Abstract
Background
The lack of effective “in vivo” and “in vitro” models to predict success of pharmacological therapy for patients with renal cell carcinoma, as well as, the variety of cancer cell types demands the development of better experimental models to understand the pathophysiology of the disease and evaluate drug sensitivity in vitro.
Purpose
To develop primary renal cancer cell culture irrespective of tumor grade and tumor type, harvested from the patient's pathological specimen immediately after the laparoscopic radical nephrectomy to study potential “in vitro” pharmacological sensitivity.
Materials and Methods
A total of 24 patients (17 males and 7 females). Mean age of 63.1±3.1 y.o. The mean size of the renal masses was 7.56±3.1 cm. Normal and pathological renal tissue was collected immediately after the specimen was extracted and submitted to enzymatic digestion for 16–24 hours. Clear cell carcinoma cells were selected through multiple passages in DMEM medium supplemented with glucose and antibiotics.
Results
Establishment of cell line culture from all the patients' specimens irrespective of tumor grade and tumor type was achieved successfully. In addition to the tumor cell line culture, normal parenchyma tissue yielded primary cell lines to allow testing the response of tumor types to various pharmacological therapeutic agents and toxicity of such treatments to healthy tissue. From the initial collection of the specimens obtained after the removal of the kidney to the development of cell lines took occurred in average 32+6 hrs. The cells in culture showed characteristics of epithelial cells; like expression on cytokeratin and were maintained in culture for more than 20 passages.
Conclusion
The development of renal cancer cell cultures in vitro is labor intense but may yield a more realistic model to tailor pharmacological therapies and predict therapeutic success prior to “in vivo” application—a step in the direction of individualized medicine for RCC.
Introduction
Renal cell carcinomas (RCCs) are the most frequent tumors of the kidney. More than 30,000 new cases are diagnosed a year in USA and mortality due to RCC is approximately over 12,000 cases a year.1 Despite new drugs targeting specific pathways an optimal medical therapy is sought after.2
RCC is characterized by different subtypes and account for about 3% of all human adult malignant diseases. The etiology of RCC is obscure and although the majority of renal tumors develop as sporadic forms rare familial forms were described3 and distinct genetic abnormalities in the different subtypes were observed. These tumors that differ also for natural history and prognosis may be adequately defined by a systematic investigation of gene expression and/or proteomic profile that allows for the identification of protein changes caused by the disease process.
Clear cell carcinoma, the most frequent subtype of RCC, originates from the proximal tubular epithelium in the renal cortex.3 Molecular analysis and proteome profile of this solid tumor is however complex and complicated due to the mixture of tumor cells and normal cells, such as leukocytes and connective tissue cells. To better understand this problem, the tumor cells may be adapted to grow in vitro, as primary cell cultures, to provide a more homogeneous cellular material for studying the biochemical and molecular changes associated to the neoplastic status. The disadvantage of establishing primary cell cultures is that the cells tend to quickly dedifferentiate as they are maintained in culture. Therefore, it may be difficult to observe altered marker expression which can be unambiguously assigned to the respective ancestor cell.
We have previously described successful establishment of primary cultures of epithelial cells from kidney tissue.4 The aim of the present study was to investigate whether it is feasible to establish primary cell cultures of RCC and normal renal cortex which maintain the same phenotype as seen in the tissue, and obtain a more homogeneous and enriched cytological material. The RCC and normal cortex primary cell cultures were characterized by morphology and histology.
Materials and Methods
A total of 24 patients (17 males and 7 females) underwent laparoscopic radical nephrectomy after evaluation for possible metastatic disease; including chest computed tomography (CT) without contrast and abdominal and pelvic CT with and without intravenous contrast. Patient mean age was 63.1±3.1 y.o. and the mean size of the renal masses was 7.56 ±3.1 cm. Normal and pathological renal tissue was collected immediately after the specimen was extracted and submitted to enzymatic digestion for 16–24 hours.
