Abstract
Cathepsins B and L contribute to Ebola virus (EBOV) entry into Vero cells and MEFs. However, the role of cathepsins in EBOV-infection of human dendritic cells (DCs), important targets of infection in vivo, remains undefined. Here, EBOV-like particles containing a beta-lactamase-VP40 fusion reporter and Ebola virus were used to demonstrate the cathepsin-dependence of EBOV entry into human monocyte-derived DCs. However, while DC-infection is blocked by cathepsin B inhibitor, it is insensitive to cathepsin L inhibitor. Furthermore, DCs pretreated for 48 hours with TNFα were generally less susceptible to entry and infection by EBOV. This decrease in infection was associated with a decrease in cathepsin B activity. Thus, cathepsin L plays a minimal, if any, role in EBOV infection in human DCs. The inflammatory cytokine TNFα modulates cathepsin B activity and affects EBOV entry into and infection of human DCs.
Introduction
Ebola viruses (EBOV) are members of the family Filoviridae and are the etiologic agents of severe hemorrhagic fever in infected humans. Infection with the Zaire EBOV causes a rapid hemorrhagic fever that can result in death within the second week after the onset of symptoms (Mahanty et al., 2004). Fatal human cases of Ebola hemorrhagic fever (EHF) are associated with a deregulated immune response (Bray et al., 2005, Hoenen et al., 2006) that lacks an effective cellular immune response (Sanchez et al., 2004) and in comparison with nonfatal cases, demonstrates a poor humeral response (Baize et al., 1999). Antigen presenting cells such as DCs act as a vital link between the early innate and later adaptive immune response (Reis e Sousa, 2004) and in vivo serve as early targets of EBOV infection (Ryabchikova et al., 1999, Geisbert et al., 2003, Bray et al., 2005). Furthermore in vitro studies demonstrate that upon EBOV infection, DCs become deregulated and fail to secrete cytokines or to stimulate T cells (Bosio et al., 2003, Mahanty et al., 2003). Altogether, DCs likely play a prominent role in the pathogenesis of EHF (Bray et al., 2005). Because the earliest interactions of the virus with the innate immune response are expected to influence the outcome of disease, a complete understanding of the requirements for DC infection may facilitate the development of therapeutic approaches toward these deadly pathogens (Bray et al., 2005).
The EBOV glycoprotein (GP) mediates EBOV attachment and entry via an endosomal pathway. Endosome acidification activates cathepsin-mediated cleavage of GP which is required for entry (Takada et al., 1997, Chan et al., 2000, Wool-Lewis et al., 1998, Brindley et al., 2007, Chandran et al., 2005, Kaletsky et al., 2007, Sanchez, 2007, Schornberg et al., 2006). Therefore, cathepsins may be a viable target for therapeutic intervention. Although the mechanisms by which cathepsins promote EBOV entry have not been completely resolved, studies performed with chemical inhibitors, knock-out cells and siRNA knockdowns demonstrate a role for both cathepsins B and L in EBOV entry into Vero cells and mouse embryonic fibroblasts (Chandran et al., 2005, Kaletsky et al., 2007, Schornberg et al., 2006). However, the entry and infection requirements of human DCs remain unexplored. Human monocyte-derived DCs (DCs) reportedly express both cathepsin B and L (Zavasnik-Bergant et al., 2005, Kessler et al., 2008). Moreover, humans DCs contain active cathepsin B, and some studies suggest that cathepsin L activity is comparatively lower than cathepsin B activity or is lacking in DCs (Burster et al., 2005a, Fiebiger et al., 2001). The possible difference in cathepsin activity in Vero cells as compared to human DCs suggests that the cathepsin requirements might differ for EBOV entry into DCs as compared to fibroblast-like cells. Therefore, this study addressed the role of cathespins B and L in EBOV infection of human DCs.
Results and Discussion
Ebola virus-like particles (VLPs) were produced by co-expressing the EBOV matrix protein, VP40, fused to β-lactamase (Simmons et al., 2003) and the EBOV GP. EBOV VLPs possess a structure and biochemical composition similar to authentic EBOV (Jasenosky et al., 2001, Timmins et al., 2001), and have previously been used to study the initial interactions of EBOV with dendritic cells and to examine EBOV budding (e.g. (Yasuda et al., 2003, Jasenosky et al., 2001, Licata et al., 2003, Harty et al., 2000, Bosio et al., 2004, Ye et al., 2006, Martinez et al., 2007). The introduction of β-lactamase by VLPs into the cytoplasm of cells is measured by fluorescence emission of a membrane-permeable β-lactamase substrate (CCF-2AM, Invitrogen). Cells are loaded with the substrate whereupon cytoplasmic esterases cleave the substrate generating a charged β-lactamase substrate which is retained in the cell. Initially, this substrate fluoresces green. However, upon cleavage by β-lactamase in the cell cytoplasm, it fluoresces blue. The enzymatic activity of the β-lactamase-tag in the VLPs can be detected using a fluorescence microscope, fluorescence plate reader or flow cytometry to measure VLP entry within 4 hours of infection and, unlike pseudotyped virus systems, does not require post-entry steps in the virus replication cycle (Cavrois et al., 2002).
