Abstract
Hormone-related symptoms are common in breast cancer survivors and many aspects of these symptoms are currently under study. Reliable and valid assessment tools are needed to successfully study hormone-related symptoms in breast cancer survivors; however, no gold standard currently exists for measuring these symptoms. This study evaluated the psychometric properties of a shortened version of the Breast Cancer Prevention Trial (BCPT) symptom checklist in a sample of 803 breast cancer survivors. Principal factor analysis with Promax oblique rotation revealed a five-factor structure, identifying five separate hormone-related symptoms scales: vasomotor symptoms, urinary incontinence, cognitive/mood changes, vaginal symptoms, and weight gain/appearance concern. Hormone-related symptom scale scores differed by demographic and clinical characteristics according to expectations, suggesting that these five scales from the shortened BCPT checklist are reasonably reliable and valid. Symptom scale scores were only weakly correlated with health-related quality of life scores; however, the pattern of results generally supported the validity of the symptom scales. This study adds to the evidence that breast cancer survivors experience a significant number of hormone-related symptoms. Future clinical trials and quality of life and symptom management intervention studies would benefit from accurate assessment of hormone-related symptoms with the five scales from the shortened BCPT checklist.
Introduction
Hormone-related symptoms are common after treatment for breast cancer and include vasomotor symptoms (hot flashes, sweats, palpitations), urinary incontinence, vaginal dryness, and cognitive and mood changes. These symptoms occur at higher rates in breast cancer survivors than in age-matched healthy peers for several reasons [Ganz, et al., 1995; Ganz, et al., 1998a; Meyerowitz, et al., 1999]. Breast cancer occurs primarily in post-menopausal women and the diagnosis may coincide with ongoing menopausal symptoms. Hormone replacement therapy (HRT) is typically stopped abruptly at the time of breast cancer diagnosis, which may intensify symptoms. Additionally, adjuvant therapy including tamoxifen or chemotherapy is associated with increased symptoms of estrogen deficiency [Ganz, et al., 1998b]. Tamoxifen has been associated with more frequent hot flashes, night sweats, and vaginal discharge than placebo in women of all ages [Day, et al., 1999]. Ovarian failure secondary to chemotherapy is associated with early menopause in younger women, and past chemotherapy is associated with sexual dysfunction in breast cancer survivors [Ganz, et al., 1999].
Many aspects of hormone-related symptoms are currently under study among breast cancer survivors, including their prevalence, duration, and impact on quality of life (QOL). At the same time, symptom management interventions are also being studied. Reliable and valid assessment tools are needed to study hormone-related symptoms in breast cancer survivors successfully. However, currently no gold standard exists for measuring these symptoms.
Various forms of the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial (BCPT) 43-item checklist have recently been used in four analyses of breast cancer survivors. [Bower, et al., 2000; Ganz, et al., 2002; Ganz, et al., 2000; Ganz, et al., 1998a]. This checklist asks about physical and psychological symptoms as well as symptoms associated with menopause and tamoxifen use. However, psychometric data on this instrument in breast cancer survivors are sparse. One recent study evaluated the psychometric properties of the original form of the instrument in breast cancer survivors [Stanton, et al., 2005]. However, further investigation of the psychometric properties of this scale is needed to ensure reliable and valid measurement of hormone-related symptoms in breast cancer survivors so that the scale can be used as a predictor or outcome variable in future studies. Additionally, a shorter version of the instrument could be more easily integrated into clinical trials if it was found to be adequately reliable and valid.
The purpose of this study was to evaluate the psychometric properties of a modified 16-item version of the BCPT checklist in a large multiethnic sample of breast cancer survivors. In this paper, we present the results of a factor analysis identifying groups of items that provide separate symptom scales, internal consistency reliability estimates for these scales, symptom scale scores within subgroups of women defined by tamoxifen use, breast cancer treatment type, and menopausal status, and associations between the hormone-related symptom scales and health-related QOL subscales of physical functioning and mental health. We hypothesized that (1) post-menopausal women and those who received adjuvant therapy with either tamoxifen or chemotherapy would have higher hormone-related symptom scale scores since these factors may contribute to the development of these symptoms, (2) factors indicating symptoms of cognition or mood problems would be more strongly associated with mental and emotional health than physical functioning since these constructs are conceptually similar, (3) factors representing physical symptoms such as vasomotor symptoms or incontinence would be more strongly associated with physical functioning than mental or emotional health since these constructs are conceptually similar, and that (4) both factors indicating difficulties with mood or cognition and factors indicating physical symptoms would be associated with vitality in breast cancer survivors since vitality may be influenced by both physical and psychological factors.
Methods
Study Design
Participants in this study are women enrolled in the Health, Eating, Activity, and Lifestyle (HEAL) Study, a population-based, multicenter, multiethnic, prospective study of women newly diagnosed with in situ or Stages I to IIIA breast cancer. HEAL study participants are being followed to determine the impact of weight, physical activity, diet, hormones, and other exposures on breast cancer prognosis. Written or documented verbal informed consent was obtained from each participant for participation in the original HEAL Study and at each subsequent assessment. All study protocols were approved by the Institutional Review Boards of each participating center, in accordance with an assurance filed with and approved by the United States Department of Health and Human Services.
Data for the current study derive from three data collection points. The baseline interview occurred on average 6.1 months following diagnosis. A second interview was conducted with HEAL participants on average 24.4 months following the baseline interview. We refer to this assessment as the 24-month assessment. A third assessment consisted of the QOL survey and was administered on average 34.5 months following the baseline interview.
