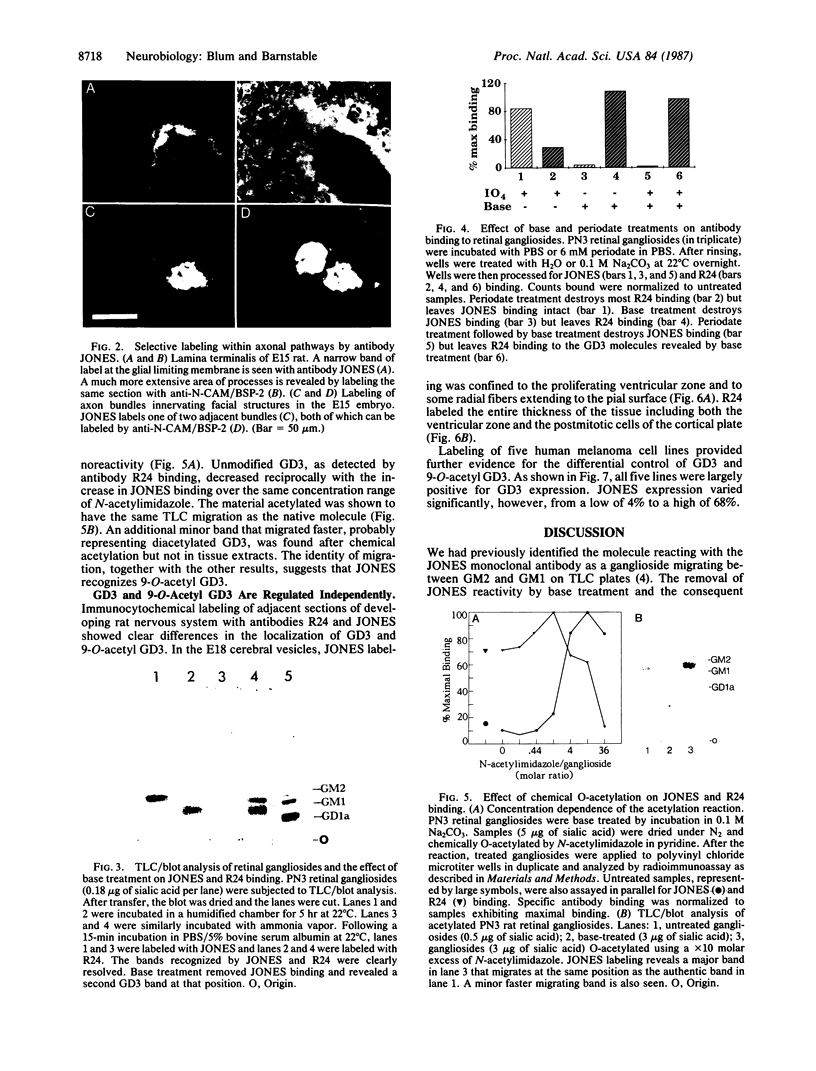
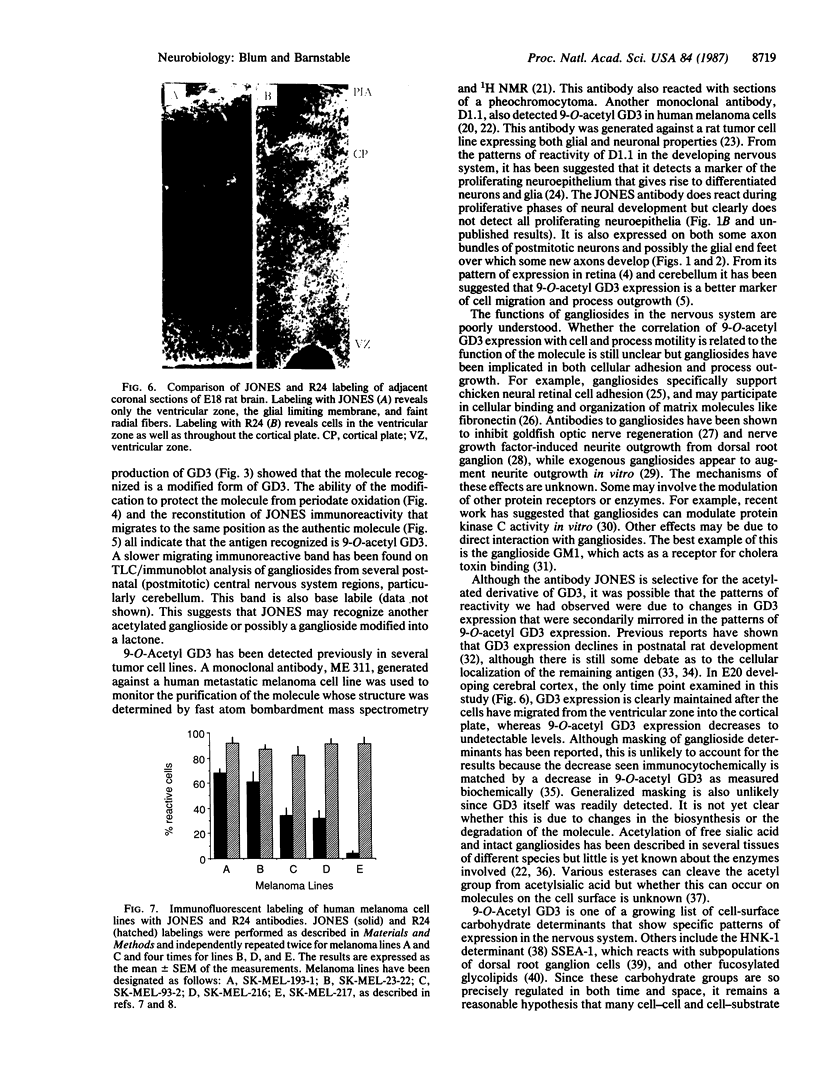
The cell-surface antigen detected by the monoclonal antibody JONES is expressed in the retina and a number of other central nervous system regions of the rat during the latter part of embryonic development and the early postnatal period. In addition to the expression on certain neuroblast populations it is found on some but not all axons and is also expressed at high levels on the end feet of radial glia in regions through which axons actively grow. In the perinatal rat retina, almost all the antigenic activity was carried on a ganglioside migrating between GM1 and GM2. The epitope recognized by antibody JONES was base labile and treatment with 0.1 M sodium carbonate or ammonia vapor converted the antigen into GD3. Resistance to oxidation by sodium periodate and reformation of the epitope by chemical acetylation of base-treated gangliosides with N-acetylimidazole identify the antigen as 9-O-acetyl GD3. The acetylation of GD3 seems to be regulated independently from GD3 expression itself since acetylated and nonacetylated GD3 do not have identical immunocytochemical distributions in the developing central nervous system. In addition, five independent human melanoma cell lines varied substantially in their expression of 9-O-acetyl GD3, even though they all expressed high levels of GD3. Acetylation of ganglioside-linked sialic acid provides a mechanism for generating unique patterns of surface carbohydrates, which may influence cell interactions in development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Lloyd K. O., Houghton A. N., Oettgen H. F., Old L. J. Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. J Exp Med. 1981 Dec 1;154(6):1764–1778. doi: 10.1084/jem.154.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimatsu Y., Naegele J. R., Barnstable C. J. Molecular markers of neuronal subpopulations in layers 4, 5, and 6 of cat primary visual cortex. J Neurosci. 1987 Apr;7(4):1250–1263. doi: 10.1523/JNEUROSCI.07-04-01250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera A. J., Marchase R. B., Roth S. Adhesive recognition and retinotectal specificity. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2482–2486. doi: 10.1073/pnas.70.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Dräger U. C. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984 Apr;11(4):847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Blackburn C. C., Swank-Hill P., Schnaar R. L. Gangliosides support neural retina cell adhesion. J Biol Chem. 1986 Feb 25;261(6):2873–2881. [PubMed] [Google Scholar]
- Bonhoeffer F., Huf J. In vitro experiments on axon guidance demonstrating an anterior-posterior gradient on the tectum. EMBO J. 1982;1(4):427–431. doi: 10.1002/j.1460-2075.1982.tb01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Reisfeld R. A., Varki A. P. O-acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984 Aug 24;225(4664):844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Varki A. P., Varki N. M., Stallcup W. B., Levine J., Reisfeld R. A. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984 Jun 25;259(12):7453–7459. [PubMed] [Google Scholar]