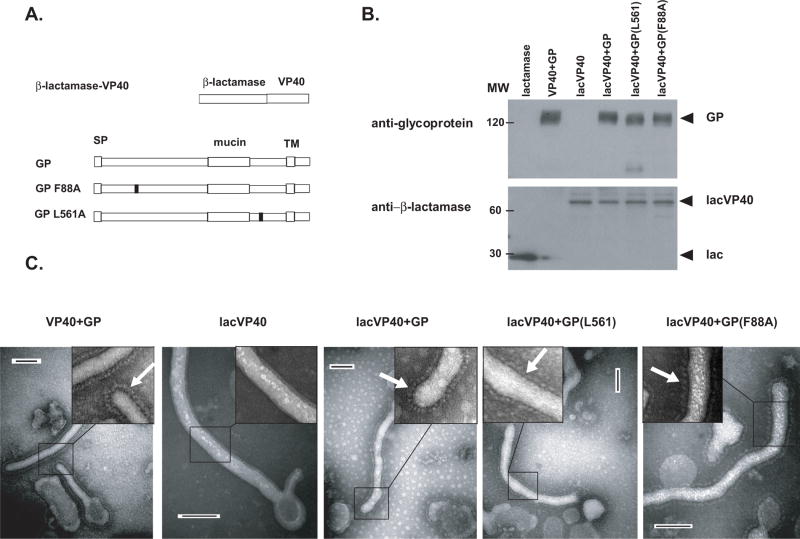
Wild-type VP40 or the VP40-β-lactamase fusion construct (lacVP40) were then co-expressed with wild-type or one of two mutant forms of the EBOV GP, L561A and F88A (Figure 1A). These GP-mutants are defective in mediating entry into target cells because of presumed defects in fusion (Watanabe et al., 2000) and receptor binding (Brindley et al., 2007, Manicassamy et al., 2005, Mpanju et al., 2006), respectively. Protein equivalents of purified VLPs were then analyzed by western blot with an anti-β-lactamase antibody. B-lactamase-VP40 fusion protein (Figure 1B) was detected in the lacVP40 (lane 3), lacVP40+GP (lane 4), lacVP40+GP L561A (lane 5) or lacVP40+GP F88A (lane 6) VLPs, but not the VP40+GP (lane 2). Similar levels of wild type GP (lanes 2, 4), mutants GP F88A (lane 5) and GP L561A (lane 6) were detected in VLPs, as determined by blotting with the 9C11 anti-GP antibody. Purified VLPs were then examined by electron microscopy (Figure 1C, compare VP40+GP, lacVP40, lacVP40+GP, lacVP40+GP L561A mutant or lacVP40+GP F88A). Each of the VLP preparations except for the lacVP40 (second panel from left) demonstrated a decorated surface (denoted by white arrows) that presumably represents the surface GP (Figure 1C).
Figure 1. Purified Ebola VLPs.
(A) Illustrations of the proteins used to produce Ebola VLPs. The first protein was constructed by fusing β-lactamase to the N-terminus of the Ebola virus VP40 protein (lacVP40). The next three illustrations depict the wild type ebola virus attachment and fusion glycoprotein (GP) and two other GP mutants unable to mediate entry containing single amino acid mutations at positions 88 and 561. SP=signal peptide. TM=transmembrane domain. (B) VLPs produced by expressing VP40 + GP (lane 2), lacVP40 (lane 3), lacVP40 and GP (lane 4), lacVP40 + GP L561A mutant (lane 5) and lacVP40 + GP F88A mutant (lane 6) were purified and equivalent amounts subjected to SDS-PAGE and western blotting. Purified TEM-1 (β-lactamase) was included as a control (lane 1). Blotted proteins were then stained for glycoprotein and lactamase enzyme using anti-GP monoclonal antibody 9C11 and anti-lactamase antibody, respectively. (C) VP40+GP, lacVP40, lacVP40+GP, lacVP40+GP (L561A) and lacVP40+GP (F88A) VLPs VLPs were purified, stained with 1% phosphotungstic acid and subjected to electron microscopy. White arrows point to decorated surface of the VLPs (see inset). Bar length represents 100nm.
Protein equivalents of the VP40+GP (lane 2), lacVP40 (lane 3), lacVP40+GP (lane 4), lacVP40+GP L561A mutant (lane 5) or lacVP40+GP F88A (lane 6) were lysed and tested for total β-lactamase activity (Figure 2A). Similar β-lactamase activity was detected for each of the lac-VP40 containing VLPs, while buffer (lane 1) and VP40+GP VLPs (lane 2) demonstrated background levels. These data (Figure 1B and Figure 2A) demonstrate equivalent incorporation of the lac-VP40 protein into the different VLP preparations and similar incorporation of wild type and mutant GPs.
Figure 2. Wild type Ebola GP mediates entry into human DCs.
