Summary
The effect of the ketogenic diet on behavior and cognition is unclear. We addressed this issue in rats behaviorally and electrophysiologically. We fed postnatal day 21 rats a standard (SD), ketogenic (KD), or calorie-restricted (CR) diet for 2–3 weeks. CR controlled for the slower weight gain experienced by KD fed rats. We assessed behavioral performance with a locomotor activity and a conditioned fear test. To evaluate possible parallel effects of diet on synaptic function, we examined paired-pulse modulation (PPM) and long-term potentiation (LTP) in the medial perforant path in vivo. KD fed rats performed similarly to SD fed rats on the behavioral tests and electrophysiological assays. These data suggest that the KD does not alter behavioral performance or synaptic plasticity.
Keywords: Calorie-restricted, conditioned fear test, locomotor activity, medial perforant path, paired-pulse modulation
The KD effectively treats medically refractory epilepsy, but its neuropsychological effects are unclear. It may improve neuropsychological function in children (Pulsifer et al., 2001), though a study in adults fed a KD for weight loss found a detrimental effect (Wing et al., 1995). The cognitive effects of KDs in animal studies have also been variable (Hori et al., 1997; Todorova et al., 2000; Zhao et al., 2004). Similarly, KDs have had variable effects on rat activity levels (Hori et al., 1997; Ziegler et al., 2005; Murphy et al., 2005). Another approach to determine whether KDs alter cognitive function is to examine their effect on measures of synaptic plasticity such as LTP, a physiological correlate to learning and memory. Here we examine the behavioral performance of KD fed rats along with short-term and long-term plasticity in the medial perforant path in vivo.
Methods
Standard, published methods were used (Supplementary Methods).
Results
KD fed rats show slower weight gain
Juvenile rats fed a KD had slower weight gain and higher serum β-hydroxybutyrate (βHB) concentrations than their littermates fed a SD (Fig. S1A–B). CR rats weight-matched to the KD fed rats did not have high serum βHB levels (Fig. S1B). These findings confirmed our previous findings (Thio et al., 2006), so CR rats served as a control for the effects of the KD on weight.
KD fed rats do not show altered behavior
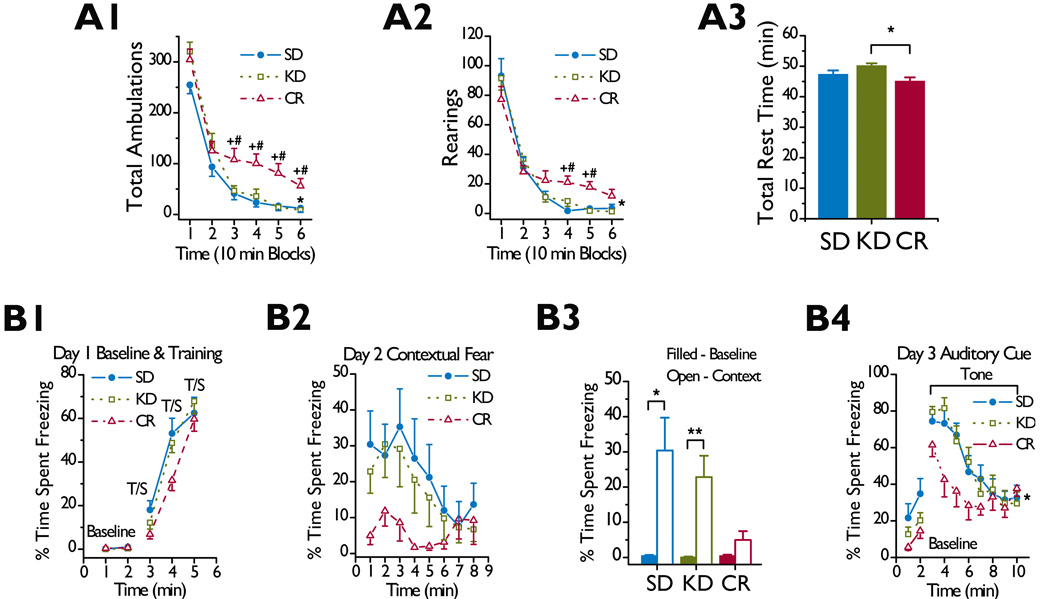
The KD and SD fed rats performed similarly on the total ambulations and rearing variables from the 1 h locomotor activity test (Fig. 1A1–1A2). In contrast, CR rats exhibited significant hyperactivity relative to the other two groups on these variables (Fig. 1A1–1A2). Accordingly, a repeated measures ANOVA on the total ambulation data showed a significant overall effect of diet [F(2,40) = 6.32, p = 0.004] and time [F(5,200) = 205.78, p < 0.0005], and diet by time interaction [F(10,200) = 2.92, p = 0.006]. Subsequent pairwise comparisons showed