Abstract
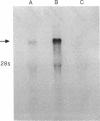
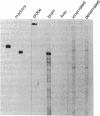
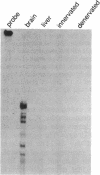
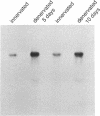
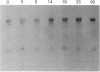
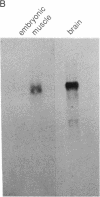
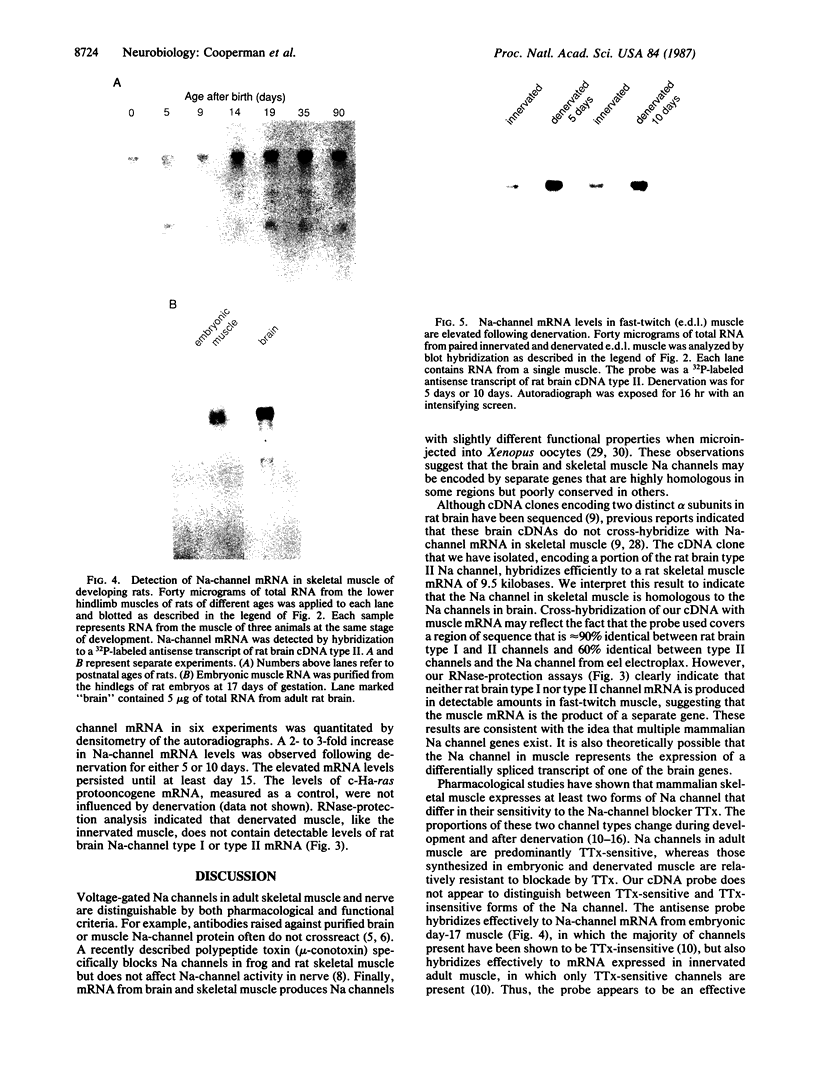
Action potentials in many types of excitable cells result from changes in permeability to Na ions. Although these permeability changes in nerve and muscle are mediated by voltage-gated Na channels that are functionally similar, we found that the Na-channel gene expressed in skeletal muscle is different from the genes coding for two Na channels (type I and type II) in brain. Despite the structural differences between muscle and brain Na-channel genes, a cDNA clone derived from rat brain hybridizes to skeletal muscle Na-channel mRNA of approximately 9.5 kilobases. We used this cDNA probe to measure changes in Na-channel mRNA levels in skeletal muscle during development and following denervation. By blot hybridization analysis of electrophoretically fractionated RNA, we found that Na-channel mRNA can be detected as early as embryonic day 17 and that mRNA levels increase 2-fold between birth and postnatal day 35. Denervation of adult muscle causes a further 2- to 3-fold increase in muscle Na-channel mRNA levels, suggesting that expression of Na-channel genes in fast-twitch muscle may be regulated by the state of innervation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei J. M., Barchi R. L. Monoclonal antibodies against the voltage-sensitive sodium channel from rat skeletal muscle: mapping antibody binding sites. J Neurochem. 1987 Mar;48(3):773–778. doi: 10.1111/j.1471-4159.1987.tb05584.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. Na+ channels with high and low affinity tetrodotoxin binding sites in the mammalian skeletal muscle cell. Difference in functional properties and sequential appearance during rat skeletal myogenesis. J Biol Chem. 1983 Jun 25;258(12):7256–7259. [PubMed] [Google Scholar]
- Goldin A. L., Snutch T., Lübbert H., Dowsett A., Marshall J., Auld V., Downey W., Fritz L. C., Lester H. A., Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Boulter J., Heinemann S., Patrick J. Muscle denervation increases the levels of two mRNAs coding for the acetylcholine receptor alpha-subunit. J Neurosci. 1985 Sep;5(9):2553–2558. doi: 10.1523/JNEUROSCI.05-09-02553.