Eligibility and Recruitment
Patients diagnosed with their first primary breast cancer were recruited from National Cancer Institute sponsored Surveillance Epidemiology and End Results (SEER) registries in three geographic regions of the United States. In the first site (New Mexico), patients were recruited into the HEAL study between July 1996 and March 1999 from Bernalillo, Sante Fe, Sandoval, Valencia, or Taos counties, New Mexico. Eligible participants had to be (1) diagnosed at age 18 years or older, (2) able to participate in an interview within a 12-month period after diagnosis, and (3) have no prior breast cancer diagnosis. The second site (Western Washington) recruited patients between September 1997 and September 1998 from King, Pierce, or Snohomish counties, Washington. Eligible participants (1) were diagnosed at age 40-64 years, (2) were able to participate in an interview within a 12-month period after diagnosis, (3) had no prior diagnosed breast cancers, and (4) were willing to travel to the study site for measures. Incident breast cancer patients at the third site (Los Angeles County, California) were initially recruited to participate in one of two population-based case-control studies, a study of in situ breast cancer [Meeske, et al., 2004; Patel, et al., 2003] and a study of invasive breast cancer [Marchbanks, et al., 2002a; Marchbanks, et al., 2002b]. Women were eligible to participate in these two case control studies if they were age 35 to 64 years at diagnosis, Caucasian or Black, and born in the United States. Los Angeles County participants in these studies were included in the HEAL study if they were Black, diagnosed between May 1995 and May 1998, and satisfied the HEAL stage eligibility criterion.
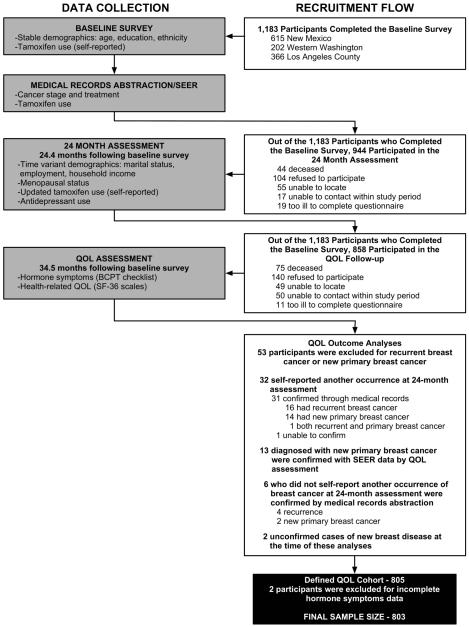
Figure 1 details the flow of participant recruitment into the HEAL QOL cohort used in these analyses. A total of 1,183 participants completed the baseline survey including 615 from New Mexico, 202 from Western Washington, and 366 from Los Angeles County. Of those women who completed the baseline survey, 944 (80%) participated in the 24-month assessment. Of the women who did not participate, 44 were deceased, 104 refused to participate, 55 could not be located, 17 could not be contacted within the study period, and 19 were too ill to complete the questionnaire. Of those women who completed the baseline survey, 858 (73%) participated in the QOL follow-up. Of the women who did not participate, 75 were deceased, 140 refused to participate, 49 could not be located, 50 could not be contacted, and 11 were too ill to complete the questionnaire. For these analyses of QOL outcomes, we excluded 53 women diagnosed with recurrent breast cancer or a new primary breast cancer by the date of the QOL assessment, identified as follows. Thirty-two women self-reported another occurrence of breast disease at the 24-month assessment. We confirmed 31 of these occurrences via medical record abstraction: 16 had recurrent breast cancer, 14 had a new primary breast cancer, and one had both. One could not be confirmed by medical record abstraction. Another thirteen women had been diagnosed with a new primary breast cancer, confirmed with SEER data, by the date of the QOL assessment. An additional six women who did not self-report another occurrence of breast disease at the 24-month follow-up were confirmed by medical record abstraction to have recurrence (n = 4) or new primary breast cancer (n = 2). We further excluded two women who had unconfirmed cases of new breast disease at the time of these analyses. This defined a QOL cohort of 805 women. However, for the analyses presented here, we also excluded an additional two participants because they did not have complete data for the symptom questions. The final sample size for the factor analysis was 803 women.
Figure 1.
Participant Recruitment and Timing of Data Collection for Variables used in this Analysis
Data collection
Figure 1 shows the timing of the three data collection points including the information collected at each assessment point. The baseline assessment was conducted via in-person interview at all three sites and included information on demographic and clinical variables. The baseline interview occurred from 1 to 9 months following diagnosis (mean = 5.3 months) in New Mexico, from 3 to 23 months following diagnosis (mean = 7.5 months) in Western Washington, and from 2 weeks to 17 months following diagnosis (mean = 6.0 months) in Los Angeles County. The 24-month assessment was conducted via in-person interview at all three sites and included information on demographic and clinical variables. The 24-month assessment was conducted 17-32 months after the baseline interview (mean = 22.9 months) in New Mexico, 12-29 months after the baseline interview (mean = 24.2 months) in Western Washington, and 23-45 months after the baseline interview (mean = 27.3 months) in Los Angeles County. The QOL assessment was administered by telephone interview and mailed questionnaire in New Mexico, by mailed questionnaire plus telephone follow-up in Washington, and by telephone interview in Los Angeles County. The same QOL assessment, a standardized questionnaire that included information on hormone-related symptoms, physical functioning and mental health, and QOL measures, was used at all sites. The QOL assessment was conducted 26-54 months post-baseline (mean = 36.4 months) in New Mexico, 15-37 months post-baseline (mean = 26.0) in Western Washington, and 28-54 months post-baseline (mean = 37.3 months) in Los Angeles County.
Measures
Hormone-related symptoms checklist
The BCPT hormone-related checklist was originally based on a checklist of 52 menopausal symptoms and estrogen side effects developed for use in a clinical trial assessing the effects of hormone replacement therapy associated with cardiac risk factors in 875 postmenopausal women [Greendale, et al., 1998]. A reduced version, based on 43 items, was subsequently used in the BCPT [Ganz, et al., 1995], a multi-center chemoprevention trial on the efficacy of tamoxifen. We selected 15 items representing the most relevant symptoms for a population of breast cancer survivors from the BCPT checklist to measure hormone-related symptoms reported by women in the HEAL QOL study. We reworded one item, changing “early awakening” to “interrupted sleep” and added one item measuring “irritability or mood swings.” The timeframe was changed from “last 4 weeks” to “during the past year” to gain a better understanding of the symptoms breast cancer survivors experience over a longer period of time. We retained the five-point Likert response scale ranging from 0 (not at all) to 4 (extremely) that was used in the original instrument. The hormone-related symptoms checklist was given at the QOL assessment.