Chemicals and reagents
The tissue digesting enzymes collagenase, hyaluronidase, and DNasel, other chemicals, and EDTA and Ficoll were obtained from Sigma. Tissue culture medium, PBS, the antibiotics penicillin and streptomycin, fluorophore tagged secondary antibodies and DAPI were obtained from Invitrogen®. MEM D-valine modification medium was obtained from United States Biochemical Corp. (Cleveland, OH). Antibodies against Cytokeratin and Desmin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell isolation and purification from tissue
Tissues were collected from consenting patients in accordance with a Colorado Multiple Institutional Review Board approved protocol. A total of 5.5 cm3 of normal renal parenchyma and equal amount of tumoral tissue was obtained from each nephrectomy specimen. All specimens underwent histological evaluation and corresponded to the gross anatomy (normal or tumoral). Immediately following surgery the tissue was transported from the operating room on ice. The tissue was processed within 20 minutes of surgical removal of the kidney. All different specimens were dissected under sterile conditions in the laboratory and diced into small pieces on ice using sterile blade (Fig. 1). Cells were isolated from tissue as previously described.4
FIG. 1.

Renal tissue sterile preparation prior cell isolation.
Briefly, cells were isolated by digesting tissue in sterile DMEM containing collagenase type IV (2 mg/ml), hyaluronidase type V (0.2mg/ml) and DNaseI (24 ng/ml). Digestion was continued for 16 to 20 hours at room temperature with constant gentle shaking. After digestion, cells were filtered through a nylon cell strainer (BD Falcon). The cells were washed twice in sterile PBS and recovered by centrifugation, as described. A Ficoll density gradient centrifugation step was done to remove cell debris and red blood cells from the cell suspension. Renal cells separated in the gradient were washed with PBS and recovered by centrifugation (Fig. 2).
FIG. 2.
Cell gradient/isolation—28 hrs post collection of specimen.
Cell culture and passage
Tumor cells were seeded in 25 mm flasks in DMEM supplemented with 10% FBS and antibiotics and maintained at 37°C in a humidified 5% CO2 environment. Cells obtained from histological “normal” tissue were maintained in D-Valine supplemented DMEM as described.4 After three days non-adhering cells were removed by washing the cells with sterile PBS. A confluent monolayer of epithelial cells was passaged with 0.25% trypsin—EDTA and incubation at 37°C for 5 minutes. The cells were removed by gently tapping the sides of the flask and then seeded at appropriate cell density in a new flask (Fig. 3).
FIG. 3.

Renal cell culture.
Expression of specific markers
Marker protein expression was visualized by antibodies directed against the markers. In these studies mouse monoclonal antibody against human Cytokeratin proteins (cytokeratins 4, 5, 6, 8, 10, 13, and 18), rabbit polyclonal antibody against human Desmin were used.
Briefly, cells were grown to about 50% confluence on 8-well chamber slides (Nalge® Nunc™), washed with sterile PBS, fixed for 20 minutes in 3.7% formaldehyde and permeabilized for 5 minutes at −20°C in acetone and methanol (7:3) mixture. After blocking with 10% Donkey serum the cells were incubated with an appropriate dilution of primary antibody for one hour at room temperature. Excess antibody was removed by washing the cells twice in PBS for five minutes each. Bound antibody was detected by incubation with Cy3 conjugated secondary antibodies. Nuclei were counterstained with DAPI (10 ng/ml) for five minutes and then washed with PBS again. Images were captured using an RXDA immunofluorescence microscope (Leica, Solms, Germany). Appropriate negative controls were included to ascertain positive antibody reactivity.