- Constantine-Paton M., Blum A. S., Mendez-Otero R., Barnstable C. J. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature. 1986 Dec 4;324(6096):459–462. doi: 10.1038/324459a0. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Solter D., Jessell T. M. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature. 1984 Oct 4;311(5985):469–472. doi: 10.1038/311469a0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fishman P. H. Recent advances in identifying the functions of gangliosides. Chem Phys Lipids. 1986 Dec 15;42(1-3):137–151. doi: 10.1016/0009-3084(86)90049-6. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Geier S. S., Hirano M. Differentiation of astrocytes and oligodendrocytes from germinal matrix cells in primary culture. J Neurosci. 1986 Jan;6(1):52–60. doi: 10.1523/JNEUROSCI.06-01-00052.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Hirano M., Yu R. K., Seyfried T. N. GD3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J Neuroimmunol. 1984 Dec;7(2-3):179–192. doi: 10.1016/s0165-5728(84)80017-x. [DOI] [PubMed] [Google Scholar]
- Gozes I., Barnstable C. J. Monoclonal antibodies that recognize discrete forms of tubulin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2579–2583. doi: 10.1073/pnas.79.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F., Cordon-Cardo C., Houghton A. N., Melamed M. R., Old L. J. Distribution of the ganglioside GD3 in the human nervous system detected by R24 mouse monoclonal antibody. Brain Res. 1984 Dec 17;324(1):190–194. doi: 10.1016/0006-8993(84)90642-5. [DOI] [PubMed] [Google Scholar]
- Haverkamp J., Schauer R., Wember M., Kamerling J. P., Vliegenthart J. F. Synthesis of 9-O-acetyl- and 4,9-di-O-acetyl derivatives of the methyl ester of N-acetyl-beta-D-neuraminic acid methylglycoside. Their use as models in periodate oxidation studies. Hoppe Seylers Z Physiol Chem. 1975 Oct;356(10):1575–1583. doi: 10.1515/bchm2.1975.356.2.1575. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R., Skaper S. D., Varon S. Interaction of GM1 ganglioside with PC12 pheochromocytoma cells: serum- and NGF-dependent effects on neuritic growth (and proliferation). J Neurosci Res. 1984;12(2-3):299–310. doi: 10.1002/jnr.490120217. [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Goldenring J. R., DeLorenzo R. J., Yu R. K. Gangliosides inhibit phospholipid-sensitive Ca2+-dependent kinase phosphorylation of rat myelin basic proteins. J Neurosci Res. 1986;15(2):159–166. doi: 10.1002/jnr.490150205. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Levine J. M., Beasley L., Stallcup W. B. Localization of a neurectoderm-associated cell surface antigen in the developing and adult rat. Brain Res. 1986 Jun;392(1-2):211–222. doi: 10.1016/0165-3806(86)90247-6. [DOI] [PubMed] [Google Scholar]