(A) Protein equivalents of purified VP40+GP (lane 2), lacVP40 (lane 3), lacVP40+GP (lane 4), lacVP40+GP L561A mutant (lane 5) or lacVP40+GP F88A (lane 6) VLPs were lysed and tested for total β-lactamase activity using a fluorogenic substrate (Lytic Blazer, Invitrogen, Carlsbad, CA). The y-axis denotes the RFU (relative fluorescence units) × 1000. (B and C) Mock (histogram 1) and lactamase equivalents of VP40+GP (histogram 2 and second dotplot), lacVP40 (histogram 3 and third dotplot), lacVP40+GP (histogram 4 and fourth dotplot), lacVP40+GP (L561A) (histogram 5 and fifth dotplot) and lacVP40+GP (F88A) (histogram 6 and sixth dotplot) VLPs were incubated with human DCs for 3 hours to allow infection after which DCs were loaded with fluorogenic CCF2-AM substrate. Ebola VLP entry was assayed by measuring the amount of cytoplasmic substrate (fluoresces ~520 nm) that was β-lactamase-cleaved into its product (fluoresces ~460nm) using a (B) FLUOstar OPTIMA plate reader (expressed as a ratio) and (C) using an LSR II flow cytometer (data expressed as a percentage of cells that have an increased blue fluorescence and decreased green fluorescence). Entry was detected only with the VLPs produced with lacVP40 and wildtype GP (fourth panel).
Human monocyte-derived DCs (CD14-, CD11c+ and HLA-DR+) were then infected with wild-type- or mutant-GP VLPs (equivalent amounts of β-lactamase were used), to test whether GP mutants defective in mediating entry into Vero cells (Manicassamy et al., 2005, Watanabe et al., 2000, Brindley et al., 2007, Mpanju et al., 2006) would also be impaired in entry into human DCs. β-lactamase activity in the cytoplasm of cells was measured by fluorescence emission of the membrane-permeable β-lactamase substrate (CCF-2AM, Invitrogen). DCs were infected for 3 hours with β-lactamase equivalents of VP40+GP (lane 2), lacVP40 (lane3), lacVP40+GP (lane 4), lacVP40+GP L561A (lane 5) or lacVP40+ GP F88A (lane 6) VLPs. Shown in Figure 2B is the ratio of blue to green fluorescence as read by a fluorometer (DTX 880). Increased blue/green fluorescence was detected from the human DCs treated with lacVP40+ GP VLPs (lane 4) relative to the other samples. Similarly, DCs were incubated with the same VLPs and analyzed using an LSR II flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Only lacVP40+GP VLPs exhibited entry (4th dotplot, Figure 2C). Also shown in the 4th dotplot of Figure 2C is the percentage of DCs (47%) into which VLPs entered. Taken together, these entry assay data confirm previous studies, performed in other cell types, where F88A and L561A GP mutants were defective for entry. Therefore, the general requirements for entry into human DCs are similar to the requirements for entry into fibroblasts.
To assess the general role of cathepsins for EBOV entry into DCs, lac-VP40 VLPs were prepared with either EBOV GP or VSV-G as the surface glycoprotein (Figure 3A and B). EBOV GP-entry into Vero cells is cathepsin-dependent whereas VSV-G-mediated entry is not (Kallstrom et al., 2005, Schornberg et al., 2006, Chandran et al., 2005). Western blotting of purified VLPs produced by expression of lac-VP40+GP or lacVP40+VSV-G in 293T cells (Figure 3A) demonstrates that both contain β-lactamase and either GP or VSV-G, respectively. Also, unlike VLPs produced by lac-VP40 in the absence of any additional viral glycoprotein (Figure 1C), electron microscopy of lacVP40+VSV-G VLPs demonstrate a decorated VLP surface, suggesting glycoprotein incorporation into the VLPs (white arrow in Figure 3B). Entry of lacVP40+GP VLPs into DCs was inhibited (Figure 3C top panel) by pretreatment of DCs for 1 hour with non-toxic levels of pan-cathepsin inhibitor E64d (50-100μM). However, the pretreatment had no effect on entry of VSV-G containing VLPs (Figure 3C). To confirm the role of cathepsins in EBOV infection, DCs were then infected, in BSL4 containment, with EBOVGFP, an EBOV engineered to express GFP ((EBOVGFP) at MOI=1 or MOI=5 (Figure 3D top panel and bottom panel, respectively). It is interesting to note that the efficiency with which DCs were infected varied from donor to donor, and the infection efficiency was typically less than that observed in Vero cells (Figure 3D). The reasons for this are unclear, but as demonstrated below, exposure of DCs to select cytokines can influence EBOV entry and infection. Forty-eight hours post-infection, DCs were harvested, and gated live cells were measured for GFP expression by flow cytometry. Pretreatment of DCs with E64d greatly inhibited infection (>80% inhibition, Figure 3D), indicating that EBOV requires cathepsin activity to infect DCs.
Figure 3. Cathepsin activity is required for EBOV entry into human DCs.