that CR rats had more total ambulations than KD (p < 0.008) and SD (p < 0.0005) fed rats during the last four, 10-minute blocks of testing, while the KD and SD groups did not differ. The rearing data showed a significant effect of time [F(5,200) = 126.83, p < 0.0005] and diet by time interaction [F(10,200) = 2.46, p = 0.035]. The CR group showed greater rearing during time blocks 4 and 5 compared to the KD (p < 0.009) and SD (p < 0.0005) groups, but the KD and SD groups did not differ. We also examined the time spent at rest to determine whether the heightened levels of activity and exploration exhibited by the CR rats resulted from faster movements, the amount of time spent moving, or both. A one-way ANOVA on the time spent at rest over the 60 min test session [F(2,40) = 5.82, p = 0.006] and subsequent pairwise comparisons indicated that CR rats spent less time at rest than the KD fed rats (p = 0.002) but not the SD fed rats, while the latter two groups did not differ (Fig. 1A3).
Figure 1.
KD fed rats do not show altered locomotor behavior or performance on the conditioned fear test. (A) KD did not alter general activity levels during a 1 h locomotor activity test. (A1) Mean number of ambulations during six, 10 min intervals in SD fed (n = 14), KD fed (n = 15), and CR (n = 14) rats. *p < 0.007 for overall effects of diet and time and a diet by time interaction. +p < 0.008 for CR versus KD. #p < 0.0005 for CR versus SD. (A2) Mean number of rearings during six, 10 min intervals. *p < 0.05 for overall effects of time and a diet by time interaction. +p < 0.009 for CR versus KD. #p < 0.0005 for CR versus SD. (A3) Mean time at rest during a 1 h test session. *p = 0.002. (B) KD did not impair conditioned fear using contextual and auditory cues. (B1) Mean percent time spent freezing at baseline and during training with tone-shock (T/S) pairings on Day 1. (B2) Mean percent time spent freezing during contextual fear testing on Day 2. (B3) Contextual fear conditioning occurred in KD and SD fed but not CR rats. Freezing during the first minute of the contextual fear test on Day 2 (Context) compared to the level averaged across the 2 min baseline period on Day 1 (Baseline). *p = 0.007. **p = 0.002. (B4) Mean percent time spent freezing during auditory cue testing on Day 3. *p < 0.05 for overall effects of time and a diet by time interaction. All data in this figure are from the same rats, and the numbers of rats on each diet are the same throughout.
Two days after completing the locomotor activity testing, we assessed performance on a conditioned fear test. In general, the behavioral measures from the conditioned fear test were similar in KD and SD fed rats, though the contextual fear and auditory cue findings from the CR rats suggested poor performance. Diet had no effect on freezing during baseline measures or training with tone-shock (T/S) pairings on Day 1 (Fig. 1B1).
