Abstract
Senile plaques are a major pathological hallmark of Alzheimer’s Disease (AD). Compelling evidence suggests that senile plaques lead to structural alterations of neuronal processes and that local toxicity may be mediated by increased oxidative stress. Anti-oxidant therapy can alleviate the neuronal abnormalities in APP mice, but the time-course of this beneficial effect is unknown. We used multiphoton microscopy to assess in vivo the characteristics of antioxidant treatment on senile plaques and neurites in AD model mice (APPswe/PS1dE9). We observed that α-phenyl-N-tert-butyl nitrone (PBN), Ginkgo biloba extract (EGb 761) and Trolox had no effect on the size of existing senile plaques. However, all anti-oxidants had a straightening effect on curved neurites. This effect was detected as soon as 4 days after commencing the treatment, and was maintained after 1 month of daily treatment, with no further increase in the effect. The straightening of neurites persisted 15 days after stopping the treatment. These data indicate that neuronal plasticity is fast and still active in adult animals, and suggest that amelioration of the neuritic distortions associated with senile plaques with antioxidants is both rapid and long lasting.
Keywords: Alzheimer’s Disease, oxidative stress, α-phenyl-N-tert-butyl nitrone (PBN), Ginkgo biloba extract (EGb 761), Trolox, multiphoton microscopy (MPM), neurite
1. INTRODUCTION
Alzheimer’s disease (AD) is the most common cause of dementia among elderly people, characterized by memory loss, cognitive impairment and behavioral deterioration. Although the ultimate neurotoxic mechanisms have not been completely elucidated, strong evidence suggests that senile plaques and amyloid-β (Aβ), a major component of senile plaques, play an important role in the process (Hyman and Gomez-Isla, 1997; Selkoe, 1994; Walsh and Selkoe, 2004). In this sense, previous studies have shown that senile plaques are a source of focal neurotoxicity in AD and in transgenic mice (Bolmont et al., 2007; Meyer-Luehmann et al., 2008; Urbanc et al., 2002). Senile plaques have been associated with abnormal curvature of nearby neurites (D'Amore et al., 2003; Garcia-Alloza et al., 2006a; Spires et al., 2005), synaptic loss (Masliah et al., 1989; Scheff et al., 1990; Spires-Jones et al., 2007) and neuritic dystrophies (Brendza et al., 2005; Lombardo et al., 2003), and these alterations have been shown to be at least partially responsible for the disruption of cortical synaptic integration (Stern et al., 2004).
Although the direct effect of senile plaques in their immediate environment is not completely understood, extensive evidence suggests that oxidative stress may underlie some of the observed alterations (El Khoury et al., 1998; Garcia-Alloza et al., 2006a; McLellan et al., 2003; Moreira et al., 2008) and it seems that the altered microenvironment around plaques is responsible to some extent for the pathological neurites present in AD brains (for review see Hashimoto and Masliah (Hashimoto and Masliah, 2003)). Taking these considerations into account, new efforts have been directed towards combating oxidative stress damage. We tested the idea that antioxidant therapy would improve the neuropathological alterations associated with AD.
α-phenyl-N-tert-butyl nitrone (PBN) is a well characterized spin trap both in vitro and in vivo with potent pharmacological activity (for review see Floyd et al. (Floyd et al., 2002). PBN increases cell survival in cortical cultured neurons (Massieu et al., 2004) and reduces oxidative stress associated with senile plaques (McLellan et al., 2003). Following this idea, natural antioxidants are particularly attractive and numerous studies have shown positive effects of vitamins and bioflavonoids (Garcia-Alloza et al., 2007b; Joseph et al., 2003; Kanowski and Hoerr, 2003; Sung et al., 2004). Of particular interest are Ginkgo biloba extract (EGb 761) and vitamin E. EGb 761 is a natural extract rich in bioflavonoids with well-characterized antioxidant and free radical scavenging properties (Colciaghi et al., 2004; Ramassamy et al., 2007; Wu et al., 2006). It has also been shown that Ginkgo biloba has a positive effect in demented patients (Napryeyenko and Borzenko, 2007). Vitamin E is commonly administered to AD patients (Kontush and Schekatolina, 2004; Zandi et al., 2004) and it has been shown that patients with lower levels of intake of vitamins, including vitamin E, had a greater acceleration of cognitive decline (Wengreen et al., 2007). Similarly, it has been described in animal models that oxidative stress-mediated neurotoxicity can be reduced by vitamin E (Dias-Santagata et al., 2007). It has been shown previously that both Ginkgo biloba extract and Trolox, a water soluble version of vitamin E, led to a reduced abnormal neuritic curvature in transgenic mice after short term treatment, supporting a neuroprotective role for antioxidant treatments (Garcia-Alloza et al., 2006a). Although PBN, Ginkgo biloba extract, and vitamin E are structurally unrelated, the 3 compounds seem to have similar neuroprotective effects (Garcia-Alloza et al., 2006a; McLellan et al., 2003). However, the time-course of plastic changes in neurites associated with senile plaques when treated with antioxidants is not known. How quickly can anti-oxidants result in beneficial effects? Do long-term treatments lead to increased improvement of neuritic alterations? How persistent is the effect? In order to develop more effective antioxidant strategies, it will be important to understand the time-course of reactive oxygen species (ROS) inhibition in the mouse models and the kinetics of plastic changes to altered neuritic processes.
In the present work we used in vivo multiphoton microscopy to assess the effect of the antioxidants PBN, Ginkgo biloba extract and vitamin E on neuronal abnormalities, plaque size and Aβ levels in APPswe/PS1dE9 mice. We observed that antioxidant treatment led to a significant reduction of neuritic curvature after only 4 days of commencing the treatment. When we assessed long-term treatments we observed a significant improvement in neuritic curvature after one month of treatment that persisted even after stopping the treatment for 15 days. These data suggest that the antioxidant effect is rapid and long-lasting. Moreover these data support previous studies suggesting that neuronal plasticity is still dynamic in adult animals.
2. MATERIAL AND METHODS
2.1. Animals
APPswe/PS1dE9 or APPswe/PS1dE9xYFP mice aged 7–8 months were obtained from Jackson Laboratories (Bar Harbor, Maine). All studies were conducted with approved protocol from the Massachusetts General Hospital Animal Care and Use Committee and in compliance with NIH guidelines for the use of experimental animals.
2.2. Reagents
Reagents were obtained from the following sources: Texas Red dextran 70,000 D molecular weight (Molecular probes, Eugene, OR), methoxy-XO4 (gift from Dr. Klunk, U. Pittsburgh), ELISA Aβ40–42 kit (Takeda, Deerfield, IL), Ginkgo biloba extract (EGb 761) (Beaufour Ipsen, Paris, France), cremophor EL, Trolox, PBN, thioflavin S and common chemical reagents w ere obtained from Sigma (St. Louis, MO).