Hematoxylin and eosin staining
Cells in culture on eight well chamber slides were stained with Hematoxylin and Eosin was done using standard procedures.5
Nomenclature
We have denoted generically the primary cell line cultures as KIKO (Kim, Koul), the last name initials of the first and senior author of this manuscript. Then, the different histological types were classified as: Renal Cell Carcinoma (RCC) Clear Cell—KIKO CC; RCC Chromophobe-KIKO CHROMO; and the RCC Sarcomatoid type—KIKO SARC. The benign cell types were defined as: Angiomyolipoma (AML)—KIKO AML, and Oncocytoma-KIKO ONCO.
The different Fuhrman grades were defined as: 100 for grade 1; 200 for grade 2; 300 for grade 3; and 400 grade 4 (Table 1). Therefore, if the cell line was characterized as Renal cell cancer Chromophobe type grade 4, the cell line culture would be called KIKO Chromo 400.
Table 1.
Nomenclature of Primary Renal Cell Line Cultures
| Histology | Renal cell line culture type |
|---|---|
| RCC clear cell | KIKO—CC |
| RCC chromophobe | KIKO—CHROMO |
| RCC sarcomatoid | KIKO—SARC |
| Angiomyolipoma | KIKO—AML |
| Oncocytoma | KIKO—ONCO |
| FG | Renal cell line culture grade |
| Grade 1 | 100 |
| Grade 2 | 200 |
| Grade 3 | 300 |
| Grade 4 | 400 |
FG, Fuhrman grade; RCC, renal cell cancer; CC—RCC clear cell; PAP, RCC papillary; Chromo, RCC chromophobe; AML, angiomyolipoma; ONCO, oncocytoma.
Results
Establishment of cell line culture from all the patients' specimens irrespective of tumor grade and tumor type was achieved successfully. Cells in culture showed uniform morphology as observed by hematoxylin and eosin staining (Fig. 4).
FIG. 4.
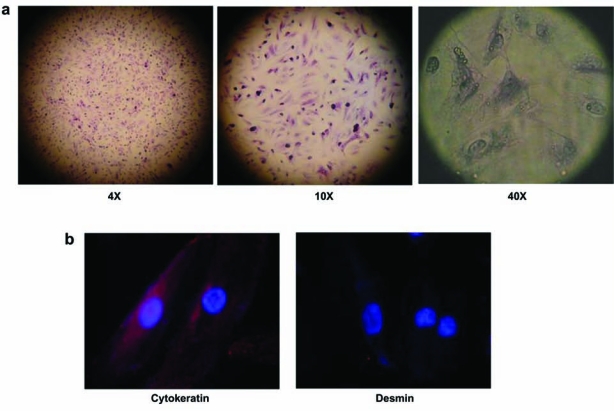
(a) Representative hematoxylin and eosin staining of renal clear cell carcinoma (Fuhrman Grade 4) cells in culture; (b) Cells from the same culture as in (a) staining for cytokeratin proteins and negative for desmin (63× objective view).
Expression of specific markers
To confirm that the cells in primary culture maintained their epithelial phenotype, we immuno-stained the cells with antibody against Cytokeratin. All the cells in culture stained positive for Cytokeratin, showing that the cells maintained their epithelial nature (Fig. 1). Staining for Desmin (a marker for muscle cells)6 was negative, confirming that the cell culture was not contaminated by any cells from muscle tissue.
A total of 48 different renal primary cell line cultures were obtained (Table 2). Among these cultures, nineteen were RCCs (14 clear cell type, one Papillary, one Sarcomatoid and 2 Chromophobe type). Among the benign cell types: 3 were Oncocytoma and 3 AMLS. All tumoral cells lines had their corresponding normal cell lines. In addition to the tumor cell line culture, normal parenchyma tissue yielded primary cell lines to allow testing the response of tumor types to various pharmacological therapeutic agents and toxicity of such treatments to healthy tissue. From the initial collection of the specimens obtained after the removal of the kidney to the development of cell lines took occurred in average 32±6 hrs.
Table 2.