Although the CR rats froze less than the KD or SD fed rats during the contextual fear test, the latter two groups showed a large degree of variability, and no overall effects involving diet were found by a two-way repeated measures ANOVA (Fig. 1B2). However, differences emerged when we assessed the likelihood that conditioning occurred in each group. Specifically, a repeated measures ANOVA conducted within each group indicated that the KD [F(1,14) = 14.05, p = 0.002] and SD [F(1,13) = 10.30, p = 0.007] fed rats showed large increases in the percent time spent freezing during the first minute of the contextual fear test on Day 2 compared to levels averaged across the 2 min baseline period on Day 1 (Fig. 1B3). In comparison, the CR rats did not show significant changes. Thus, the KD and SD fed groups showed evidence of robust contextual fear conditioning while the CR fed rats either did not condition to the contextual cues or did not retain such conditioning over 24 hours.
We found no significant overall effects involving diet by an ANOVA on the freezing data from the “altered context” baseline period on Day 3 (Fig. 1B4). During the auditory cue phase, however, a two-way repeated measures ANOVA yielded a significant effect of time [F(7,280) = 23.00, p < 0.0005] and diet by time interaction [F(14,280) = 2.13, p = 0.032] but only a marginally nonsignificant effect of diet (p = 0.053). Subsequent contrasts revealed that the KD and SD fed rats performed similarly during the 8 min test session. By comparison, the CR rats showed substantially but not significantly less (Bonferroni corrected level is p < 0.016) freezing on average across the 8 min test session than the SD (p = 0.040) or KD (p = 0.030) fed rats.
A MANOVA of the shock sensitivity data indicated that, on average, diet did not influence the level of shock required to elicit behaviors such as flinching, running, vocalizing, or jumping.
KD fed rats do not show altered in vivo synaptic transmission
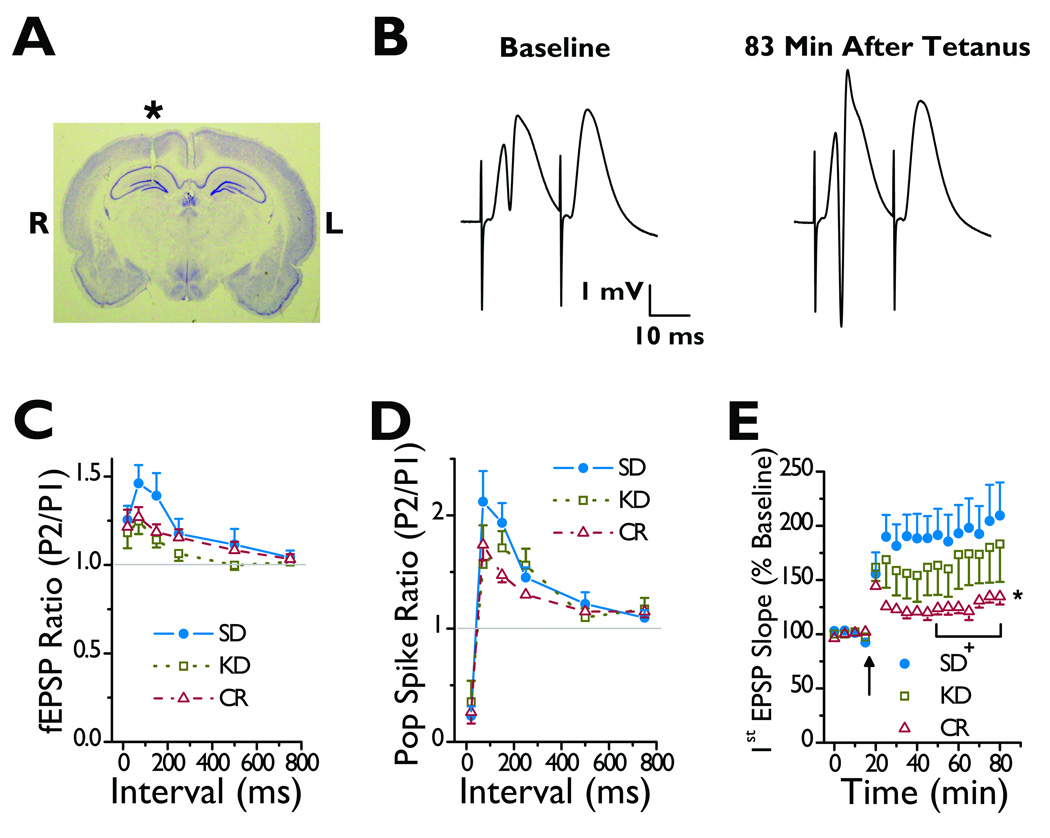
To assess the effect of the KD on short-term plasticity, we compared paired-pulse modulation in the medial perforant path in SD fed, KD fed, and CR rats (Fig. 2A and 2B). Field excitatory postsynaptic potentials (fEPSPs) showed paired pulse potentiation, which did not differ among the diets (Fig. 2C). This finding suggests that neither the KD nor CR altered short-term plasticity. Similarly, rats fed all three diets showed the same paired-pulse modulation of the population spike (PS) (Fig. 2D). This result suggests that neither the KD nor CR altered γ-aminobutyric acidA receptor (GABAAR) mediated inhibition within the dentate gyrus (Bough et al., 2003).