2.3. Ex vivo assessment of natural antioxidants
We assessed the capacity of the compounds under study: PBN, Ginkgo biloba and Trolox to reduce Amplex Red (AR) oxidation associated with senile plaques as previously described (D'Amore et al., 2003; Garcia-Alloza et al., 2006a; McLellan et al., 2003). Paraformaldehyde fixed brain sections were treated for 45 minutes with the antioxidants; EGb 761 (0.01 and 1 mg/ml), Trolox (10nM and 100 mM) and PBN (10nM and 100 mM). Sections were washed and incubated for 45 minutes with 200 µM AR (in the presence of 0.5mg/ml peroxidase) with the antioxidants, whereas Control tissue was incubated in AR and peroxidase only. The tissue was covered to minimize light and air exposure. Sections were washed in PBS to rinse excess reagent, aqueously coverslipped, and imaged with multiphoton microscopy. Afterwards, the tissue was washed in PBS and incubated for 20 minutes in thioflavin S (0.01%). After washing, the sections were covered and imaged again. Images were analyzed with Image-J software (NIH, freeware) and the intensity of the immediate surroundings of the dense-core plaques was subtracted from the dense core fluorescence for AR and thioflavin S to correct for background levels. A ratio between AR intensity and thioflavin S intensity was calculated to obtain an AR oxidation index for each plaque to normalize across images and mice. This ratio provides a quantitative, dimensionless index of plaque-associated AR oxidation. Results are expressed as a percentage of Control values.
2.4. In vivo treatment and surgical preparation
APPswe/PS1dE9 received bilateral intracortical injections of adeno-associated virus (AAV) containing the gene for enhanced GFP (titer, 4.2×1012 viral genomes/ml) as previously described (Spires et al., 2005). The construct rAAV-DBA-EGFP-WPRE was prepared and purified as described before (Spires et al., 2005). Imaging was initiated 3 weeks after injection of the virus. Alternatively, some animals were crosses of APPswe/PS1dE9 with the YFP expressing mice (thy-1:YFP line H+/− Tg mice (Brendza et al., 2003)).
For the short term experiments, APPswe/PS1dE9xYFP or APPswe/PS1dE9-GFP received PBN i.p. (100 mg/Kg) once a day for 7 consecutive days, starting the treatment at the end of the first imaging session (day 0). For the long term treatments, APPswe/PS1dE9-YFP received gavage administrations once a day for 30 days of EGb 761 (100 mg/Kg in water) or Trolox (210 mg/Kg 25% cremophor in distilled water). Control animals followed similar procedures but received water instead of antioxidant treatment.
Surgery was performed as previously described (Skoch et al., 2004) with minor modifications. Fluorescent angiograms were performed with ~12.5 mg/ml i.v. injection of Texas Red dextran (70KD) and served as a guide to find the same sites in the brain between longitudinal imaging sessions. Animals received methoxy-XO4 i.p. (~3 mg/Kg in 3% DMSO, 6% cremophor and 91% PBS), a fluorescent Congo Red derivative that crosses the blood-brain barrier and binds fibrillar Aβ (Klunk et al., 2002) 24 hours before each imaging session. A wax ring was placed around the coverslip of the cortical window and filled with distilled water to create a well.
2.5. Multiphoton imaging and processing
As previously described (Spires et al., 2005) two-photon fluorescence was generated with 800 nm excitation from a mode-locked Ti:Sapphire laser (MaiTai, Spectra-Physics, Mountain View, CA mounted on a multiphoton imaging system (Bio-Rad 1024ES, Bio-Rad, Hercules, CA). A custom-built external detector containing three photomultiplier tubes (Hamamatsu Photonics, Bridgewater, NJ) collected emitted light in the range 380–480, 500–540 and 560– 650 nm. 3-color images were acquired for plaques, neurites, and angiography simultaneously using a 20X objective (NA=0.95, Olympus). In vivo images at low resolution (615×615µm; z-step, 5µm, depth, 200 µm approximately) were acquired to provide a map of the area, using the angiogram as a 3-D fiducial. Higher resolution images were captured to identify single neurites and plaques (125×125 µm; z-step, 0.8 µm, depth, 20 µm approximately). To exclude motion artifacts induced by heartbeat and breathing, image stacks were aligned using AutoDeblur software (AutoQuant). Images from the green channel (YFP or GFP neurites) were further processed with the blind 3D deconvolution function in AutoDeblur to remove background noise. 2D projections of stacks from the three channels were combined in Adobe Photoshop 7 (Adobe Systems). Stacks were used to measure neurite curvature, neurite diameter, spine density, neuritic dystrophy size and plaque size. Observations and measurements of plaques and neurites were made by taking images of the same plaques and neurites in subsequent imaging sessions (Meyer-Luehmann et al., 2008; Spires et al., 2005). Neurite curvature ratio was calculated by dividing the end-to-end distance of a neurite segment by the total length between the two segment ends. We measured as many neurites as we could confidently follow for at least 20 µm (D'Amore et al., 2003; Knowles et al., 1999; Lombardo et al., 2003). Since the imaging was limited to cortical layers 1 and 2, most of the measured segments, from both APPswe/PS1dE9xYFP or APPswe/PS1dE9-GFP mice, were probably dendrites, however, we cannot exclude that a minor amount of axons were included in the measurements. Therefore, we refered to the measured segments as neurites, as previously described in other studies (Garcia-Alloza et al., 2006a; Meyer-Luehmann et al., 2008). We used 2D maximum intensity projections of the imaged volumes (20µm depths) instead of 3D determinations of neurite curvature for simplicity, and it is possible that we have underestimated the absolute value of the curvature of individual neurites. However, the approach was used systematically in treated and untreated animals so that appropriate comparisons could be made, similar to previous studies (D'Amore et al., 2003; Garcia-Alloza et al., 2006a; Lombardo et al., 2003; Meyer-Luehmann et al., 2008; Spires et al., 2005). Neurite shaft diameters were measured at each end and the midpoint of each segment to provide an average diameter. To determine the effect of proximity to plaques, the average distance between the nearest methoxy-XO4 stained amyloid plaque and each neuritic segment was calculated using the average of the distance from the plaque edge to each end and the midpoint of the neuritic segment on the three-channel images. Only neurites located in the proximity of senile plaques (up to 50 µm from plaque border) were included in the study. Spine density analysis was performed in a selected population of dendrites that had identificable spine protrusions. Dendritic spines were counted in maximum intensity projections of the green channel, and dendritic spine density was calculated as spines/µm along the dendritic shaft (Spires et al., 2005).
Neuritic dystrophies, defined as the areas of swelling in the immediate surrounding of the senile plaques (up to 15 µm from plaques border) (Brendza et al., 2005; Tsai et al., 2004), were measured from maximum intensity projections from the green channel by thresholding, segmenting, and measuring using Image-J software. Senile plaque size was measured by thresholding, segmenting, and measuring using the blue fluorescence channel and Image J software. We measured the background intensity and threshold the image at a value 2 standard deviation above the mean of the background. This approach also allowed delineation of the plaque borders for measurements of size and proximity.
PBN treated animals were imaged before the commencement of the treatment (day 0) and reimaged on a daily basis for the next 4 consecutive days (day 1–4), as well as one last time 7 days after the commencement of the treatment (day 7). EGb 761 and Trolox treated animals were imaged for the first time 30 days after the beginning of the treatment and once more 15 days after the treatment was over. Control treated animals followed similar imaging schedules.