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B., Schotland D. L., Fieles W. E., Barchi R. L. Localization of sodium channel subtypes in adult rat skeletal muscle using channel-specific monoclonal antibodies. J Neurosci. 1987 Sep;7(9):2957–2966. doi: 10.1523/JNEUROSCI.07-09-02957.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Marshall M. W. Tetrodotoxin-resistant action potentials in newborn rat muscle. Nat New Biol. 1973 Jun 6;243(127):191–192. doi: 10.1038/newbio243191a0. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Studies on tetrodotoxin resistant action potentials in denervated skeletal muscle. Acta Physiol Scand. 1971 Nov;83(3):382–388. doi: 10.1111/j.1748-1716.1971.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Jaimovich E., Chicheportiche R., Lombet A., Lazdunski M., Ildefonse M., Rougier O. Differences in the properties of Na+ channels in muscle surface and T-tubular membranes revealed by tetrodotoxin derivatives. Pflugers Arch. 1983 Apr;397(1):1–5. doi: 10.1007/BF00585159. [DOI] [PubMed] [Google Scholar]
- Kraner S. D., Tanaka J. C., Barchi R. L. Purification and functional reconstitution of the voltage-sensitive sodium channel from rabbit T-tubular membranes. J Biol Chem. 1985 May 25;260(10):6341–6347. [PubMed] [Google Scholar]
- Lawrence J. C., Catterall W. A. Tetrodotoxin-insensitive sodium channels. Ion flux studies of neurotoxin action in a clonal rat muscle cell line. J Biol Chem. 1981 Jun 25;256(12):6213–6222. [PubMed] [Google Scholar]
- Lechan R. M., Wu P., Jackson I. M., Wolf H., Cooperman S., Mandel G., Goodman R. H. Thyrotropin-releasing hormone precursor: characterization in rat brain. Science. 1986 Jan 10;231(4734):159–161. doi: 10.1126/science.3079917. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Olivera B. M., Gray W. R., Strichartz G. R. Discrimination of muscle and neuronal Na-channel subtypes by binding competition between [3H]saxitoxin and mu-conotoxins. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5321–5325. doi: 10.1073/pnas.83.14.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Sherman S. J., Lawrence J. C., Messner D. J., Jacoby K., Catterall W. A. Tetrodotoxin-sensitive sodium channels in rat muscle cells developing in vitro. J Biol Chem. 1983 Feb 25;258(4):2488–2495. [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]
- Wollner D. A., Catterall W. A. Antigenic differences among the voltage-sensitive sodium channels in the peripheral and central nervous systems and skeletal muscle. Brain Res. 1985 Apr 1;331(1):145–149. doi: 10.1016/0006-8993(85)90724-3. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]