Figure 2.
KD fed rats do not show altered in vivo medial perforant path short-term and long-term synaptic plasticity. (A) Nissl stain of a coronal slice showing the track of the electrode (*) used to record fEPSPs from the dentate gyrus (R, right; L, left). Track of stimulating electrode is not visible in this slice. (B) Average of five paired fEPSPs before and 83 minutes after applying a 1 s 100 Hz tetanus to the right medial perforant path. The first fEPSP and PS of the pair are larger after the tetanus, and the second fEPSP did not elicit a PS. (C) Diet did not alter paired-pulse modulation of the fEPSP. Mean ratio of the slope of the second fEPSP to that of the first fEPSP at various interstimulus intervals in rats fed a SD (n = 7), a KD (n = 7), or a CR diet (n = 7). (D) Diet did not alter paired-pulse modulation of the PS. Mean ratio of the amplitude of the second PS to that of the first PS at various interstimulus intervals. Traces analyzed in C and D were the same and were recorded before the tetanus. (E) CR rats but not KD fed rats exhibited reduced in vivo medial perforant path LTP. Mean slope of the first fEPSP of a pair of fEPSPs before and after a 1 s 100 Hz tetanus (arrow). *p = 0.049 for an overall effect of diet over time. +p = 0.02 for CR versus SD fed rats. Pairs of fEPSPs were evoked at a 20 ms interval. Data in C–E are from the same rats, and the numbers of rats are the same in C–E.
We used medial perforant path LTP to determine the effect of diet on long-term synaptic plasticity (Fig. 2B). LTP, as assessed by pairs of fEPSPs elicited before and after the tetanus, was similar in KD and SD fed rats. A two-way repeated measures ANOVA showed no significant interaction between diet and time on the first [F(12,18) = 1.06, p = 0.45] or second fEPSP slope [F(12,18) = 1.24, p = 0.33] or PS amplitude [F(12,18) = 1.02, p = 0.47] during the last 30 minutes of recording (Fig. 2E and S2). Therefore, we used a two-way repeated measures ANOVA model without interaction to compare the diets. The slopes of the first fEPSP showed a significant main effect of diet over time (p = 0.049) (Fig. 2E). Further pairwise comparisons revealed that the least square mean for the CR rats was smaller than that for SD fed rats (p = 0.02), whereas the KD fed rats did not differ from SD (p = 0.13) fed or CR (p = 0.30) rats. However, a similar analysis showed no significant main effect of diet on PS amplitude (p = 0.30) (Fig. S2A) or second EPSP slope (p = 0.15) (Fig. S2B). This analysis indicates that diet did not significantly alter these parameters, though CR rats tended to show the smallest potentiation. Thus, the KD did not alter, though CR might reduce LTP in the medial perforant path.