2.6. Aβ ELISA measurements
At the end of the experiments, the mice were killed, and the brains hemisected. Soluble and insoluble Aβ40 and Aβ42 were quantified in animals treated with Ginkgo biloba and trolox for 30 days. Colorimetric ELISA kits were used as previously described (Kawarabayashi et al., 2001) with minor modifications. Hemibrains were homogenized for 45s at speed 20 (BioSpec Tissue-Teminaror™) in extraction buffer (10uL/mg brain mass) with protease inhibitor (Complete Protease Cocktail, Roche Diagnostics GmbH, Mannheim, Germany). Extraction buffer consisted of deionized water with 50mM Tris HCl, 2mM EDTA 2Na, 0.01% Merthiorate Na, 400mM NaCl, and 1%BSA. One milliliter of each homogenized brain was centrifuged at 15,000 RPM for 5 minutes at 4° C. The supernatant was removed (soluble Aβ, 1:10 final dilution), and the pellet was diluted 1:8 and homogenized in 70% formic acid (FA) (800µL FA for a 100mg pellet) and centrifuged at 15,000 RPM for 5 minutes at 4° C. Supernatant removed again (insoluble Aβ), neutralized and diluted 1:25 in Tris buffer with pH=11 (1M Tris with 70% FA). Final dilution of insoluble Aβ was 1:200. All samples were analyzed in duplicate. Standard curves were made using human Aβ40 and Aβ42 standards provided in the ELISA kit diluted in Triton-X extraction buffer (for soluble Aβ) or Tris-neutralized FA (for insoluble Aβ). Absorbance was measured by Wallac Victor 2 1420 Multilabel Counter (PerkinElmer Life & Analytical Sciences, Shelton, CT) and data expressed as pmol/g wet tissue.
3. RESULTS
3.1 Ex vivo activity of natural antioxidants
We used AR, a fluorogenic reporter of oxidation to measure the effectiveness of the antioxidants PBN, Ginkgo biloba, and Trolox at inhibiting the plaque derived AR oxidation activity, as previously described (Garcia-Alloza et al., 2006a; McLellan et al., 2003). An AR oxidation index was calculated from the signal obtained from AR divided by the signal obtained after thioflavin S staining from the same identified senile plaques within a tissue section. This ratio normalizes for plaque morphology and allows quantitative measurements of plaque derived AR oxidation. All three of the antioxidants were capable of significantly reducing the AR oxidation associated with senile plaques (Garcia-Alloza et al., 2006a; McLellan et al., 2003) (table 1) suggesting that the antioxidant activity observed may account for the effects observed on neurites in vivo.
Table 1.
Quantitative inhibition of Amplex Red oxidation from senile plaques in tissue sections from mouse brain.
| Treatment | Amplex Red oxidation index (% Control) |
|---|---|
| Control | 100.2±4.3 |
| Ginkgo biloba (1mg/ml) | 53.4±4.2* |
| Ginkgo biloba (0.01 mg/ml) | 82.2±3.9 |
| Trolox (100 µM) | 47.0±5.0* |
| Trolox (10 nM) | 82.5±4.0 |
| PBN (100 µM) | 44.8±1.6* |
| PBN (10 nM) | 86.1±5.0 |
Amplex Red oxidation index derived from Amplex Red-Thioflavin S intensity ratio, resulting from plaque mediated oxidation is expressed as percentage of Control values. Data are representative of 20–98 plaques from 3 animals. Significant differences were determined by one-way ANOVA followed by Tamhane test. ({F6,306=42.086; *p<0.001} vs. Control Group, Ginkgo biloba (0.01 mg/ml), Trolox (10 nM) and PBN (10nM)).
3.2. In vivo effect of antioxidants on neuronal abnormalities and senile plaques
APPswe/PS1dE9xYFP and APPswe/PS1dE9-GFP mice were injected with methoxy-XO4 to allow simultaneous multiphoton imaging of neuronal processes and Aβ deposits. We have previously shown in APPswe/PS1dE9xYFP mice that the toxic effect of senile plaques on neurites leads to a significant increase in neurite curvature when compared to YFP alone mice, and therefore this is a useful model to study neuritic abnormalities. However, this effect can be at least partially reversed by short term treatment (2 weeks) with antioxidants (Garcia-Alloza et al., 2006a). In the present study we assessed how early the straightening effect can be detected and whether longer term treatments leads to more effective straightening.
In order to assess how early the straightening process begins we used our most effective and bioavailable antioxidant, PBN, and imaged the animals using multiphoton microscopy. Mice were imaged before starting the treatment (day 0) and for the next 4 consecutive days (days 1–4), as well as one last time 7 days after the commencement of the treatment (day 7). Since we used APPswe/PS1dE9xYFP and APPswe/PS1dE9-GFP injected mice in this study we compared the neuritic populations in both models. We detected no differences in plaque size, neurite distance to the border of the closest senile plaque, or dystrophy size between APPswe/PS1dE9xYFP and APPswe/PS1dE9-GFP injected mice (data not shown), and therefore both populations were analyzed as one group. Plaque size or dystrophy size were not affected by PBN treatment (table 2). Similarly shaft diameter was not affected by antioxidant treatment (table 2). When we assessed spine density we did not detect a significant PBN effect after 7 days of treatment (table 3), ruling out the possibility of a compensatory mechanism at this level.
Table 2.
Effect of Ginkgo biloba extract (EGb 761), Trolox and α-phenyl-N-tert-butyl nitrone (PBN), on plaque size, dystrophy size and neurite diameter in APPswe/PS1dE9 mice
| Treatment | Plaque size (µm2) |
Dystrophy size (µm2) |
Shaft diameter (µm) |
|---|---|---|---|
| Control day 30 | 286±39 | 8±1 | 1±0.1 |
| EGb 761 day 30 | 331±24 | 7±1 | 1±0.1 |
| Trolox day 30 | 362±37 | 9±1 | 1±0.1 |
| Control day 30+15 days | 285±53 | 9±1 | 1±0.1 |
| EGb 761 day 30+15 days | 362±37 | 9±1 | 1±0.1 |
| Trolox day 30+15 days | 384±46 | 8±1 | 1±0.1 |
| Control day 0 | 313±27 | 8±1 | 1±0.1 |
| PBN day 0 | 310±26 | 9±1 | 1±0.1 |
| Control day 7 | 241±6 | 12±5 | 1±0.1 |
| PBN day 7 | 313±4 | 8±1 | 1±0.1 |
Ginkgo biloba and Trolox had no effect on senile plaque size, dystrophy size or neuritic diameter. Ginkgo biloba extract (EGb 761) (100mg/Kg/day p.o.) or Trolox (210 mg/Kg/day p.o.) had no significant effect on plaque size after 30 days of treatment (30 days: n=20–31 plaques, {F2,73=1.602; p=0.209}, 30 days+15 days washing out: n=8–17 plaques {F2,44=0.872; p=0.425}). No effect was observed on dystrophy size (30 days: n=93–126 dystrophies, {F2,340=1.093; p=0.336}, 30 days+15 days washing out: n=54–159 distrophies{F2,1296=0.290; p=0.749}), or on neurite diameter (30 days: n=36–74 neurites, {F2,171=1.200; p=0.304}, 30 days+15 days washing out: n=16–464 neurites {F2,105=2.303; p=0.105}). Data are mean ± standard deviation from 3–5 animals and differences were assessed by one way ANOVA.