(A) Equivalent amounts of purifed lacVP40+GP and lacVP40+VSV-G VLPs produced by expressing lacVP40 and GP or VSV-G in 293T cells were subjected to SDS-PAGE and western blotted. Top, middle and bottom panels demonstrate anti-GP, anti-VSV-G and anti-β-lactamase stains, respectively. Top to bottom, arrows denote position of GP, VSV-G and lacVP40, respectively. (B) lacVP40+VSV-G VLPs were purified, stained with 1% phosphotungstic acid and subjected to EM. Shown are two examples of the VSV-G containing VLPs. White arrows point to decorated surface of the VLPs. Bar length represents 100nm. (C) Entry assays. Histograms represent percentage of dendritic cells infected with (top panel) lacVP40+GP VLPs and (bottom panel) lacVP40+VSV-G VLPs. Control lane represents DCs pretreated with diluent and E64d lane represents DCs pretreated with pan-cathepsin inhibitor E64d. (D) Histograms represent the percentage of DCs infected and expressing GFP from EBOVGFP at 48 hours post-infection at MOI of 1 (top panel) and MOI of 5 (bottom panel) as determined by flow cytometry. First lane represents DCs only, second lane is EBOVGFP infected DCs, third lane represents mock-treated EBOVGFP infected DCs and the last lane represent E64d pretreated EBOVGFP infected DCs.
Specific inhibitors of cathepsin B and L inhibit EBOV infection of Vero cells (Brindley et al., 2007, Chandran et al., 2005, Kaletsky et al., 2007, Sanchez, 2007, Schornberg et al., 2006). Therefore, the effect of specific cathepsin B inhibitor CA-074 Me (Calbiochem) and cathepsin L inhibitor Z-FY(t-BU)dmk (Calbiochem) on entry of lac-VP40+GP VLPs and on EBOVGFP infection was compared in Vero cells (Figure 4, A, C, and E) and DCs (Figure 4, B, and D). Vero cells and DCs were plated in DC media supplemented with 1% DMSO and either mock-treated or pretreated with decreasing concentrations of inhibitor (Figure 4 A-E) for 2 hours prior to infection at the indicated MOIs. Overall infection efficiencies of Vero and DC infections ranged from 40-80% (data not shown). Shown in Figure 4A and 4B is the relative entry of Ebola VLPs as well as the relative infection (as measured by GFP expression from EBOVGFP) either 24 hours (in the case of Veros, MOI=1) and 48 hours (in the case of DCs, MOI=5) post-incubation. In Figure 4A, a dose dependent inhibition of both Ebola VLP entry and EBOV infection into Vero cells was detected in the presence of cathepsin B- or L-inhibitor. In contrast, although there is a dose-dependent effect of cathepsin B inhibitor on Ebola VLP entry and EBOV infection into DCs, cathepsin L inhibitor did not adversely affect either Ebola VLP entry or EBOV infection (Figure 4B). This observed lack of Ebola VLP entry and EBOV infection inhibition has been confirmed using DCs from five different donors.
Figure 4. Effect of cathepsin B and L inhibitors on Ebola VLP entry and EBOV infection of VEROs and DCs.
VERO (A, C, E) and DCs (B, D) were pretreated with mock and decreasing concentrations of cathepsin B and cathepsin L, respectively. Also, VERO (A) and DCs (B) were mock and VLP treated or EBOVGFP-infected (denoted by bar under graphs). Solid bars represent relative percentage of VEROs (A) and DCs (B) infected by VLPs and white bars represent relative percentage of VEROs (A) and DCs (B) infected by EBOVGFP. Cathepsin B (C, D) and cathepsin L (E) activity was measured in duplicate (solid and white bars) using a fluorogenic substrate and expressed as relative fluorenscence units (RFU) using equivalent amounts of pretreatred VEROs (C, E) and DCs (D). (F) Equivalent amounts of VERO (lane 1) and DC (lane 2) lysates (same lysates used to determine cathepsin activity) were subjected to SDS-PAGE and western blotting using anti-cathepsin L antibody (204106, R&D systems, Minneapolis, MN). Arrow denotes positions of Cathepsin L species. (G) VP40+GP VLPs were incubated for 1.5 hours with decreasing concentrations of recombinant cathepsin L (5, 1, 0.2, 0.04 μg/mL) and subjected to western blotting using a polyclonal anti-GP antibody. Arrow denotes positions of GP species.