General functioning
We used the Medical Outcomes Study short form 36 (SF-36) health status measure created to measure physical functioning and mental health aspects of health-related QOL in healthy populations [Hays, et al., 1993; Ware, 1996]. This widely used measure includes 36 items, scored into eight subscales: Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role-Emotional, and Mental Health, and summarized into a physical component and a mental component summary scale. All SF-36 subscales ranged from zero to 100 with increasing scores indicating better functioning, per standard coding protocol. Also per protocol, the two component summary measures have a mean of 50 and a standard deviation of 10. Considerable psychometric analyses have been published on the SF-36, and our own analyses indicate high internal consistency among items in the eight subscales (Cronbach’s alphas ranged from 0.78-0.91). The SF-36 was given at the QOL assessment.
Demographic variables
We used standard measures of age in years, education, and ethnicity collected at baseline. We collected information on marital status, household income, and employment at the 24-month assessment.
Menopausal status
Menopausal status was determined at the 24-month assessment using an algorithm that assigned women into pre, post, or unclassifiable menopausal status based on the following questionnaire data: age, date of last menstruation, hysterectomy and oophorectomy status. Because of the inability to define menopausal status for women without a uterus or those taking HRT, we first considered all women in these two groups who were over age 55 as postmenopausal. This decision was made based on the very low proportion of women over age 55 who are premenopausal. Women who were 55 years of age or older, and who had not menstruated in the last year or who did not know the date of their last menstruation but reported having had a hysterectomy, were categorized as postmenopausal. Women less than age 55 were also categorized as postmenopausal if they had not menstruated in the last year prior to their interview. The following groups of women were categorized as unknown menopausal status: women less than age 55, who had a hysterectomy, but had at least one ovary remaining; and women 55 years of age or older with an intact uterus, who were still menstruating but had used HRT within a year or more prior to interview. The remaining women were classified as premenopausal. Women in the QOL study who did not complete a 24-month follow-up assessment are missing menopausal status (n = 27).
Stage of breast cancer and treatment
Stage of disease was based on SEER data. Treatment data were obtained from medical record abstraction and SEER registry records. We abstracted details of surgeries, radiation therapy, chemotherapy, and use of tamoxifen therapy. Treatment data were recoded as: surgery only; surgery with chemotherapy; surgery with radiation, or the combination of all three treatments. A separate variable dichotomized treatment as any chemotherapy versus no chemotherapy. These variables were abstracted before or concurrent with the 24-month assessment. Use of tamoxifen was collected by a combination of medical record abstraction and self-reported use collected from the baseline and 24-month assessments. We used these data to create a three-level variable of tamoxifen use: use between baseline and 24-months, use at or before baseline only, and no use during the study period.
Antidepressant use
Self-reported use of antidepressant medication was collected at the 24-month assessment. Participants were asked whether they were currently taking any prescription medications. If yes, participants enumerated each medication name, the dose, and how often they took the medication. We coded these data to form a dichotomous variable indicating current use (currently taking at 24 mo) versus no current use (not currently taking at 24 months).
Overview of Analysis
The analyses presented in this paper use data collected through November 18th, 2004. Responses to the 16-item symptom questionnaire were subjected to an exploratory factor analysis using weighted least square parameter estimates since we had no a priori notions regarding the number or specification of the underlying latent variables. We used the principal factor method followed by Promax (oblique) rotation to extract components in order to yield optimal results given our belief that the underlying factors would be correlated. For interpretation of the rotated factor pattern, an item was said to load on a given component if the factor loading was at least 0.30 for that component and had factor loadings less than 0.30 for all other components.
Determination of the number of factors to retain in the final solution was based on a number of indices. First, eigenvalues were examined using scree plots and also by looking at the corresponding percent of variance explained by each factor. Second, the root mean square error of approximation (RMSEA) was examined with 0.06 serving as a rule of thumb for good model fit [Hu and Bentler, 1999]. Third, scale interpretability was evaluated to determine the dimensionality of the menopausal symptom scale. The factor analysis was conducted using Mplus, which is specialized software that is designed for analyzing ordinal level data [Muthen and Muthen, 1998].
Symptom scale scores for each participant were estimated based on the five factors by computing a mean of the items that loaded highly on each component. Hormone-related symptom scale scores, stratified by tamoxifen use, treatment type, and menopausal status, were compared using analysis of covariance (ANCOVA) while controlling for age, breast cancer stage, and antidepressant use. Additionally, differences were tested using a bootstrap re-sampling method with analysis of variance to account for non-normality when estimating p-values (based on 1,000 bootstrap samples per model) since the symptom scales exhibited positive moderate-to-high skewness [Davison and Hinkley, 2003]. The bootstrap method produced results that were similar to those that were produced by our standard analysis of variance approach; thus, the results of the normal theory-based linear models are presented here. Symptom scale scores were correlated with the SF-36 subscales and summary component scales to investigate convergent and discriminate validity. These analyses were performed using SAS/STAT software, Version 9 of the SAS System for Windows [SAS Institute, 2002].
Results
Sample Characteristics
Table 1 presents the baseline and 24-month follow-up demographic and clinical characteristics of the sample. Where demographic characteristics may have changed from the baseline survey to the 24-month assessment (marital status, employment, income, menopausal status), we present the data from the 24-month assessment, where available. At baseline, participants ranged in age from 29-86 years with a mean of 56 years (sd =10). The majority of women were non-Hispanic White (60%), followed by Black (25%) and Hispanic (12%) participants. Most participants had been diagnosed with localized breast cancer (56%) and received some chemotherapy and/or radiation treatment in addition to surgery. At the 24-month follow-up, 76% of women were post-menopausal, 45% were taking tamoxifen, and over half were married and currently employed.
Table 1.