Similarly PBN treatment had no effect on senile plaque size, dystrophy size or neuritic diameter. No differences were detected between PBN (100mg/Kg/day i.p) and Control treated animals before the commencement of the treatment (day 0) or after 7 days of treatment (day 7) on senile plaque size (n=21–58 plaques; p=0.918, p=0.279 respectively). No effect was observed on dystrophy size (n=16–21 dystrophies; p=0.566, p=0.410 respectively) or neurite diameter (n=55–185 neurites; p=0.627, p=0.824 respectively). Data are mean ± standard deviation from 3–6 animals and differences were assessed by Student T test
Table 3.
Ginkgo biloba extract (EGb 761), Trolox and α-phenyl-N-tert-butyl nitrone (PBN), on spine density in APPswe/PS1dE9 mice
| Treatment | Spine density (number/µm) |
|---|---|
| Control day 30 | 0.43±0.04 |
| EGb 761 day 30 | 0.44±0.02 |
| Trolox day 30 | 0.48±0.04 |
| Control day 0 | 0.40±0.08 |
| PBN day 0 | 0.37±0.03 |
| Control day 4 | 0.31±0.05 |
| PBN day 4 | 0.40±0.04 |
| Control day 7 | 0.30±0.06 |
| PBN day 7 | 0.39±0.04 |
Ginkgo biloba (EGb 761) (100mg/Kg/day p.o.) and Trolox (210 mg/Kg/day p.o.) had no significant effect on spine density from dendrites located up to 50 µm from senile plaques after 30 days of treatment (30 days: n=13–21 dendrites, {F2,51=0.513; p=0.602}). Data are mean ± standard deviation from 3–5 animals and differences were assessed by one way ANOVA. Similarly no differences were detected between PBN (100mg/Kg/day i.p) and Control treated animals on spine density before the commencement of the treatment (day 0), after 4 (day 4) or after 7 days of treatment (day 7) (n=4–54, dendrites; p=0.9782, p=0.249, p=0.317 respectively). Data are mean ± standard deviation from 3–6 animals and differences were assessed by Student T test
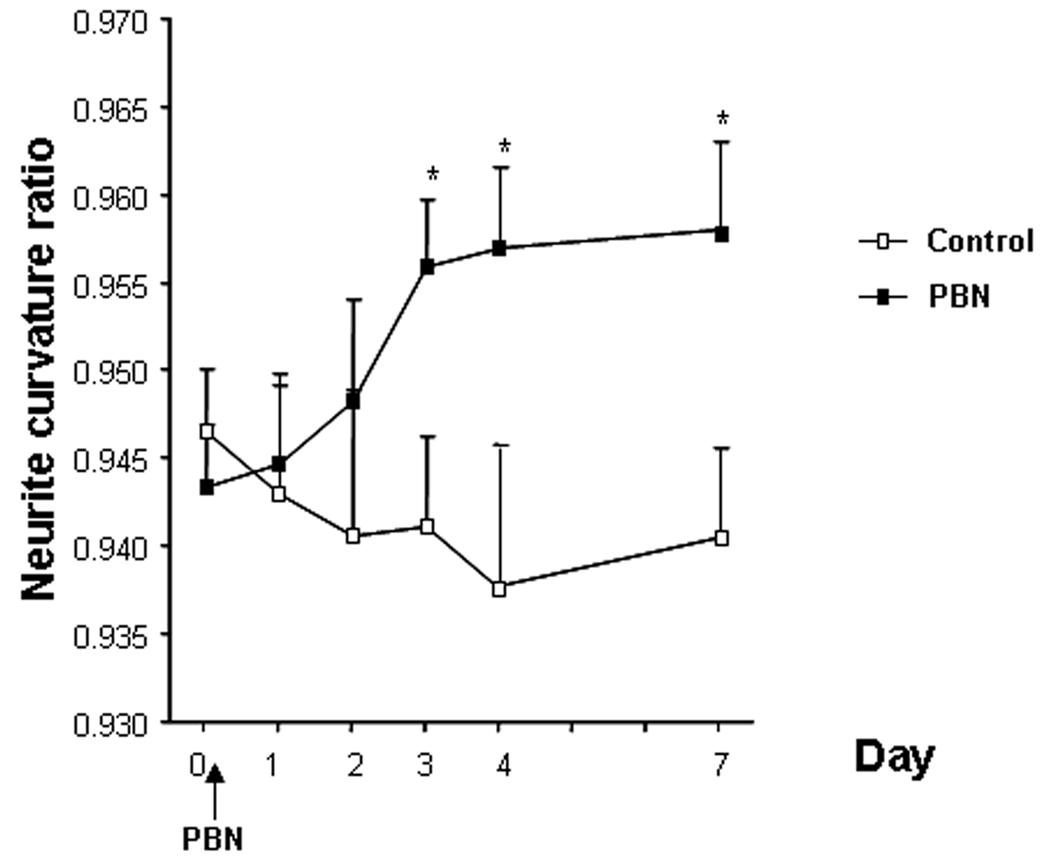
We examined the curvature of the neurites located in close proximity to senile plaques (within 50 µm) for each daily imaging session. PBN showed an early effect on curvature ratio that could be detected as soon as 4 days after commencing PBN treatment. Although we did not detect a treatmentXsession effect, when sessions were individually compared we detected a significant positive effect of the antioxidant treatment (figure 1, and illustrative example in figure 2). Moreover, the differences detected between treated and Control groups were maintained for the duration of the treatment (up to 7 days).
Figure 1.
Effect of PBN treatment on neuritic curvature when neurites up to 50 µm from plaque border were examined. Data shown are the means±SD of all measured neurites per condition (15–176 neurites from 3–6 animals). Animals were imaged on a daily basis before commencing the treatment (day 0) and for the next 4 consecutive days (days 1–4) as well as one last time 7 days after beginning the treatment (day 7). Individual, identified neurites were analyzed at each serial imaging session. The curvature ratio approaches 1.0 for straight neurites, and is less than 1 for “curvy” neurites. Significant differences were determined by Student T test. Statistically significant differences were observed between Control and PBN treated animals as soon as 4 days after commencing the treatment and differences were maintained until the end of the treatment (day7) (*p<0.05 vs. Control group).