The lysates of treated Vero cells and DCs were tested for specific cathepsin B and L activities (cathepsin activity kits from Calbiochem, Gibbstown, NJ). Notably, these inhibitors show some cross inhibition, with the highest concentrations of cathepsin L inhibitor causing a modest inhibition of cathepsin B activity (Figure 4C and D) and with the cathepsin B inhibitor causing an inhibition of cathepsin L activity in Vero cells (Figure 4E). At the concentrations of cathepsin B inhibitor (1, 0.3, 0.1μM) used, little cathepsin B activity was detected in Vero cell lysates (Figure 4C), while the lysates retained a somewhat reduced cathepsin L activity (Figure 4E). This residual cathepsin L activity might contribute to the Ebola VLP entry and EBOV infection seen in Vero cells when cathepsin B is inhibited (Figure 4A). At the concentrations of cathepsin L inhibitor (1, 0.3, 0.1μM) used, Vero lysates demonstrated little cathepsin L activity (Figure 4E) but retained cathepsin B activity (Figure 4C) which could account for the small amount of Ebola VLP entry and EBOV infection seen under cathepsin L inhibition (Figure 4A). Likewise, cathepsin B inhibitor-treated DCs contained little if any cathepsin B activity (Figure 4D). Strikingly, even in untreated DCs, no cathepsin L activity was initially detected using the cathepsin L enzyme activity assay which has a minimum detectable activity (sensitivity) of 1.2 ng/mL. When greater concentrations of DC lysates were tested, approximately 1000-fold less cathepsin L activity was present in DCs as compared to Vero cells (not shown), despite the apparent similar levels of cathepsin L protein in the lysates of DCs and Vero cells (Figure 4F). Of note, western blotting demonstrates that the predominant form of cathepsin L found (Burster et al., 2005b) in human DCs (≤25 kDa) is runs on SDS-PAGE at a significantly lower molecular weight that that found in the Vero lysates (~26 kDa). The Vero cell cathepsin L band corresponds in size to the active form of cathespin L (Honey et al., 2001). We also confirm that commercially purchased cathepsin L can process the Ebola GP (Figure 4G) contained in our preparations of VP40+GP VLPs to the small ~20KDa species seen in previous studies (Kaletsky et al., 2007, Chandran et al., 2005, Brindley et al., 2007, Schornberg et al., 2006). At the concentrations of cathepsin L inhibitor used (1, 0.3, 0.1 μM) there was some decrease in cathepsin B activity in DCs (Figure 4D), but this was insufficient to decrease VLP entry and EBOV infection (Figure 4B and D). The effect of the cathepsin L inhibitor on cathepsin B activity would suggest that the inhibitor was functional. Altogether, the data suggest that cathepsin L activity in not required for EBOV entry into DCs. Additionally, these data suggest a prominent role for cathepsin B in EBOV entry into DCs. We therefore asked whether entry into DCs can be modulated by factors that alter cathepsin B activity in DCs.
We asked whether inflammatory cytokines TNFα, IL-6 or IL-1 could modulate cathepsin B activity in DCs (Figure 5). Immature DCs were mock, TNFα, IL-6 and IL-1 treated for 2, 24 and 48 hours after which DCs were lysed and relative cathepsin B activity was measured. As shown in the Figure 5A, TNFα, but not IL-6 nor IL-1 (not shown) treatment of DCs consistently led to a modest decrease in cathepsin B activity in lysates tested at both 24 and 48 hour timepoints. Interestingly, TNFα increased cathepsin B activity of the DCs tested at the 2 hour timepoint. Furthermore, 48 hour treatment of DCs with TNFα but not IL-6 increased surface expression of DC maturation marker CD80 (not shown). We then tested VLP entry (Figure 5B) and EBOVGFP infection (Figure 5C) of DCs pretreated for 2, 24 and 48 hours with mock, TNFα and IL-6. The relative entry of Ebola VLPs (lacVP40+GP) and EBOV infection of 48 hour TNFα-treated DCs was decreased as compared to mock (DC) and IL-6 treated DCs. The inhibition of Ebola VLP mediated entry into TNFα treated DCs showed similar results when repeated with the monocyte-derived DCs harvested from six different donors. The fact that the 2 hour TNFα-treated DCs time-point showed less of an effect on entry or infection as compared to control suggests that the inhibition of entry and infection may require differentiation of the DCs.
Figure 5. Effect of TNFα and IL-6 treatment on DC cathepsin B activity and susceptibility of DCs to EBOV entry and infection.
DCs were pretreated for 2 hours, 24 hours and 48 hours (A, B, C) with mock, TNFα and IL-6 cytokines. (A) DC cell lysate was then tested for total cathepsin B activity and is represented as relative cathepsin B activity. This experiment was repeated with similar results. (B and C) Pretreated DCs were subjected to VLP (lacVP40+GP) entry assay (B) and EBOVGFP infection (C). Shown is the relative entry and infection if the mock control is set to 1. This experiment has been repeated with similar results with at least six different donors.
These experiments strongly suggest that cathepsin L is dispensable for EBOV infection of DCs. This is in contrast to data obtained in Vero cells where inhibition or knock-down of either cathepsin B or L profoundly inhibits EBOV entry (Kaletsky et al., 2007, Sanchez, 2007, Schornberg et al., 2006, Chandran et al., 2005). However, our results are consistent with data reported by Chandran et al. which studied cathepsin B/cathepsin L double knock-out mouse embryo fibroblasts reconstituted with either cathepsin B or L. In these cells, cathepsin L could enhance entry. However, in this system cathepsin L was neither necessary nor sufficient for EBOV entry (Chandran et al., 2005). The DCs used in the present study appear to be the functional equivalent of cathepsin L knock-outs (Hsing et al., 2005). Further experiments are required to understand the basis for the lack of cathepsin L activity in DCs and to determine whether cathepsin L activity might be inducible in these cells. It remains to be determined whether, in human DCs, cathepsin B is sufficient for entry or whether additional cellular proteases may also be required. We also tested the impact of cathepsin S inhibitors on Ebola VLP entry into DCs, but found no inhibition (data not shown). However, it is clear that GP can also be appropriately cleaved by another protease, thermolysin (Schornberg et al., 2006, Kaletsky et al., 2007). We also show that treatment of DCs with TNFα can modestly decrease cathepsin B activity in these cells. Following the 2 hour TNFα-treatment, VLP entry into DCs and EBOV infection of DCs is increased while 24 and 48 hour TNFα-treatment leads to decreased VLP entry and infection of DCs. Although the association is intriguing, mechanisms in addition to modulation of cathepsin B activity likely also contribute to the decrease in entry and infection. Although the inhibitions observed in DCs are modest, they suggest that it may be possible to modulate EBOV infection of this important cell type in vivo. They also raise the possibility that TNFα, if expressed at appropriate times and at appropriate levels during EBOV infection, might have a beneficial effect upon the outcome of disease. Such a role would contrast with the role of TNFα as a factor which promotes deleterious effects, such as vascular leakage (Feldmann et al., 1996), in the infected host.