- Levine J. M., Beasley L., Stallcup W. B. The D1.1 antigen: a cell surface marker for germinal cells of the central nervous system. J Neurosci. 1984 Mar;4(3):820–831. doi: 10.1523/JNEUROSCI.04-03-00820.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchase R. B. Biochemical investigations of retinotectal adhesive specificity. J Cell Biol. 1977 Oct;75(1):237–257. doi: 10.1083/jcb.75.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry R. C., Riopelle R. J., Roder J. C. Accelerated regenerative neurite formation by a neuronal surface epitope reactive with the monoclonal antibody, Leu 7. Neurosci Lett. 1985 May 14;56(2):95–100. doi: 10.1016/0304-3940(85)90113-2. [DOI] [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Spirman N. Sprouting from chicken embryo dorsal root ganglia induced by nerve growth factor is specifically inhibited by affinity-purified antiganglioside antibodies. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6080–6083. doi: 10.1073/pnas.79.19.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnino S., Ghidoni R., Chigorno V., Masserini M., Tettamanti G. Recognition by two-dimensional thin-layer chromatography and densitometric quantification of alkali-labile gangliosides from the brain of different animals. Anal Biochem. 1983 Jan;128(1):104–114. doi: 10.1016/0003-2697(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Sparrow J. R., McGuinness C., Schwartz M., Grafstein B. Antibodies to gangliosides inhibit goldfish optic nerve regeneration in vivo. J Neurosci Res. 1984;12(2-3):233–243. doi: 10.1002/jnr.490120211. [DOI] [PubMed] [Google Scholar]
- Suzuki K. The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem. 1965 Jul;12(7):629–638. doi: 10.1111/j.1471-4159.1965.tb04256.x. [DOI] [PubMed] [Google Scholar]
- Thurin J., Herlyn M., Hindsgaul O., Strömberg N., Karlsson K. A., Elder D., Steplewski Z., Koprowski H. Proton NMR and fast-atom bombardment mass spectrometry analysis of the melanoma-associated ganglioside 9-O-acetyl-GD3. J Biol Chem. 1985 Nov 25;260(27):14556–14563. [PubMed] [Google Scholar]
- Towbin H., Schoenenberger C., Ball R., Braun D. G., Rosenfelder G. Glycosphingolipid-blotting: an immunological detection procedure after separation by thin layer chromatography. J Immunol Methods. 1984 Sep 4;72(2):471–479. doi: 10.1016/0022-1759(84)90015-2. [DOI] [PubMed] [Google Scholar]
- Varki A., Muchmore E., Diaz S. A sialic acid-specific O-acetylesterase in human erythrocytes: possible identity with esterase D, the genetic marker of retinoblastomas and Wilson disease. Proc Natl Acad Sci U S A. 1986 Feb;83(4):882–886. doi: 10.1073/pnas.83.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Boyer A. M., Schwarting G. A. Fucose-containing glycolipids are stage- and region-specific antigens in developing embryonic brain of rodents. Proc Natl Acad Sci U S A. 1985 May;82(9):3045–3049. doi: 10.1073/pnas.82.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]