Demographic and clinical characteristics of HEAL participants with baseline and QOL follow-up data (n = 803)
| Characteristic | n | % |
|---|---|---|
| Baseline Characteristics | ||
| Location | ||
| New Mexico | 439 | 54.7 |
| Western Washington | 166 | 20.7 |
| Los Angeles | 198 | 24.7 |
| Age (yr) | ||
| 29-49 | 238 | 29.6 |
| 50-59 | 301 | 37.5 |
| 60-69 | 178 | 22.2 |
| 70+ | 86 | 10.7 |
| (mean ± sd) | (55.5 ± 10.4) | |
| Education | ||
| HS or less | 205 | 25.6 |
| Some college | 293 | 36.5 |
| College grad | 155 | 19.3 |
| Grad school | 149 | 18.6 |
| (missing) | (1) | |
| Race/Ethnicity | ||
| Non-Hispanic White | 485 | 60.4 |
| Black | 199 | 24.8 |
| Hispanic | 95 | 11.8 |
| Other | 24 | 3.0 |
| Stage at diagnosis | ||
| in situ | 178 | 22.2 |
| Local | 453 | 56.4 |
| Regional | 172 | 21.4 |
| Treatment type | ||
| Surgery only | 259 | 32.3 |
| Surgery/Radiation | 296 | 36.9 |
| Surgery/Chemotherapy | 74 | 9.2 |
| Surgery/Radiation/Chemotherapy | 174 | 21.7 |
| 24-Month Assessment Follow-up Characteristics | ||
| Marital status | ||
| Currently married or in a partnered relationship | 450 | 58.2 |
| Widowed/divorced/separated | 272 | 35.2 |
| Never married | 51 | 6.6 |
| (missing)* | (30) | |
| Current employment | ||
| Currently working | 449 | 58.0 |
| Unemployed (on leave, looking for work) | 26 | 3.4 |
| Not working outside the home/retired/disabled | 299 | 38.6 |
| (missing)* | (29) | |
| Income ($) | ||
| <= 10K | 54 | 7.5 |
| >10K – 20K | 86 | 11.9 |
| >20K – 30K | 93 | 12.9 |
| >30K – 50K | 168 | 23.2 |
| >50K – 70K | 210 | 29.0 |
| >70K | 113 | 15.6 |
| (missing)* | (79) | |
| Menopausal status | ||
| Pre | 142 | 18.3 |
| Post | 589 | 75.9 |
| Unclassifiable | 45 | 5.8 |
| (missing)* | (27) | |
| Months from diagnosis to QOL survey | ||
| 23 - 35 | 192 | 23.9 |
| 36 - 41 | 274 | 34.1 |
| 42 – 47 | 209 | 26.0 |
| 48 – 63 | 128 | 15.9 |
| (mean ± sd) | (40.5 + 6.5) | |
| Tamoxifen | ||
| Use between baseline & 24 mo | 350 | 45.1 |
| Use at or before baseline only | 69 | 8.9 |
| No use during study period | 357 | 46.0 |
| (missing)* | (27) | |
| Antidepressants | ||
| Currently taking at 24 mo | 119 | 15.3 |
| Not currently taking at 24 mo | 657 | 84.7 |
| (missing)* | (27) |
Includes women with baseline and QOL data who did not complete a 24-month assessment (n = 27).
Self-reported symptoms
Table 2 reports the prevalence (past year) and mean severity for the 16 hormone-related symptoms assessed in this study. Over 98% of women reported experiencing at least one hormone-related symptom in the past year and 50% of women reported between eight and 12 different symptoms. The most prevalent symptoms were forgetfulness, interrupted sleep, hot flashes, unhappiness with body appearance, and weight gain. Women in the sample reported being “moderately” bothered by symptoms, on average, with greater severity ratings for hot flashes and night sweats.
Table 2.
Prevalence (past year) and mean severity of hormone-related symptoms in 803 breast cancer survivors
| Symptom | Prevalence (% past year) |
Mean Severity‡ (SD) |
|---|---|---|
| Forgetfulness | 79.6 | 1.8 (0.9) |
| Interrupted sleep | 77.7 | 2.1 (1.0) |
| Hot flashes | 73.5 | 2.5 (1.1) |
| Unhappy with appearance of my body | 68.7 | 2.1 (1.0) |
| Weight gain | 64.5 | 2.1 (1.0) |
| Irritability or mood swings | 61.9 | 1.7 (0.8) |
| Difficulty concentrating | 60.8 | 1.7 (0.8) |
| Night sweats | 60.0 | 2.2 (1.1) |
| Breast sensitivity/tenderness | 59.5 | 1.7 (0.9) |
| Easily distracted | 58.7 | 1.7 (0.8) |
| Difficulty with bladder control at other times | 58.2 | 1.8 (1.0) |
| Tendency to take naps; stay in bed | 53.7 | 1.7 (0.9) |
| Difficulty with bladder control when laughing or crying |
46.0 | 1.8 (0.9) |
| Pain with intercourse † | 40.7 | 1.9 (1.0) |
| Vaginal discharge | 37.5 | 1.6 (0.8) |
| Genital itching/irritation | 34.4 | 1.5 (0.8) |
Among those who reported having intercourse in the past year (n = 492).
Severity coded from 1 (slightly) to 4 (extremely).
Factor Structure
Principal factor analysis with Promax rotation revealed a five-factor structure accounting for 72.1% of the variance in symptoms (Table 3: factor loadings used to interpret the meaning of each factor are in bold type). This five-factor solution satisfied all of the criteria for establishing meaningful components, including good interpretability. Results of a scree test suggested that there were five meaningful components and each showed good interpretability. The RMSEA for the five-factor solution was 0.06, indicating good model fit. One item, pain with intercourse, was dropped from analysis due to a low number of respondents, as 313 women did not report having intercourse within the past year.
Table 3.
Promax (oblique) rotated factor pattern from analysis of the hormone-related symptom checklist
| Item | Vasomotor Factor 1 |
Urinary Incontinence Factor 2 |
Cognitive/ Mood Factor 3 |
Vaginal Symptoms Factor 4 |
Weight/ Appearance Factor 5 |
|---|---|---|---|---|---|
| Night sweats | 0.95 | 0.00 | 0.05 | −0.04 | −0.07 |
| Hot flashes | 0.82 | −0.04 | −0.11 | 0.08 | 0.03 |
| Interrupted sleep | 0.38 | 0.09 | 0.28 | −0.02 | 0.08 |
|
| |||||
| Difficulty with bladder control at other times (ie: coughing, sneezing) |
−0.05 | 0.98 | −0.02 | −0.01 | −0.02 |
| Difficulty with bladder control when laughing or crying |
0.04 | 0.92 | −0.05 | −0.01 | −0.01 |
|
| |||||
| Difficulty concentrating | 0.02 | −0.08 | 1.07 | −0.09 | −0.07 |
| Easily Distracted | −0.05 | 0.00 | 0.94 | 0.01 | −0.05 |
| Forgetfulness | −0.01 | 0.02 | 0.73 | 0.00 | 0.07 |
| Irritability or mood swings | 0.10 | 0.01 | 0.45 | 0.11 | 0.14 |
| Tendency to take naps; stay in bed | −0.12 | 0.10 | 0.35 | 0.19 | 0.10 |
|
| |||||
| Vaginal discharge | 0.02 | −0.01 | −0.09 | 0.79 | −0.05 |
| Genital itching/irritation | −0.01 | −0.02 | −0.02 | 0.78 | −0.02 |
|
| |||||
| Unhappiness with the appearance of my body | −0.10 | −0.05 | 0.00 | −0.05 | 0.91 |
| Weight gain | 0.07 | 0.01 | −0.12 | −0.02 | 0.76 |
| Breast sensitivity/tenderness † | 0.08 | 0.03 | 0.14 | 0.08 | 0.23 |
Note: n = 803; Factor loadings used to interpret the meaning of each factor are in bold.