Overall, our data suggest that strategies to inhibit EBOV infection based upon its cathepsin-dependence should take into account the cell-type specific activity of these enzymes and the activation status of the target cell.
Materials and methods
Cells, plasmids and antibodies
293T (human embryonic kidney) and Vero cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Invitrogen), 2 mM L-glutamine (Invitrogen), 100 units/ml of penicillin, 100 μg/ml streptomycin at 37°C in 7% CO2. The anti-GP mucin domain antibody 9C11 was developed in collaboration with the Hybridoma Center located in the Mount Sinai Department of Microbiology. The anti-GP polyclonal antibody was a kind gift from Lijun Rong. The anti-β-lactamase antibody (AB3738) was purchased from Chemicon International and anti-cathepsin L antibody (204106) purchased from R&D systems (Minneapolis, MN).
Isolation and culture of human DCs
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation (Histopaque; Sigma Aldrich, St. Louis, MO) from buffy coats of healthy human donors (New York Blood Center). CD14+ cells were immunomagnetically purified using anti-human CD14 antibody-labeled magnetic beads and iron-based Midimacs LS columns (Miltenyi Biotec, Auburn, CA). After elution from the columns, cells were plated (0.7-1×106 cells/ml) in DC media (RPMI (Invitrogen, Carlsbad, CA) supplemented with, 100 units/ml of penicillin, 100 g/ml streptomycin, 55μM β-mercaptoethanol) supplemented with 4% human serum AB (GemCell, Gemini Bio-Products, West Sacramento, CA), 500 U/ml human granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ), 500 U/ml human interleukin-4 (IL-4; Peprotech), 1ng/mL ciproflaxocin (SIGMA) and incubated for 5 to 7 days at 37°C to produce immature DCs. By day 5 immature DCs expressed surface CD11c and HLA-DR (MHC II), but not CD14.
Virus-like particle production
VLPs were produced by transfecting 18 μg of expression plasmids into 107 293T cells in 10 cm plates using lipofectamine 2000 (Invitrogen) at a 1:1 ratio of DNA to lipofectamine as recommended by the manufacturer. The EBOV Zaire VP40 or beta-lactamaseVP40 expression plasmid was transfected (12 μgs) in combination with EBOV Zaire GP, GPF88A, GPL561A or VSV-G expression plasmids (6 μgs). 48 hours post-transfection, cells and cellular debris were pelleted away from the harvested VLP-containing supernatant with a cell spin. Then, VLPs were centrifuged through a sucrose cushion at 26,000 rpm in an SW-28 rotor for 2 hours at 4°C, washed in ice-cold NTE buffer (10 mM Tris pH7.5, 100mM NaCl, 1mM EDTA) by centrifuging at 26,000 rpm for 2 hours at 4°C and then gently tapped 100 times to resuspend in 50-100 μL of NTE buffer. VLP protein content was quantified using the DC protein assay (Bio-Rad, Hercules, CA). VLPs were left on ice for up to 48 hours until used.
Electron Microscopy
10 μL of VLPs were pipetted onto 300 mesh copper grid coated with carbon film and incubated for 15 minutes at RT. Grids were then washed twice with water and negatively stained for 15 seconds using 1% phosphotungstic acid buffered to pH 7.0 with 1M ammonium hydroxide. Particles were then visualized using a Hitachi H7000 transmission electron microscope.
Flow cytometry
Flow cytometry was performed using an LSR II flow cytometer equipped with a violet laser (BD bioscience). Data were analyzed using WinMDI Version 2.8 (http://facs.scripps.edu/software.html) or Flo Jo software (Tree Star, Ashland, OR).
DC treatments
Day 5 DCs plated in 24 well dishes (0.4×106cells/well) were used for all treatments. For cathepsin inhibitor treatments, Vero cells and DCs were plated in DC media supplemented with 1% DMSO and 1% human sera AB (GemCell) and either mock-treated or pretreated with decreasing concentrations of inhibitor for 2 hours prior to infection at the indicated MOIs. For cytokine treatment DCs were stimulated with 50ng/mL TNFα or 100ng/mL of IL-6 for the indicated amount of time in DC media supplemented with 1% human serum AB (GemCell).