List of Primary Cultures Obtained from Renal Cell Carcinomas—Demographic and Histological Data
| Pt | Sex | Age | Diagnosis | FG | size (cm) | Tumor stage |
|---|---|---|---|---|---|---|
| 01 | M | 34 | RCC CC | 2 | 6.5 | pT1b, Nx, Mx |
| 02 | M | 63 | ONCO | na | 4.0 | na |
| 03 | F | 43 | RCC SARC | 4 | 29.0 | pT2, N0, M0 |
| 04 | M | 53 | RCC CC | 3 | 10.0 | pT3a, Nx, Mx |
| 05 | M | 31 | CHROMO | 4 | 3.0 | pT1a, Nx, Mx |
| 06 | M | 67 | RCC CC | 4 | 7.5 Multiple masses | pT4, Nx, Mx |
| 07 | M | 80 | RCC CC | 3 | 8.0 | pT2, N0, Mx |
| 08 | M | 73 | AML | na | na | na |
| 09 | F | 59 | RCC CC | 1 | 8.3 | pT2, N0, Mx |
| 10 | F | 53 | RCC CC | 2 | 6.0 | pT1b, Nx, Mx |
| 11 | F | 83 | RCC CC | 3 | 3.7 | pT3a, Nx, Mx |
| 12 | M | 55 | RCC CC | 2 | 4.0 | pT3b, Nx, Mx |
| 13 | M | 67 | RCC CC | 2 | 3.5 | pT1a, Nx, Mx |
| 14 | M | 85 | CHROMO | 1 | 5.1 | pT3a, Nx, Mx |
| 15 | M | 65 | RCC Pap | 3 | 3.5 | pT3a, Nx, Mx |
| 16 | M | 55 | RCC CC | 3 | 10.0 | pT3a, N0, Mx |
| 17 | F | 65 | AML | na | 4.0 | na |
| 18 | M | 65 | RCC CC | 3 | 12.0 | pT3b, N0, Mx |
| 19 | F | 86 | RCC CC | 1 | 10.1 | pT2b, N0, Mx |
| 20 | F | 56 | RCC CC | 2 | 5.0 | pT1b, Nx, Mx |
| 21 | M | 71 | ONCO | na | 4.8 | na |
| 22 | M | 83 | RCC CC | 2 | 5.3 | pT1b, Nx, Mx |
| 23 | M | 75 | RCC CC | 2 | 5.5 | pT3b, Nx, Mx |
| 24 | M | 48 | ONCO | na | 6.2 | na |
FG, Fuhrman grade; RCC, renal cell cancer; CC, RCC clear cell; PAP, RCC papillary; CHROMO, RCC chromophobe; SARC, sarcomatoid; AML, angiomyolipoma; ONCO, oncocytoma.
Discussion
The purpose of the present study was to evaluate the feasibility to develop primary renal cancer cell culture irrespective of tumor grade and tumor type, harvested from the patient's pathological specimen immediately after the laparoscopic radical nephrectomy.
The renal epithelial primary cell cultures, both normal and tumoral, preserve in vivo properties that potentially could tailor adjuvant medical therapy, allowing pharmacological evaluation of sensitivity/toxicity and therapeutic responses that could predict “in vivo” responses in the same patient to a specific drug. Several criteria must be considered and fulfilled in order to attain this goal. Indeed, the primary cells must proliferate well in culture, the origin within the nephron of the cells must be defined, and the cytological composition must be the most homogeneous as possible to help in defining the proteomic pattern underlying normal kidney and RCC. This study confirmed that normal kidney and RCC cells can be successfully adapted to in vitro growth for a few passages.
The rate of success obtained in establishing primary cell line cultures was surprisingly high. Kidney epithelial cells have a propensity to be adapted to in vitro growth and, as it has been reported2, the normal cells grow even better than the tumoral ones. This propensity was exploited during the establishment of primary cultures. In fact, the plated tissues were removed from dishes after seven days when a number of epithelial cells had spread out but the fibroblasts that can easily be distinguished from renal epithelial cells, had not yet started to massively grow out from the frustules. In this manner, fibroblast overgrowth was not a significant problem as in agreement with others.8,9,10
The cytological homogeneity of our primary cultures was evaluated by immunocytochemical stains at passage three, and in all instances the reactions were homogeneous in intensity and had identical profiles. This characterization showed that the cells in cultures was more than 90% homogeneous and was composed of tubular cells. The reason tubular cells are the most represented in the cultures is probably due to their high proliferative potential.