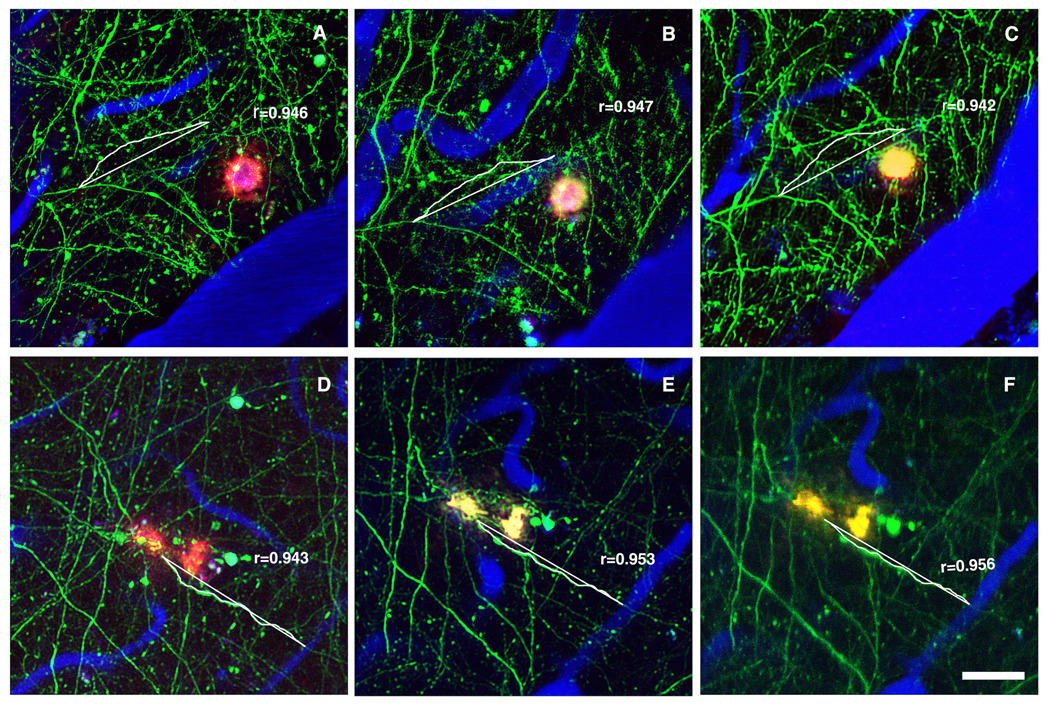
Figure 2.
Representative example of PBN treatment on neuritic curvature. In vivo images with multiphoton microscopy were taken before, as well as 4 and 7 days after commencing the treatment (A, Control day 0; B, Control day 4; C, Control day 7; D, PBN day 0; E, PBN day 4; F, PBN day 7). Neurons expressing YFP or GFP are green, blood vessels (red) contain Texas Red dextran and dense-core Aβ plaques are histochemically labeled by methoxy-XO4 (blue). Single neurites were identified and measured for curvature at each time point. Scale bar: 50 µm
We also assessed the effect of long-term treatment with antioxidants. For this we used Ginkgo biloba extract EGb761 and Trolox. A similar profile to that observed for PBN treated mice was observed: there was no significant effect on plaque size or dystrophy size after 30 days of treatment (table 2) and neurite shaft diameter was not affected at the end of the treatment (table 2). Spine density was not affected by long term antioxidant treatment with Ginkgo biloba extract or Trolox (table 3) as previously shown with PBN.
spine density, senile plaque size or dystrophy size after 30 days of treatment when compared to untreated mice (tables 2 and 3).The treatment was stopped and the animals were re-imaged 15 days later. There was also no effect on plaque size, dystrophy size or neurite diameter (table 2) when we analyzed the same animals after 15 days of concluding the treatment.
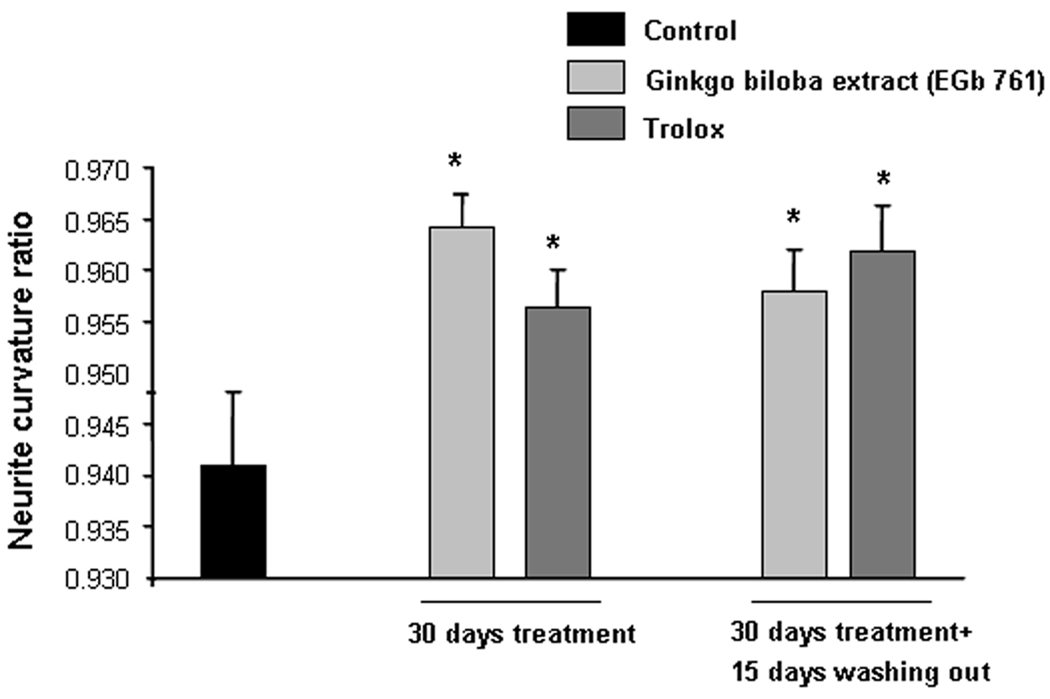
When neurite morphology was examined, a restorative effect on curvature ratio for Ginkgo biloba and Trolox was found (figure 3 and figure 4 for illustrative example). This straightening effect was similar in magnitude to previous work treating animals with the compounds for 15 days (Garcia-Alloza et al., 2006b). We also assessed the effect of long-term antioxidant treatment on neurites 15 days after concluding the treatments. Both Ginkgo biloba and Trolox maintained the straightening profile observed after 30 days of treatment (figure 3 and figure 4 for illustrative example).
Figure 3.
Effect of long term treatment with Ginkgo biloba extract (EGb 761) (100mg/Kg/day p.o.) and Trolox (210 mg/Kg/day p.o.) on neuritic curvature when neurites up to 50 µm from plaque border were examined. Data shown are the means±SD of all measured neurites per condition (4–73 from 3–5 animals). Ratio of curvature for each group was analyzed by one way ANOVA followed by Tuckey B test, with distance to senile plaque as independent variable. There was no significant effect, of distance to the closest plaque, on the curvature ratio between groups {F4,241=0.877, p=0.571}. Statistically significant differences were observed between animals treated with Ginkgo biloba (100 mg/Kg) and Trolox (210 mg/Kg) for 30 days or after 15 days of washing out and Control mice ({F4,273=3.890, *p=0.004} vs. Control group).
