Abstract
Cell microarrays with culture sites composed of individually removable microstructures or micropallets have proven benefits for isolation of cells from a mixed population. The laser energy required to selectively remove these micropallets with attached cells from the array depends on the microstructure surface area in contact with the substrate. Laser energies sufficient to release micropallets greater than 100 μm resulted in loss of cell viability. A new 3-dimensional culture site similar in appearance to a table was designed and fabricated using a simple process that relied on a differential sensitivity of two photoresists to UV-mediated photopolymerization. With this design, the larger culture area rests on four small supports to minimize the surface area in contact with the substrate. Microtables up to 250 × 250 μm were consistently released with single 10 μJ pulses to each of the 4 support structures. In contrast, microstructures with a 150 × 150 μm surface area in contact with the substrate could not be reliably released at pulse energies up to 212 μJ. Cassie-Baxter wetting is required to provide a barrier of air to localize and sequester cells to the culture sites. A second asset of the design was an increased retention of this air barrier under conditions of decreased surface tension and after prolonged culture of cells. The improved air retention was due to the hydrophobic cavity created beneath the table and above the substrate which entrapped air when an aqueous solution was added to the array. The microtables proved an efficient method for isolating colonies from the array with 100% of selected colonies competent to expand following release from the array.
Keywords: microtable, micropallet, microfabrication, 1002F, SU-8, cell separation, cell array
INTRODUCTION
The ability to select and isolate individual cells with unique characteristics from a mixed population plays an important role in biomedical research. A variety of strategies including fluorescence-activated cell sorting (FACS), limiting dilution, panning, column chromatography, and magnetic sorting are currently employed to accomplish this task [1]. These techniques require cells to be in suspension in order to perform the analysis and sorting steps. To sort cells that grow in an adherent manner, cells must be suspended by disaggregating or stripping the cells from their growth surface; however, this process imposes drawbacks including loss of cell morphology, removal of cell surface markers, damage to cell membranes, alterations in cellular physiology and loss of viability [2–9]. As a result, new technologies enabling cells to be separated while they remain adherent to their growth surface have been sought. One such approach is laser microdissection (LM) [10]. LM techniques have proven valuable for selecting cells from fixed or frozen tissue sections for genetic and proteomic studies, but have only partially met the needs of investigators for the positive selection of live cells due to a low throughput and poor cell viability [11].
The Allbritton group has pursued an alternative strategy for cell separations based on an array of microfabricated structures on a microscope slide created by photolithography using a photoresist composed of SU-8 or 1002F [12–18]. The microarray-based platforms have been called micropallet or microcup arrays depending on the shape of the arrayed microstructures. These arrays permit the sorting of cells based on a wide range of cellular attributes, including morphology, growth rate, immunofluorescence, localization and translocation of subcellular proteins or organelles, and many others [18]. The transparent arrays with cultured cells are scanned by conventional microscopy to identify target cells or colonies. Walls of air between the micropallets created by Cassie-Baxter wetting insure that the cells attach only to the upper surface of these microfabricated structures [12]. This air barrier directs cells to the culture site and contributes to sequestration of the cells in culture. A number of variables exist that contribute to its stability, but loss of the air wall during culture remains an issue. A pulsed laser beam is used to release a single micropallet with its attached cell(s) from the array for subsequent collection [12, 19]. The laser energy required to release a micropallet is proportional to the surface area in contact with the underlying glass substrate [20]. Thus a large micropallet (≥150 μm), which may be needed to culture cell types with extended processes, very large cells, or a cell colony, may require a high laser pulse energy to achieve release from the array. Such energies can fragment the pallet rather than release it from the surface. In addition these high energies can dislodge or damage cells on the surface of the structures. For this reason, most work to date has employed small pallets with surface areas that are less than 100 μm on a side, thus limiting their applications to the isolation of small cells or colonies with a low number of cells.