Discussion
Our results suggest that the KD does not impair certain behavioral and cognitive processes in seizure naïve rats. We found that the KD did not alter locomotor activity as Hori et al. (1997) did, though others have observed a decrease (Murphy et al., 2005) or a transient increase (Zhao et al., 2004; Ziegler et al., 2005) in locomotor activity in seizure naïve animals. Diet composition, rat strain, rat age, and time on the diet do not appear to account for the different results. We found that the KD did not impair contextual fear conditioning, consistent with its lack of effect on visuospatial learning and memory in seizure naïve animals (Hori et al., 1997; Todorova et al., 2000). In contrast, a study similar to ours found that the KD impaired performance on the Morris water maze test (Zhao et al., 2004). One explanation for this difference is that performance on the water maze and contextual fear conditioning tasks involve different types of cognitive processing (instrumental learning and Pavlovian conditioning, respectively). Thus, experimental treatments can differentially affect these two types of learning as Shuman et al. (2009) observed. Together, our results and those of earlier studies suggest that the KD may impair cognitive function in a domain specific manner rather than globally.
The variable effects of the KD on cognitive function in rats led us to examine the effect of the KD on short-term and long-term synaptic plasticity. We found that the KD did not alter in vivo paired-pulse modulation of the fEPSP or PS in the medial perforant path of seizure naïve rats in accordance with results from hippocampal slices (Stafstrom et al., 1999). The relative preservation of paired-pulse modulation and LTP in the medial perforant path in KD fed rats is consistent with the lack of behavioral performance deficits we observed.
In contrast, CR rats showed increased activity, evidence of impaired contextual fear conditioning, and possibly weaker medial perforant path LTP compared to SD fed rats. Thus, CR may have adverse effects on cognition in the developing animal at least when associated with slower growth. The results raise the possibility that the KD may protect against the adverse cognitive and synaptic effects associated with impaired growth in young animals. More importantly, our results provide no evidence for the KD having deleterious effects on cognition in young animals.
Supplementary Material
Acknowledgement
Support for this work came from NIH grants NS32636, NS42774, DK20579, NS58597, and a Neuroscience Blueprint Interdisciplinary Center Core Grant P30 NS057105 to Washington University; Juvenile Diabetes Research Foundation grant 1-2004-594; and the Diabetes Research Training Center at Washington University. The work cited in this publication was performed in a facility supported by NCRR grant C06 RR015502. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
References
- Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminishneuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- Hori A, Tandon P, Holmes GL, Stafstrom CE. Ketogenic diet: effects on expression of kindled seizures and behavior in adult rats. Epilepsia. 1997;38:750–758. doi: 10.1111/j.1528-1157.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Murphy P, Likhodii SS, Hatamian M, McIntyre BW. Effect of the ketogenic diet on the activity level of Wistar rats. Pediatr Res. 2005;57:353–357. doi: 10.1203/01.PDR.0000150804.18038.79. [DOI] [PubMed] [Google Scholar]
- Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM. Effects of ketogenic diet on development and behavior: preliminary report of a prospective study. Dev Med Child Neurol. 2001;43:301–306. doi: 10.1017/s0012162201000573. [DOI] [PubMed] [Google Scholar]
- Shuman T, Wood SC, Anagnostaras SG. Modafinil and memory: effects of modafinil on Morris water maze learning and Pavlovian fear conditioning. Behav Neurosci. 2009;123:257–266. doi: 10.1037/a0014366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Wang C, Jensen FE. Electrophysiological observations in hippocampal slices from rats treated with the ketogenic diet. Dev Neurosci. 1999;21:393–399. doi: 10.1159/000017389. [DOI] [PubMed] [Google Scholar]
- Thio LL, Erbayat-Altay E, Rensing N, Yamada KA. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatr Res. 2006;60:413–417. doi: 10.1203/01.pdr.0000238244.54610.27. [DOI] [PubMed] [Google Scholar]
- Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–940. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Wing RR, Vazquez JA, Ryan CM. Cognitive effects of ketogenic weight-reducing diets. Int J Obes Relat Metab Disord. 1995;19:811–816. [PubMed] [Google Scholar]
- Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Gamaro GD, Araujo E, Bassani MG, Perry ML, Dalmaz C, Goncalves CA. Nociception and locomotor activity are increased in ketogenic diet fed rats. Physiol Behav. 2005;84:421–427. doi: 10.1016/j.physbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.