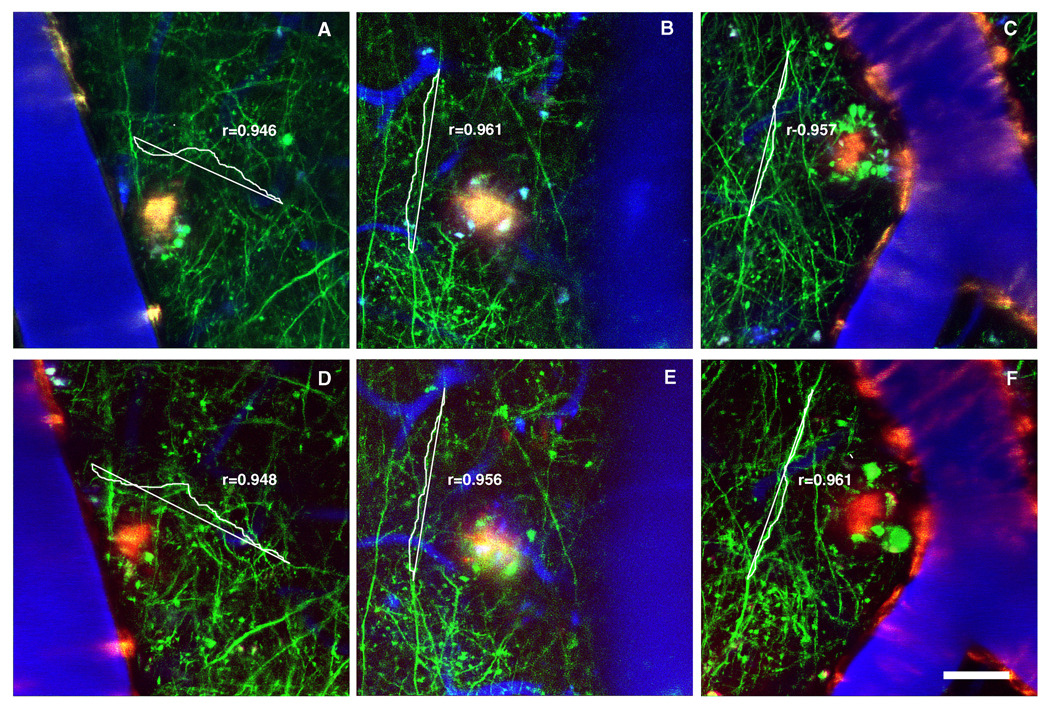
Figure 4.
Representative example of long term treatments with Ginkgo biloba extract (EGb 761) and Trolox on neuritic curvature. In vivo images with multiphoton microscopy were taken 30 days after commencing the treatment (A, Control; B, Ginkgo biloba extract; C, Trolox). Neurons are green, blood vessels (red) contain Texas Red dextran and dense-core Aβ plaques are histochemically labeled by methoxy-XO4 (blue). Scale bar: 25 µm
3.3. Effect of antioxidants on Aβ40 and 42 levels
Hemisected brains were flash frozen, homogenized, and used for measurements of Aβ. ELISA determinations showed that neither Ginkgo biloba extract nor trolox affected soluble or insoluble levels of Aβ40 and 42 when compared to control values (table 2) and data are in the range previously described for this transgenic AD model (Garcia-Alloza et al., 2006b; Jankowsky et al., 2003)
DISCUSSION
AD has a dramatic effect in an aging population that leads to exorbitant health care costs as well as societal and personal burdens. Moreover, the currently available therapeutic treatments are based on manipulation of neurotransmitter systems, including acetylcholinesterase inhibitors or N-methyl-D-aspartate receptor (NMDAR) antagonist memantine (Alexopoulos et al., 2005), and there is no compelling evidence to suggest that these can interfere with the underlying pathogenic process (Siemers et al., 2006). Due to the limited success of these available treatments, new efforts are being directed towards the study of antioxidants as an alternative approach to delay or slow the illness (for reviews see (Frank and Gupta, 2005; Ramassamy, 2006). Supporting this approach, substantial evidence suggests that the microenvironment surrounding plaques is a local source of oxidative stress in AD (Behl and Moosmann, 2002). In vitro studies have shown that oxidative stress may play an important role in Aβ induced toxicity (Heinitz et al., 2006; Matsumoto et al., 2006; Tamagno et al., 2006). Moreover, in vivo studies in transgenic mice suggest that senile plaques are a source of oxidative stress and that the use of antioxidants can ameliorate oxidative stress associated with the plaques (Garcia-Alloza et al., 2007a; Garcia-Alloza et al., 2006a; McLellan et al., 2003).
In this study we have included 3 well characterized antioxidants: PBN, Ginkgo biloba extract and trolox. PBN is a spin-trap compound with neuroprotective properties both in vitro (Massieu et al., 2004) and in vivo (McLellan et al., 2003) (for review see. (Floyd et al., 2000). On the other hand, Ginkgo biloba extract can alleviate Aβ-induced pathological behaviors and memory deficits in AD models including Tg2576 mice (Stackman et al., 2003) and Caenorhabditis elegans (Wu et al., 2006). Moreover, clinical trials have shown some promising effects of Ginkgo biloba, supporting its use in the treatment of AD (Birks et al., 2002; Le Bars et al., 2002; Mazza et al., 2006). Vitamin E has also been shown to reduce Aβ levels and amyloid deposition in young transgenic mice (Sung et al., 2004) and in one clinical trial vitamin E appeared to slow the progression of the disease (Sano et al., 1997). We have used PBN, Ginkgo biloba extract and trolox administration in combination with direct, in vivo imaging to assess the possible effects on senile plaques and the locally disrupted neurite morphology in the proximity of senile plaques.
We did not detect any significant effect on senile plaque size after treatment with PBN, Ginkgo biloba or trolox, and the natural antioxidants did not show any effect on Aβ levels measured postmortem by ELISA. Previous studies have shown that Ginkgo biloba can reduce amyloid precursor protein (Yao et al., 2004) and inhibit Aβ aggregation in vitro (Luo et al., 2002). However, supporting our results, Stackman et al (Stackman et al., 2003) have shown that Tg2576 mice treated with Ginkgo biloba showed cognitive improvement without any effect on Aβ levels or senile plaque burden. Similarly, vitamin E seems to have an effect on Aβ levels or senile plaques only when administered prior to the appearance of the pathology (Sung et al., 2004) whereas our animals, at 7–8 months of age already present significant AD pathology.
We assessed the effect of PBN, Ginkgo biloba extract and trolox on the neuritic dystrophies associated with senile plaques. These plaque associated swellings resemble those found in human tissue and have been previously described both in APPswe/PS1dE9 and in other AD transgenic mouse models (Garcia-Alloza et al., 2006a; Spires et al., 2005). Brendza et al. (Brendza et al., 2005) have shown that anti-Aβ antibody treatment can reduce or eliminate neuronal dystrophies associated with senile plaques. In our hands, none of the antioxidants studied altered the total amount of dystrophies located in the immediate surround of senile plaques suggesting a completely different mechanism of action of the antioxidants.