The immunocytochemical stains outlined that the cultured cells maintained the characteristics observed in the original tissue. The cultured tumor cells coexpress cytokeratin as in the tumor tissue and do not stain for Desmin confirming their epithelial origin. Additional studies with respect to characterization of these cells cultures are currently in progress to evaluate feasibility of using these cells as model systems for individualized therapeutic options.
Conclusion
In conclusion, these primary cultures at low passages seem to retain a great similarity with tissue and may be a useful model complementary to tissue specimens. Primary cultures may be employed for studies concerning low abundance and differentially expressed proteins, and altered patterns of metabolic pathways characterizing RCC. However, research dealing with primary culture utilization for proteomic investigation needs to be continued because the data utility must be validated by a wider number of reports since the risk that the cells change phenotype as they are maintained in culture is always possible. Clearly additional studies are warranted to fully characterize these cells in culture and to evaluate if they retain all the characteristics of primary tumor, or at least to distinguish their characteristics from the parent tissues.
Abbreviations Used
- CT
computed tomography
- RCC
renal cell carcinoma
Acknowledgments
The authors gratefully acknowledge support from NIH-2RO1DKO54084 (H. Koul) and the Department of Surgery, School of Medicine University of Colorado Denver AEF-seed grant funds (F. Kim and H. Koul).
Disclosure Statement
No competing interests exist.
References
- 1.Kim FJ. Rha KH. Hernandez F. Jarrett TW. Pinto PA. Kavoussi LR. Laparoscopic radical versus partial nephrectomy: assessment of complications. J Urol. 2003 Aug;170((2 Pt 1)):408–11. doi: 10.1097/01.ju.0000076017.26789.6a. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ. Hutson TE. Tomczak P. Michaelson MD. Bukowski RM. Rixe O. Oudard S. Negrier S. Szczylik C. Kim ST. Chen I. Bycott PW. Baum CM. Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Bonné AC. Bodmer D. Schoenmakers EF. van Ravenswaaij CM. Hoogerbrugge N. van Kessel AG. Chromosome 3 translocations and familial renal cell cancer. Curr Mol Med. 2004;4:849. doi: 10.2174/1566524043359593. [DOI] [PubMed] [Google Scholar]
- 4.Khandrika L. Kim FJ. Campagna A. Koul S. Meacham RB. Koul HK. Primary culture and characterization of human renal inner medullary collecting duct epithelial cells. J Urol. 2008;179(5):2057–2063. doi: 10.1016/j.juro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Maroni PD. Koul S. Meacham RB. Chandhoke PS. Koul HK. Effects of oxalate on IMCD cells: a line of mouse inner medullary collecting duct cells. Ann N Y Acad Sci. 2004;1030:144. doi: 10.1196/annals.1329.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulin D. Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Detrisac C J. Sens M A. Garvin A J. Spicer S S. Sens D A. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney International. 1984;25:383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- 8.Sordillo LM. Oliver SP. Akers RM. Culture of bovine mammary epithelial cells in D-valine modified medium: selective removal of contaminating fibroblasts. Cell Biol Int Rep. 1988;12:355. doi: 10.1016/0309-1651(88)90060-4. [DOI] [PubMed] [Google Scholar]
- 9.Hawksworth GM. Isolation and culture of human renal cortical cells with characteristics of proximal tubules. Methods Mol Med. 2005;107:283. doi: 10.1385/1-59259-861-7:283. [DOI] [PubMed] [Google Scholar]
- 10.Aub M. Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980;105:369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]