Item excluded from factor to optimize internal consistency.
The first factor represented vasomotor symptoms (night sweats, hot flashes, and interrupted sleep) and accounted for the largest percentage (37%) of the common variance in symptom scores (Table 3). The remaining factors were associated with urinary incontinence (difficulty with bladder control while laughing or crying or at other times), cognitive difficulty and mood (difficulty concentrating, distractibility, forgetfulness, irritability/mood swings, and napping/staying in bed), vaginal symptoms (vaginal discharge, genital itching/irritation), and weight and appearance (weight gain, unhappiness with the appearance of body). These factors accounted for 11%, 9%, 8% and 7% of the common variance in symptom scores, respectively. Correlations among the five factors ranged from 0.23 (vasomotor & urinary incontinence) to 0.61 (cognitive/mood & weight/appearance) and were generally low to moderate.
Internal consistency reliability
We assessed the item-total correlation coefficients and Cronbach’s alpha coefficients for the five factor-based scales created from the hormone-related symptom checklist in order to establish internal consistency reliability. For each scale, the correlations between each item and the sum of the remaining items that constitute that scale were moderate to high (vasomotor symptoms r = 0.45 to 0.68; urinary incontinence symptoms both r = 0.79; cognitive/mood symptoms r = 0.42 to 0.78; vaginal symptoms both r = 0.43; weight/appearance symptoms both r = 0.54). Internal consistency coefficients were reasonably high across the scales. Cronbach’s alpha coefficients for the scales varied from 0.60 (vaginal symptoms scale) to 0.88 (urinary incontinence scale), with 0.83 for the cognitive/mood scale and 0.75 for the vasomotor scale. The Cronbach’s alpha for the weight/appearance scale improved from 0.63 to 0.70 with the exclusion of the breast sensitivity and tenderness variable associated with a weak factor loading (0.23). All three items were retained in the vasomotor scale even though the factor loading for interrupted sleep was substantially lower than the other two symptoms in the scale for two reasons. First, the Cronbach’s alpha for the scale was not improved with the exclusion of the interrupted sleep item. Second, these items hang together conceptually since experiencing hot flashes and night sweats can interrupt sleep. Similarly, the items assessing irritability and napping had lower factor loadings than the other three items on the cognitive/mood scale but all five items were retained to optimize the Cronbach’s alpha for the scale.
Factor-based scores
Inter-correlations among the five hormone-related symptom scores were statistically significant (p ≤ 0.0001) ranging from 0.16 (vaginal symptoms and urinary incontinence scales) to 0.45 (cognitive/mood and weight/appearance scales) and were generally low.
Table 4 presents the mean scale scores for the five hormone-related symptom scales, in total, and stratified by several characteristics (tamoxifen use, menopausal status, treatment type) that may relate to their occurrence. ANCOVA results are also presented, adjusted for age, breast cancer stage at diagnosis, and 24-month antidepressant use. Scores on the vasomotor and vaginal symptoms scales were significantly higher for women who reported taking tamoxifen between baseline and 24-months compared with women who had not taken tamoxifen during the study period (p < .05). Both scale scores were also significantly higher for women taking tamoxifen between baseline and 24-months compared to women who had taken tamoxifen at or before baseline but had stopped taking tamoxifen by 24-months (p < .05).
Table 4.
Mean hormone-related symptom scale scores by tamoxifen use, menopausal status, and treatment type
| Total | Tamoxifen Use | Menopausal Status | Treatment Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptom scale | Mean (SD) |
Use between baseline & 24 mo |
Use at or before baseline only |
No use during study period |
Pre | Post | Surgery Only |
Surgery/ Radiation |
Surgery/ Chemo |
Surgery/ Radiation/ Chemo |
| Vasomotor | 1.6 (1.1) | 1.9 (1.1) | 1.5 (1.1) | 1.3 (1.0) | 1.5 (1.0) | 1.6 (1.1) | 1.4 (1.1) | 1.5 (1.1) | 2.0 (1.2) | 1.9 (1.1) |
| LSM† | 1.8 a*,b*** | 1.5 a* | 1.3 b*** | 1.2 e*** | 1.7 e*** | 1.5 | 1.6 | 1.8 | 1.7 | |
| Urinary Incontinence | 0.9 (1.1) | 1.0 (1.1) | 1.0 (1.1) | 0.8 (1.0) | 0.6 (0.8) | 1.0 (1.1) | 1.0 (1.1) | 0.9 (1.0) | 1.0 (1.0) | 1.0 (1.1) |
| LSM | 1.2 | 1.2 | 1.0 | 1.0 | 1.1 | 1.1 | 1.0 | 1.3 | 1.2 | |
| Cognitive/Mood | 1.1 (0.8) | 1.1 (0.8) | 1.1 (0.8) | 1.0 (0.8) | 1.1 (0.8) | 1.0 (0.8) | 1.0 (0.8) | 1.0 (0.7) | 1.2 (0.9) | 1.2 (0.9) |
| LSM | 1.3 | 1.3 | 1.2 | 1.2 | 1.3 | 1.2 | 1.2 | 1.4 | 1.3 | |
| Vaginal Symptoms | 0.6 (0.7) | 0.7 (0.8) | 0.4 (0.7) | 0.4 (0.6) | 0.6 (0.7) | 0.5 (0.8) | 0.5 (0.7) | 0.5 (0.7) | 0.8 (0.7) | 0.6 (0.8) |
| LSM | 0.8 c**d*** | 0.5 c** | 0.4 d*** | 0.6 | 0.6 | 0.5 | 0.6 | 0.8 | 0.6 | |
| Weight/Appearance | 1.4 (1.1) | 1.5 (1.2) | 1.4 (1.2) | 1.4 (1.1) | 1.5 (1.1) | 1.3 (1.1) | 1.2 (1.1) | 1.3 (1.1) | 1.5 (1.1) | 1.7 (1.2) |
| LSM | 1.6 | 1.6 | 1.5 | 1.4 | 1.6 | 1.4 h** | 1.5 g* | 1.5 f* | 1.8 f*,g*,h** | |
Note: n = 803 for treatment type and for total sample means; 776 for tamoxifen use at 24-month assessment; 731 for menopausal status at 24-month assessment.