We also assessed the possible neuroprotective effect of PBN, Ginkgo biloba and trolox by measuring dendritic spine density in close proximity to senile plaques. Spine density was not affected after antioxidant treatment suggesting that the positive effect observed on neuritic curvature does not translate in a compensatory mechanisms and increased amount of synapses. However, we observed a significant reduction of neuritic curvature after antioxidant treatment. It has been previously shown that transgenic mouse models of AD exhibit abnormal dendritic curvature associated with senile plaques, similar to that found in AD tissue (Garcia-Alloza et al., 2006a; Spires et al., 2005). It has also been suggested that abnormal neuronal geometry in the immediate vicinity of the senile plaques may account for the failure of neurons to successfully integrate and propagate information (Stern et al., 2004). Although the negative impact of Aβ on neuritic architecture and functionality seems well established, whether this effect is direct or indirect is unknown. We cannot establish an irrefutable cause-effect relationship between oxidative stress and abnormal neuritic curvature in AD, however our ex vivo data show the capacity of PBN, Ginkgo biloba and Trolox to reduce the oxidation associated with senile plaques, supporting previous studies and suggesting that the neuroprotective effects of antioxidants in AD may be mediated by a reduction of ROS production. These data are also in accordance with previous studies showing that neurites up to 50 µm from the plaque border have a compromised architecture (D'Amore et al., 2003). This large "halo" of neuropil alterations suggests that observed alterations extend beyond the discrete border of senile plaques (D'Amore et al., 2003) and supports the idea of a diffusible agent that mediates the neuronal changes. The presence of secondary changes due to local oxidative damage can not be excluded. It also needs to be considered that ROS may have multiple sources, including microglia (Colton et al., 2000), Aβ itself (De Felice et al., 2007), or any of the myriad molecules that compose senile plaques (Armstrong, 2006).
A previous study supports the idea that oxidative stress associated with senile plaques may contribute to local alterations in neurite trajectories since short term treatment with antioxidants had a straightening effect on neuritic curvature (Garcia-Alloza et al., 2006a). However, it remains unclear how early the neuroprotective changes appear once the antioxidant treatment starts or for how long the positive effect can be detected once the treatment is concluded. In order to address the first point, PBN was administered i.p. and in vivo multiphoton imaging was performed on a daily basis. The same identified neurites, located within 50 µm from a plaque, were imaged daily. After the first administration, a straightening effect was observed and this effect reached statistical significance as soon as four days after commencing the treatment. The effect was maintained for as long as the treatment was administered (up to 7 days) but reached a plateau and did not continue to improve. These data suggest that the neuroprotective effect observed after antioxidant treatment occurs rapidly and is maintained for as long as the treatment is continued. Moreover, these data support a great degree of plasticity in the adult nervous system, as previously described in other transgenic mouse models (Lombardo et al., 2003).
We also assessed the effect of long-term antioxidant treatment on neurite curvature. Mice were treated for one month with 2 well characterized antioxidants, Gingko biloba extract and trolox. In our hands both compounds showed a neuroprotective effect that led to a significant straightening of the distorted neurites within 50 µm from the plaque border, in a similar pattern to that observed after short-term PBN treatment. It seems that PBN can more effectively reduce AR oxidation associated with senile plaques (Garcia-Alloza et al., 2006a; McLellan et al., 2003). However due to the fact that Ginkgo biloba and Trolox are naturally derived antioxidants, widely used and with very limited toxic effects, we performed long-term treatments using these 2 compounds. Moreover, previous studies demonstrated that both Ginkgo biloba and Trolox also had an early (2 weeks) positive straightening effect on neurite curvature (Garcia-Alloza et al., 2006a). This effect suggests a common pathway for all antioxidants under study, as previously described in other paradigms (Garcia-Alloza et al., 2006a; McLellan et al., 2003). The straightening effect observed after long-term treatment was in the range observed after PBN treatment and after shorter antioxidant treatments previously described (Garcia-Alloza et al., 2006a).
The positive straightening effect was still present 15 days after stopping the treatment with both Ginkgo biloba and Trolox, suggesting that the effects were long lasting. These data , together with the limited toxic effects associated with vitamin E and Ginkgo biloba support the long-term chronic treatments with antioxidants. It has been demonstrated that long-term treatment with Ginkgo biloba can improve performance of Tg2576 mice in the Morris water maze (Stackman et al., 2003). Similarly, vitamin E depletion seems to exacerbate memory impairment (Nishida et al., 2006) and improve behavioral performance in association with preservation of the dendritic structure, supporting the implication of oxidative stress in neuronal degeneration in AD (Veinbergs et al., 2000), although other mechanisms including toxic oligomeric species of Aβ or secondary inflammatory responses cannot be excluded. All together, it would appear that antioxidant treatment can not only reduce oxidative stress and improve abnormal neurite curvature in transgenic mice, but that these effects could translate into more meaningful functional consequences. Taking these considerations into account, our data support a neuroprotective effect of antioxidants that is not only fast but sustained in time, supporting further studies to asses a possible role for antioxidant therapy in the treatment of AD patients.
Table 4.
Effect of Ginkgo biloba extract (EGb 761) and Trolox on soluble and insoluble amyloid-beta 40 and 42 in APPswe/PS1dE9 mice
| Treatment | Soluble Aβ40 | Soluble Aβ42 | Insoluble Aβ40 | Insoluble Aβ42 |
|---|---|---|---|---|
| Control | 3.7±0.7 | 4.3±0.9 | 581.3±7.6 | 1002.1±22.2 |
| EGb 761 30 days | 3.1±0.2 | 3.8±0.5 | 590.3±64.3 | 1009.6±11.0 |
| Trolox 30 days | 3.6±0.8 | 3.6±0.6 | 510.0±55.8 | 1052.7±1.9 |
Levels of both tris-extracted (soluble) and formic acid-extracted (insoluble) amyloid-β 40 and 42 in APPswe/PS1dE9, expressed as pmol/g of wet tissue were not affected by Ginkgo biloba extract (EGb 761) or Trolox after 30 days of treatment. Soluble Aβ40 {F4,9=0.513; p=0.615}, soluble Aβ42 {F4,9=0.255; p=0.781}, insoluble Aβ40 {F4,9=1.075; p=0.386}, insoluble Aβ42 {F4,7=1.853; p=0.212}. Data are mean ± standard deviation from 3–6 animals and differences were assessed by one way ANOVA.
Acknowledgements
This work was supported by NIH AG020570, EB000768, AG024688, IPSEN (BJB), NIH AG08487, and a fellowship from the AHA-Bugher Foundation fellowship (M.G-A).