Western blot
4×105 DCs/stimuli were harvested, pelleted and snap-frozen in a dry-ice ethanol bath and stored at -80°C. Cell pellets were thawed on ice, resuspended in 50μL of 1X protein sample buffer, sonicated and loaded into a 4-20% gradient or 10% gels for separation by SDS-PAGE. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, blocked in 1% milk in TBS (50mM Tris pH 7.5, 100mM NaCL) for one hour, probed overnight with appropriate antibodies diluted in 0.5% milk in TBS, washed 3 times in TBS-T (TBS + 0.075% tween-20) for 5 minutes/wash, probed with secondary antibody for 1 hour and washed a final 3 times. The Western blots were developed using the Western Lightning ECL kit (Perkin-Elmer, Boston, MA) and Kodak BioMax film (Kodak, Rochester, NY).
Entry assay
The β-lactamase enzyme was fused to the amino-terminus of the Zaire Ebola virus VP40 matrix gene, via the linker peptide GSGGGSGGT. This chimeric protein exhibited β-lactamase activity when expressed in Vero and 293T cells (data not shown). VLPs produced with this construct were purified, concentrated, resuspended in NTE buffer and measured for protein concentration, as described (Martinez et al., 2007). Beta-lactamase activity in the cytoplasm of cells (entry of lactamase-VP40) can be measured by fluorescence emission of a membrane-permeable substrate that is retained in the cytoplasm (CCF-2AM, Invitrogen) that normally fluoresces green but when cleaved by beta-lactamase will fluoresce blue. Dendritic cells or Vero cells, plated in 96 well fluorescence compatible plates or 24 well plates, were treated with beta-lactamase equivalents of different VLPs, spinoculated (2000 rpm at 4°C for 1 hour) and incubated at 37°C for 3-4 hours after which substrate was added and plate incubated for 1 hour at room temperature (RT). A fluorescence plate reader was used to measure the ratio of green to blue fluorescence or a flow cytometer was used to measure the increase in blue and decrease in green fluorescence of infected cells.
Ebola Virus infection
DCs are infected with the indicated MOI or, if not indicated, an MOI=5 (as determined by titrating on Vero cells) of EBOVGFP for 1 hour with rocking every 15 minutes. Cells are then washed and grown DC media supplemented with 2% human sera. 48 hours post infection; DCs are harvested and checked for GFP expression.
Total beta-lactamase activity
Beta-lactamase activity of purified VLPs was measured using a fluorogenic substrate (Lytic Blazer, Invitrogen, Carlsbad, CA) as determined by the manufacturer.
Cathepsin L treatment Ebola VLPs
1 μg of purified VLPs were subjected to recombinant cathepsin L (219382; Calbiochem) treatment for 1.5 hours in cathepsin L activity buffer (pH 5.5 100mM Na Acetate, 2mM EDTA, 5mM DTT) after which the VLPs were western blotted and stained with an anti-GP polyclonal antibody.
Acknowledgments
This work was supported in part by funds from the NIH, including U54 AI057158 (Northeast Biodefense Center-Lipkin) and AI059536 to CFB. We would like to thank Alejandra Galvez for producing and purifying some of the virus-like particles used in this study. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
References
- Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, et al. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis. 2003;188:1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, Aman MJ, Bavari S. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology. 2004;326:280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Bray M, Geisbert TW. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. The international journal of biochemistry & cell biology. 2005;37:1560–1566. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Brindley MA, Hughes L, Ruiz A, McCray PB, Jr, Sanchez A, Sanders DA, Maury W. Ebola Virus Glycoprotein 1: Identification of Residues Important for Binding and Postbinding Events. J Virol. 2007;81:7702–7709. doi: 10.1128/JVI.02433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burster T, Beck A, Tolosa E, Schnorrer P, Weissert R, Reich M, et al. Differential Processing of Autoantigens in Lysosomes from Human Monocyte-Derived and Peripheral Blood Dendritic Cells. J Immunol. 2005a;175:5940–5949. doi: 10.4049/jimmunol.175.9.5940. [DOI] [PubMed] [Google Scholar]
- Burster T, Beck A, Tolosa E, Schnorrer P, Weissert R, Reich M, et al. Differential processing of autoantigens in lysosomes from human monocyte-derived and peripheral blood dendritic cells. J Immunol. 2005b;175:5940–5949. doi: 10.4049/jimmunol.175.9.5940. [DOI] [PubMed] [Google Scholar]
- Cavrois M, de Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotech. 2002;20:1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- Chan SY, Speck RF, Ma MC, Goldsmith MA. Distinct Mechanisms of Entry by Envelope Glycoproteins of Marburg and Ebola (Zaire) Viruses. J Virol. 2000;74:4933–4937. doi: 10.1128/jvi.74.10.4933-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal Proteolysis of the Ebola Virus Glycoprotein Is Necessary for Infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Bugany H, Mahner F, Klenk HD, Drenckhahn D, Schnittler HJ. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol. 1996;70:2208–2214. doi: 10.1128/jvi.70.4.2208-2214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebiger E, Meraner P, Weber E, Fang I-F, Stingl G, Ploegh H, Maurer D. Cytokines Regulate Proteolysis in Major Histocompatibility Complex Class II-dependent Antigen Presentation by Dendritic Cells. J Exp Med. 2001;193:881–892. doi: 10.1084/jem.193.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. The American journal of pathology. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13871–13876. doi: 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenen T, Groseth A, Falzarano D, Feldmann H. Ebola virus: unravelling pathogenesis to combat a deadly disease. Trends in molecular medicine. 2006;12:206–215. doi: 10.1016/j.molmed.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Honey K, Duff M, Beers C, Brissette WH, Elliott EA, Peters C, et al. Cathepsin S regulates the expression of cathepsin L and the turnover of gamma-interferon-inducible lysosomal thiol reductase in B lymphocytes. J Biol Chem. 2001;276:22573–22578. doi: 10.1074/jbc.M101851200. [DOI] [PubMed] [Google Scholar]
- Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev. 2005;207:229–241. doi: 10.1111/j.0105-2896.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. Ebola virus VP40-induced particle formation and association with the lipid bilayer. Journal of virology. 2001;75:5205–5214. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky RL, Simmons G, Bates P. Proteolysis of the Ebola Virus Glycoproteins Enhances Virus Binding and Infectivity. J Virol. 2007;81:13378–13384. doi: 10.1128/JVI.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallstrom G, Warfield KL, Swenson DL, Mort S, Panchal RG, Ruthel G, et al. Analysis of Ebola virus and VLP release using an immunocapture assay. J Virol Methods. 2005;127:1–9. doi: 10.1016/j.jviromet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Kessler T, Reich M, Jahn G, Tolosa E, Beck A, Kalbacher H, et al. Human cytomegalovirus infection interferes with major histocompatibility complex type II maturation and endocytic proteases in dendritic cells at multiple levels. J Gen Virol. 2008;89:2427–2436. doi: 10.1099/vir.0.2008/001610-0. [DOI] [PubMed] [Google Scholar]
- Licata JM, Simpson-Holley M, Wright NT, Han Z, Paragas J, Harty RN. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. Journal of virology. 2003;77:1812–1819. doi: 10.1128/JVI.77.3.1812-1819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4:487–498. doi: 10.1016/S1473-3099(04)01103-X. [DOI] [PubMed] [Google Scholar]
- Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170:2797–2801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- Manicassamy B, Wang J, Jiang H, Rong L. Comprehensive Analysis of Ebola Virus GP1 in Viral Entry. J Virol. 2005;79:4793–4805. doi: 10.1128/JVI.79.8.4793-4805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O, Valmas C, Basler CF. Ebola virus-like particle-induced activation of NF-kappaB and Erk signaling in human dendritic cells requires the glycoprotein mucin domain. Virology. 2007;364:342–354. doi: 10.1016/j.virol.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpanju OM, Towner JS, Dover JE, Nichol ST, Wilson CA. Identification of two amino acid residues on Ebola virus glycoprotein 1 critical for cell entry. Virus Research. 2006;121:205–214. doi: 10.1016/j.virusres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Seminars in immunology. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Ryabchikova EI, Kolesnikova LV, Luchko SV. An Analysis of Features of Pathogenesis in Two Animal Models of Ebola Virus Infection. The Journal of Infectious Diseases. 1999;179:S199–S202. doi: 10.1086/514293. [DOI] [PubMed] [Google Scholar]
- Sanchez A. Analysis of Filovirus Entry into Vero E6 Cells, Using Inhibitors of Endocytosis, Endosomal Acidification, Structural Integrity, and Cathepsin (B and L) Activity. The Journal of Infectious Diseases. 2007;196:S251–S258. doi: 10.1086/520597. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Lukwiya M, Bausch D, Mahanty S, Sanchez AJ, Wagoner KD, Rollin PE. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78:10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. Role of Endosomal Cathepsins in Entry Mediated by the Ebola Virus Glycoprotein. J Virol. 2006;80:4174–4178. doi: 10.1128/JVI.80.8.4174-4178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Rennekamp AJ, Chai N, Vandenberghe LH, Riley JL, Bates P. Folate Receptor Alpha and Caveolae Are Not Required for Ebola Virus Glycoprotein-Mediated Viral Infection. J Virol. 2003;77:13433–13438. doi: 10.1128/JVI.77.24.13433-13438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins J, Scianimanico S, Schoehn G, Weissenhorn W. Vesicular release of ebola virus matrix protein VP40. Virology. 2001;283:1–6. doi: 10.1006/viro.2001.0860. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Takada A, Watanabe T, Ito H, Kida H, Kawaoka Y. Functional importance of the coiled-coil of the Ebola virus glycoprotein. Journal of virology. 2000;74:10194–10201. doi: 10.1128/jvi.74.21.10194-10201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool-Lewis RJ, Bates P. Characterization of Ebola Virus Entry by Using Pseudotyped Viruses: Identification of Receptor-Deficient Cell Lines. J Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda J, Nakao M, Kawaoka Y, Shida H. Nedd4 regulates egress of Ebola virus-like particles from host cells. Journal of virology. 2003;77:9987–9992. doi: 10.1128/JVI.77.18.9987-9992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, et al. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006 doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Zavasnik-Bergant T, Repnik U, Schweiger A, Romih R, Jeras M, Turk V, Kos J. Differentiation- and maturation-dependent content, localization, and secretion of cystatin C in human dendritic cells. J Leukoc Biol. 2005;78:122–134. doi: 10.1189/jlb.0804451. [DOI] [PubMed] [Google Scholar]