Note: Hormone-related symptom scales coded 0-4 with increasing scores indicating more severe symptoms.
Least-squares means (LSM) adjust for age in years at the time symptoms were measured, breast cancer stage at diagnosis, and antidepressant use at 24-month assessment follow-up; n = 776 for tamoxifen use and treatment type; n = 731 for menopausal status.
Pairwise comparisons of LSM that share a common superscript designated by the letters a through h are those that differ significantly from each other: *p < .05; **p < .01; ***p < .001.
Vasomotor scale scores were significantly higher in post-menopausal women compared to pre-menopausal women (p < .001). Scores on the urinary incontinence scale appeared higher in post-menopausal women compared to pre-menopausal women; however this was not statistically significant in the adjusted model.
Scores on several scales differed by breast cancer treatment type. Table 4 presents mean scores for each of the symptom scales by four common types of treatment: surgery, surgery with radiation, surgery with chemotherapy, and surgery with radiation and chemotherapy. However, we also wanted to directly assess any differences in symptoms potentially attributable to chemotherapy use specifically. Thus, we also tested differences in symptom scores using a dichotomous treatment variable (chemotherapy vs. no chemotherapy). Scores on the vasomotor scale were not significantly different among the four treatment categories. However, using the dichotomous chemotherapy variable, women treated with chemotherapy had significantly higher vasomotor scores than women not treated with chemotherapy (mean difference = 0.25; p = 0.03). Scores on the vaginal symptoms scale did not significantly differ among the four treatment categories. However, using the dichotomous chemotherapy variable, women treated with chemotherapy reported significantly higher vaginal symptoms scores than women not treated with chemotherapy (mean difference = 0.16; p = 0.04). Women who were treated with a combination of chemotherapy, radiation, and surgery had significantly higher scores (all p < .05) on the weight/appearance scale than any other treatment group. The difference in weight/appearance scores by any chemotherapy use did not reach statistical significance (mean difference = 0.20; p = 0.08). Scores on the cognitive/mood scale were slightly higher in the women who received any chemotherapy than in the other treatment groups but this was not statistically significant using either the four-level or two-level treatment variables.
Correlations with physical functioning and mental health
Table 5 presents the Pearson correlation coefficients between the factor-based scores on the hormone-related symptom scales and the SF-36 component summary scales as well as the eight individual subscales. Although many of the symptom scales and SF-36 scales were significantly correlated (p ≤ 0.001), the correlations were generally weak and negative (r = −0.03 to −0.52). Negative correlations indicate that fewer and less severe symptoms were associated with better functioning on the domains of the SF-36.
Table 5.
Correlations of hormone-related symptom scale scores with SF-36 summary scores and subscale scores
| SF-36 Summary Scales | SF-36 Physical Health Individual Scales | SF-36 Mental Health Individual Scales | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptom Scale |
Physical Component Summary |
Mental Component Summary |
Physical Functioning |
Role- Physical |
Bodily Pain |
General Health |
Vitality | Social Functioning |
Role- Emotional |
Mental Health |
| Vasomotor | −0.09* | −0.23*** | −0.03 | −0.10** | −0.19*** | −0.17*** | −0.25*** | −0.16*** | −0.13*** | −0.23*** |
| Urinary Incontinence |
−0.22*** | −0.18*** | −0.22*** | −0.21*** | −0.21*** | −0.22*** | −0.25*** | −0.17*** | −0.20*** | −0.20*** |
| Cognitive/ Mood |
−0.26*** | −0.51*** | −0.20*** | −0.35*** | −0.33*** | −0.40*** | −0.49*** | −0.44*** | −0.39*** | −0.52*** |
| Vaginal Symptoms |
−0.09** | −0.18*** | −0.06 | −0.09** | −0.14*** | −0.17*** | −0.20*** | −0.18*** | −0.10** | −0.18*** |
| Weight/ Appearance |
−0.11** | −0.30*** | −0.06 | −0.16*** | −0.20*** | −0.21*** | −0.32*** | −0.20*** | −0.18*** | −0.32*** |
p < .05
p < .01
p < .001
Note: n = 803; SF-36 subscales ranged from 0-100 with increasing scores indicating better functioning; SF-36 summary component scales have a mean of 50 and a standard deviation of 10; Hormone-related symptom scales were coded 0-4 with increasing scores indicating more severe symptoms.
The factor-based scores on the cognitive/mood scale correlated better with SF-36 scales than the other symptom scales. Cognitive/mood scores were more highly correlated with SF-36 mental health subscales (r = −0.39 to −0.52) and the mental component summary score (r = −0.51) than with SF-36 physical functioning subscales (r = −0.20 to −0.40) or the physical component summary score (r = −0.26). Scores on the vasomotor symptom scale were more strongly correlated with the mental than physical component summary scores (r = −0.23, −0.09) and with the vitality, mental health, and bodily pain scores specifically (r = −0.25, −0.23, −0.19). Scores on the urinary incontinence scale were weakly correlated with SF-36 scores, with correlations approximately equivalent for physical and mental domains (r ≈ −0.20). Weight/appearance scale scores were more highly correlated with the mental component summary score (r = −0.30) and the mental health subscale scores (r = −0.18 to −0.32) than with the physical component summary score (r = −0.11) or the physical functioning subscales (r = −0.06 to −0.21). Additionally, the correlations between scores on the vaginal symptom scale and SF-36 measures were generally very small. Finally, scores on all five hormone-related symptom scales were correlated weakly to moderately with the SF-36 vitality subscale, with stronger correlations for cognitive/mood scores (r = −0.49) and weight/appearance scores (r = −0.32).