Disclosure of Funding: Partial support and Ginkgo biloba extract (EGb 761) provided by IPSEN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Alexopoulos GS, Jeste DV, Chung H, Carpenter D, Ross R, Docherty JP. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgrad Med Spec No. 2005:6–22. [PubMed] [Google Scholar]
- Armstrong RA. Plaques and tangles and the pathogenesis of Alzheimer's disease. Folia Neuropathol. 2006;44:1–11. [PubMed] [Google Scholar]
- Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer's disease as preventive and therapeutic approach. Free Radic Biol Med. 2002;33:182–191. doi: 10.1016/s0891-5849(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2002:CD003120. doi: 10.1002/14651858.CD003120. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP x Tau transgenic mice. Am J Pathol. 2007;171:2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, Bacskai BJ, Cirrito JR, Simmons KA, Skoch JM, Klunk WE, Mathis CA, Bales KR, Paul SM, Hyman BT, Holtzman DM. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest. 2005;115:428–433. doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, O'Brien C, Simmons K, McKeel DW, Bales KR, Paul SM, Olney JW, Sanes JR, Holtzman DM. PDAPP; YFP double transgenic mice: a tool to study amyloid-beta associated changes in axonal, dendritic, and synaptic structures. J Comp Neurol. 2003;456:375–383. doi: 10.1002/cne.10536. [DOI] [PubMed] [Google Scholar]
- Colciaghi F, Borroni B, Zimmermann M, Bellone C, Longhi A, Padovani A, Cattabeni F, Christen Y, Di Luca M. Amyloid precursor protein metabolism is regulated toward alpha-secretase pathway by Ginkgo biloba extracts. Neurobiol Dis. 2004;16:454–460. doi: 10.1016/j.nbd.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Colton CA, Chernyshev ON, Gilbert DL, Vitek MP. Microglial contribution to oxidative stress in Alzheimer's disease. Ann N Y Acad Sci. 2000;899:292–307. doi: 10.1111/j.1749-6632.2000.tb06195.x. [DOI] [PubMed] [Google Scholar]
- D’Amore JD, Kajdasz ST, McLellan ME, Bacskai BJ, Stern EA, Hyman BT. In vivo multiphoton imaging of a transgenic mouse model of Alzheimer disease reveals marked thioflavine-S-associated alterations in neurite trajectories. J Neuropathol Exp Neurol. 2003;62:137–145. doi: 10.1093/jnen/62.2.137. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Dias-Santagata D, Fulga TA, Duttaroy A, Feany MB. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J Clin Invest. 2007;117:236–245. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer's disease. Neurobiol Aging. 1998;19:S81–S84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K, Bing G. Evidence for enhanced neuro-inflammatory processes in neurodegenerative diseases and the action of nitrones as potential therapeutics. J Neural Transm Suppl. 2000:387–414. doi: 10.1007/978-3-7091-6301-6_28. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K, Forster MJ, Kelleher-Anderson JA, Wood PL. Nitrones as neuroprotectants and antiaging drugs. Ann N Y Acad Sci. 2002;959:321–329. doi: 10.1111/j.1749-6632.2002.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Frank B, Gupta S. A review of antioxidants and Alzheimer's disease. Ann Clin Psychiatry. 2005;17:269–286. doi: 10.1080/10401230500296428. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007a;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007b;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Dodwell SA, Meyer-Luehmann M, Hyman BT, Bacskai BJ. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J Neuropathol Exp Neurol. 2006a;65:1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006b;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer's and dementia with Lewy bodies. Neurochem Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- Heinitz K, Beck M, Schliebs R, Perez-Polo JR. Toxicity mediated by soluble oligomers of beta-amyloid(1–42) on cholinergic SN56.B5.G4 cells. J Neurochem. 2006;98:1930–1945. doi: 10.1111/j.1471-4159.2006.04015.x. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Gomez-Isla T. The natural history of Alzheimer neurofibrillary tangles and amyloid deposits. Neurobiol Aging. 1997;18:386–387. doi: 10.1016/s0197-4580(97)00054-7. discussion 389–392. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Xu G, Fromholt D, Gonzales V, Borchelt DR. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:1220–1227. doi: 10.1093/jnen/62.12.1220. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Kanowski S, Hoerr R. Ginkgo biloba extract EGb 761 in dementia: intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry. 2003;36:297–303. doi: 10.1055/s-2003-45117. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT. Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer's disease. Proc Natl Acad Sci U S A. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontush K, Schekatolina S. Vitamin E in neurodegenerative disorders: Alzheimer's disease. Ann N Y Acad Sci. 2004;1031:249–262. doi: 10.1196/annals.1331.025. [DOI] [PubMed] [Google Scholar]
- Le Bars PL, Velasco FM, Ferguson JM, Dessain EC, Kieser M, Hoerr R. Influence of the severity of cognitive impairment on the effect of the Gnkgo biloba extract EGb 761 in Alzheimer's disease. Neuropsychobiology. 2002;45:19–26. doi: 10.1159/000048668. [DOI] [PubMed] [Google Scholar]
- Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT. Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci. 2003;23:10879–10883. doi: 10.1523/JNEUROSCI.23-34-10879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Smith JV, Paramasivam V, Burdick A, Curry KJ, Buford JP, Khan I, Netzer WJ, Xu H, Butko P. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci U S A. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Terry RD, DeTeresa RM, Hansen LA. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989;103:234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- Massieu L, Moran J, Christen Y. Effect of Ginkgo biloba (EGb 761) on staurosporine-induced neuronal death and caspase activity in cortical cultured neurons. Brain Res. 2004;1002:76–85. doi: 10.1016/j.brainres.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Akao Y, Yi H, Shamoto-Nagai M, Maruyama W, Naoi M. Overexpression of amyloid precursor protein induces susceptibility to oxidative stress in human neuroblastoma SH-SY5Y cells. J Neural Transm. 2006;113:125–135. doi: 10.1007/s00702-005-0318-0. [DOI] [PubMed] [Google Scholar]
- Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;13:981–985. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- McLellan ME, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci. 2003;23:2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Napryeyenko O, Borzenko I. Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung. 2007;57:4–11. doi: 10.1055/s-0031-1296579. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Yokota T, Takahashi T, Uchihara T, Jishage K, Mizusawa H. Deletion of vitamin E enhances phenotype of Alzheimer disease model mouse. Biochem Biophys Res Commun. 2006;350:530–536. doi: 10.1016/j.bbrc.2006.09.083. [DOI] [PubMed] [Google Scholar]
- Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Longpre F, Christen Y. Ginkgo biloba extract (EGb 761) in Alzheimer's disease: is there any evidence? Curr Alzheimer Res. 2007;4:253–262. doi: 10.2174/156720507781077304. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Siemers ER, Dean RA, Demattos R, May PC. New pathways in drug discovery for Alzheimer's disease. Curr Neurol Neurosci Rep. 2006;6:372–378. doi: 10.1007/s11910-996-0017-8. [DOI] [PubMed] [Google Scholar]
- Skoch J, Hyckey GA, Kajdasz ST, B.T H, Bacskai BJ. Methods in Molecular Biology. (Humana Press); 2004. In vivo Imaging of Amyloid-β Deposits in Mouse Brain with Multiphoton Microscopy; pp. 249–264. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. Impaired spine stability underlies plaque-related spine loss in an Alzheimer's disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment. Exp Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer's disease. Faseb J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Guglielmotto M, Danni O, Tabaton M. The various aggregation states of beta-amyloid 1–42 mediate different effects on oxidative stress, neurodegeneration, and BACE-1 expression. Free Radic Biol Med. 2006;41:202–212. doi: 10.1016/j.freeradbiomed.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer's disease. Proc Natl Acad Sci U S A. 2002;99:13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinbergs I, Mallory M, Sagara Y, Masliah E. Vitamin E supplementation prevents spatial learning deficits and dendritic alterations in aged apolipoprotein E-deficient mice. Eur J Neurosci. 2000;12:4541–4546. [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, Skoog I, Norton MC, Tschanz J, Breitner JC, Welsh-Bohmer KA. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11:230–237. [PubMed] [Google Scholar]
- Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci. 2006;26:13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZX, Han Z, Drieu K, Papadopoulos V. Ginkgo biloba extract (Egb 761) inhibits beta-amyloid production by lowering free cholesterol levels. J Nutr Biochem. 2004;15:749–756. doi: 10.1016/j.jnutbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]