Discussion
This study sought to investigate the psychometric properties of a modified version of the BCPT menopausal symptom checklist in breast cancer survivors. Factor analysis revealed five meaningful scales that measured vasomotor symptoms, urinary incontinence, cognitive/mood symptoms, vaginal symptoms, and weight gain/appearance concern. Results indicated that each of the five scales had good internal consistency reliability, the items within each scale were sufficiently highly related but not redundant, and each scale represents a homogeneous construct.
These five scales are consistent with the results of Stanton and colleagues on the factor analysis of the full BCPT checklist [Stanton, et al., 2005] (scales: hot flash, bladder control, vaginal problems, cognitive problems, weight problems), with the exception that the analysis of the full instrument revealed three additional scales measuring nausea, musculoskeletal pain, and arm problems. Further, these scales are consistent with the three scales of Ganz and colleagues [Ganz, et al., 2000] who divided their subset of BCPT checklist symptoms into hot flashes, vaginal symptoms, and urinary symptoms scales conceptually and report similarly high Cronbach’s alpha estimates for each scale (hot flash=0.76, vaginal=0.73, urinary=0.76). Our factor structure is also somewhat similar to the six-factor structure of the original PEPI instrument: cognitive-affective, weight-appetite, musculoskeletal, breast discomfort, anxiety, and vasomotor [Greendale, et al., 1998]. Further, our scales are similar to those of the menopause-specific QOL questionnaire used in healthy women [Hilditch, et al., 1996], conceptually divided into vasomotor symptoms, psychosocial (including cognitive and mood symptoms), physical (including weight gain and urinary symptoms), and sexual items (including vaginal symptoms). The consistency among the scale structure in these studies suggests that the five scales identified by factor analysis in the current study are measuring conceptually meaningful groups of symptoms.
We computed scale scores for each of the five symptom scales. The correlations among these scale scores were generally low, indicating that the scale scores are measuring different but related dimensions of hormone-related symptoms and can be used as separate outcomes in future research. We did not compute a total scale score in these analyses because we believed that the correlations were low enough that a total symptom score would be an amalgamation of distinct symptom groups and not a useful construct.
Vasomotor symptoms scale
Higher vasomotor symptom scores were associated with tamoxifen use, postmenopausal status, and chemotherapy exposure. These results are consistent with previous research that has shown use of tamoxifen to be associated with more frequent hot flashes and night sweats compared to placebo [Day, et al., 1999]. Furthermore, scores on the vasomotor scale should be higher in women with chemotherapy-induced early menopause or age-related natural menopause. Prior research on the menopause transition in healthy women has shown that vasomotor symptoms increase from early to late peri-menopause [Dennerstein, et al., 2000].
Urinary incontinence scale
The urinary incontinence scale score was not significantly associated with tamoxifen use, treatment type, or menopausal status. The potential effects of tamoxifen on urinary incontinence are unknown. However, the trend for the severity to be worse in post-menopausal women compared to pre-menopausal women is consistent with past research in healthy women showing that urinary incontinence may increase from the pre-menopausal and early peri-menopausal stages and peak two years post-menopause [Dennerstein, et al., 2000].
Cognitive/Mood Scale
Cognitive/mood scores were not significantly associated with tamoxifen use, chemotherapy exposure, or menopausal status. Previous research has suggested that adjuvant treatment with chemotherapy can produce cognitive deficits [Rugo and Ahles, 2003] and many patients complain of “chemobrain.” The inconsistency between these results and ours may suggest that the measure of cognitive difficulty in the present scale is not sufficiently sensitive to detect clinically significant cognitive impairment. Alternatively, it may be that cognitive deficits associated with chemotherapy cannot be detected much beyond termination of the therapy. One might expect normal age-related differences in both cognitive and mood domains in pre- versus post-menopausal women. However, since our factor encapsulates both cognition and mood, the normal age-related decline in cognitive function likely cancels out the mood elevation noted throughout the menopause transition in healthy women [Dennerstein, et al., 2000].
Vaginal symptoms scale
Scores on the vaginal symptoms scale were higher in women taking tamoxifen and women who received chemotherapy. These results are consistent with previous research that has shown use of tamoxifen to be associated with more vaginal discharge compared to placebo [Day, et al., 1999] and that adjuvant treatment with chemotherapy can produce vaginal symptoms [Ganz, et al., 1998b]. Further, scores on our vaginal symptoms scale did not differ by menopausal status, consistent with past research showing vaginal discharge symptoms were relatively stable across the menopause transition in healthy women [Dennerstein, et al., 2000].
Weight gain/appearance concern scale
The weight/appearance scale scores were similar for pre- and post-menopausal women, consistent with past research indicating weight symptoms were relatively stable across the menopause transition [Dennerstein, et al., 2000]. Our study found slightly higher scores on the weight/appearance scale for women who had been treated with chemotherapy. This is consistent with previous research showing treatment with chemotherapy to be associated with weight gain one year after breast cancer diagnosis [Goodwin, et al., 1999].
To investigate convergent and discriminative validity, we next compared the symptom scale scores to scores on the SF-36 physical functioning and mental health summary component scales and subscales. Though these correlations were moderate to weak, the patterns of results generally supported the validity of the hormone-related symptom scales. Scores on the cognitive/mood scale were more strongly associated with SF-36 mental health subscales than physical subscales. Weight/appearance scale scores were more highly correlated with the mental health scores than with physical scores. Finally, consistent with our hypothesis that physical and psychological processes likely influence feelings of vitality, scores on all five hormone-related symptom scales were correlated weakly to moderately with the SF-36 vitality and mental health subscales. Counter to our hypotheses, scores on the vasomotor and urinary incontinence symptom scales were not more strongly associated with SF-36 physical functioning subscales than mental health subscales. The SF-36 physical functioning subscales may not have been sensitive to vasomotor symptoms and urinary incontinence. Alternatively, vasomotor symptoms and urinary incontinence may contribute equally to both physical functioning and mental health.
Previous work correlating the BCPT checklist scores with SF-36 scores in breast cancer survivors [Stanton, et al., 2005] and in healthy women at high-risk for breast cancer [Ganz, et al., 1995] found similar moderate to weak negative correlations. The general patterns of correlations between hormone-related scale scores and SF-36 scores in this study lend support for the construct validity of the scales. However, the low magnitude of these correlations here and in Stanton’s and Ganz’s work suggests that these persistent and common symptoms do not relate strongly to health-related QOL.
The strengths of this study include its large ethnically and socio-economically diverse sample of breast cancer survivors and the opportunity to compare hormone-related symptoms to health-related QOL. The main limitation of this study is the limited number of psychometric instruments available for validation. Future research is needed to help provide construct validity for the hormone-related symptom scales, as well as investigate test-retest reliability, face validity, and how symptoms and the factor structure presented here change over time. Evidence is accumulating that hormone-related symptoms may change over the course of survivorship [Ganz, et al., 2002] and a valid instrument should be sensitive to these changes.
It is not ideal that three out of five of the scales determined via factor analysis are comprised of only 2 items and that none of the scales had internal consistency estimates that reached the 0.90 deemed appropriate for individual-level measurement [Sloan, et al., 2002]. Further, both the vasomotor scale and the cognitive/mood scale included items that did not cluster together well with the rest of the scale, as demonstrated by lower factor loadings (under .50). Future research should investigate the benefit of adding more items to the scales presented here until the reliability estimates reached 0.90. For example, the vaginal symptoms subscale might include an item specifically measuring vaginal dryness or the weight gain/appearance concern scale might include items about concern that weight gain may increase overall health risk or risk of breast cancer recurrence. Other sleep symptoms may be added to see whether different aspects of sleep disturbance would load differentially on the factors and help explain more of the variance in symptoms. Alternatively, future investigators wishing to use these scales may also choose not to use the items that did not load as highly on the vasomotor or cognitive/mood scales.
In conclusion, the evidence presented here suggests that the five hormone-related symptom scales from the shortened form of the BCPT checklist are reliable and valid and can be used to more accurately determine the prevalence and severity of symptoms among breast cancer patients. This shortened form cannot be uniformly substituted for the full BCPT checklist, which generates three additional scales. However, this short form may be useful to future studies in which the entire scale is not feasible. This study adds to the growing evidence that breast cancer survivors are experiencing a significant number of hormone-related symptoms. Future clinical trials, as well as QOL and symptom management intervention studies, will benefit from accurate assessment of hormone-related symptoms with these five scales from the shortened form of the BCPT checklist.
Acknowledgments
Funded by NCI contract #N01-CN-75036-20. Part of Dr. Alfano’s effort was supported by a grant from the National Cancer Institute (CA92408).
Footnotes
Some material presented in this manuscript was presented at the 2005 meeting of the American Psychosocial Oncology Society.
References
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap Methods and Their Application. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham L, Fisher B. Health related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Day R, Ware JE, Jr., Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–82. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Desmond KA, Belin TR, Meyerowitz BE, Rowland JH. Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol. 1999;17:2371–80. doi: 10.1200/JCO.1999.17.8.2371. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B, Belin TR. Managing menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Natl Cancer Inst. 2000;92:1054–64. doi: 10.1093/jnci/92.13.1054. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998a;16:501–14. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 1998b;152:396–411. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, Trudeau M, Hood N, Redwood S. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–9. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Hogan P, Barnabei VM, Shumaker S, Johnson S, Barrett-Connor E. Symptom relief and side effects of postmenopausal hormones: results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol. 1998;92:982–8. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Hilditch JR, Lewis J, Peter A, van Maris B, Ross A, Franssen E, Guyatt GH, Norton PG, Dunn E. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24:161–75. doi: 10.1016/s0378-5122(96)82006-8. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Marchbanks PA, McDonald JA, Wilson HG, Burnett NM, Daling JR, Bernstein L, Malone KE, Strom BL, Norman SA, Weiss LK, Liff JM, Wingo PA, Burkman RT, Folger SG, Berlin JA, Deapen DM, Ursin G, Coates RJ, Simon MS, Press MF, Spirtas R. The NICHD Women’s Contraceptive and Reproductive Experiences Study: methods and operational results. Ann Epidemiol. 2002a;12:213–21. doi: 10.1016/s1047-2797(01)00274-5. [DOI] [PubMed] [Google Scholar]
- Marchbanks PA, McDonald JA, Wilson HG, Folger SG, Mandel MG, Daling JR, Bernstein L, Malone KE, Ursin G, Strom BL, Norman SA, Wingo PA, Burkman RT, Berlin JA, Simon MS, Spirtas R, Weiss LK. Oral contraceptives and the risk of breast cancer. New Engl J Med. 2002b;346:2025–32. doi: 10.1056/NEJMoa013202. [DOI] [PubMed] [Google Scholar]
- Meeske K, Press M, Patel A, Bernstein L. Impact of reproductive factors and lactation on breast carcinoma in situ risk. Int J Cancer. 2004;110:102–9. doi: 10.1002/ijc.20072. [DOI] [PubMed] [Google Scholar]
- Meyerowitz BE, Desmond KA, Rowland JH, Wyatt GE, Ganz PA. Sexuality following breast cancer. J Sex Marital Ther. 1999;25:237–50. doi: 10.1080/00926239908403998. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. Muthen and Muthen; Los Angeles, CA: 1998. [Google Scholar]
- Patel AV, Press MF, Meeske K, Calle EE, Bernstein L. Lifetime recreational exercise activity and risk of breast carcinoma in situ. Cancer. 2003;98:2161–9. doi: 10.1002/cncr.11768. [DOI] [PubMed] [Google Scholar]
- Rugo HS, Ahles T. The impact of adjuvant therapy for breast cancer on cognitive function: current evidence and directions for research. Semin Oncology. 2003;30:749–62. doi: 10.1053/j.seminoncol.2003.09.008. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS OnlineDoc®, Version 9. SAS Institute, Inc.; Cary, NC: 2002. [Google Scholar]
- Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C. Assessing the clinical significance of single items relative to summated scores. Mayo Clin Proc. 2002;77:479–87. [PubMed] [Google Scholar]
- Stanton AL, Bernaards CA, Ganz PA. The BCPT Symptom Scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–56. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- Ware JE., Jr. The SF-36 health survey. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Lippincott-Raven; Philadelphia: 1996. pp. 337–45. [Google Scholar]