In the current work, an array of microstructures similar in appearance to a table was designed and fabricated in order to create large cell culture sites that could be individually released with minimal laser energy. Microtables were created using a two-layer fabrication process to create a tabletop supported by four legs attached to a glass microscope slide. With this design, the microstructure possessed a large upper surface for cell growth, but a much smaller contact area with the glass substrate than is possible with the standard micropallet or microcup designs. The release of individual microtables using single laser pulses to each leg was compared to micropallets identical in size to the microtable top. Microtable arrays were also tested for their ability to maintain a virtual air wall created by Cassie-Baxter wetting, an important attribute for the deposition and culture of cells on the arrays. Finally, the arrays were evaluated for their ability to localize cells growing on the arrays and for isolation and subsequent culture of cells.
EXPERIMENTAL SECTION
Materials
UVI-6976 photoinitiator (triarylsulfonium hexafluoroantimonate salts in propylene carbonate) was purchased from Dow Chemical (Torrance, CA) and poly(dimethylsiloxane) (PDMS) (Sylgard 184 silicone elastomer kit) was purchased from Dow Corning (Midland, MI). SU-8 photoresist and SU-8 developer (1-methoxy-2-propyl acetate) were obtained from MicroChem Corp. (Newton, MA). EPON resin 1002F (phenyl, 4,4'-(1-methylethylidene)bis-, polymer with 2,2'-[(1-methylethylidene) bis(4,1-phenyleneoxymethylene]bis[oxirane]) was obtained from Miller-Stephenson (Sylmar, CA). All other photoinitiators, resins, γ-butyrolactone (GBL), L-glutamine and poly(D-lysine)hydrobromide (MW 70,000–150,000) were from Sigma-Aldrich (St. Louis, MO). (Heptadecafluoro-1,1,2,2-tetrahydrodecyl) trichlorosilane was from Gelest Inc. (Morrisville, PA). Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA). Human plasma fibronectin was obtained from Millipore (Billerica, MA). All other reagents were obtained from Fisher Scientific (Pittsburgh, PA).
Formulation of 1002F photoresist
The 1002F photoresist was produced in house from three components: epoxy resin, solvent, and photoinitiator. The EPON epoxy resin 1002F was dissolved in GBL and was then mixed with the photoinitiator, UVI-6976. The ratio in the mixture of 1002F photoresist was 65:32.9:2.1 (resin:solvent:photoinitiator, w/w/w).
Fabrication of micropallet and microtable arrays
The fabrication of the microtables used a two-layer microfabrication process employing two different photoresists, 1002F and SU-8. The photoresist 1002F was spun on glass slides using a spin coater (WS-400B-6NPP/LITE, Laurell Technologies Corporation, North Wales, PA) by a two-step spin: an initial spin of 10 s at 500 rpm followed by a spin of 30 s at 2000 rpm. The coated slides were then soft baked in an oven at 95 °C for 50 min. After baking, the slides were slowly cooled to room temperature. To prepare an array of legs for the microtables, the 1002F film was exposed to UV light through a photomask with the appropriate design features (table legs) at 1200 mJ/cm2 using a collimated UV source (Oriel Model #97435, Newport Stratford, Inc., Stratford, CT). The post-exposure bake was performed in an oven at 95 °C for 10 min. After slowly cooling to room temperature, SU-8-10 was then spin coated on the sample as the second photoresist layer. A two step spin method was again applied. The initial spin was 500 rpm for 10 sec followed by a second spin 3000 rpm for 30 sec. The coated slides were then soft baked at 120 °C for 3 min. The sample was exposed to UV light through a photomask with the appropriate design features (a tabletop) at 150 mJ/cm2 using a mask aligner UV source (MA-6, Karl Suss, Inc., Waterbury Center, VT). The post-exposure bake was performed at 95 °C for 10 min. After slow cooling to room temperature, the samples were developed in SU-8 developer, rinsed with 2-propanol, and dried in a stream of nitrogen. Each array contained 3025 microtables. After fabrication of microtable arrays on a glass substrate, the arrays were coated with a hydrophobic perfluoroalkylsilane layer ([heptadecafluoro-1,1,2,2-tetrahydrodecyl]trichlorosilane) as described previously to enable the virtual wall to be generated (see below) [12–13, 21]. For experiments comparing microtables and micropallets, arrays of 3025 micropallets were fabricated with the same process using the masks for the microtable top to create two-layer structures with a base composed of 1002F and an upper layer of SU-8. For samples used for cell culture, a chamber was created by using a ring of molded polydimethylsiloxane (PDMS) attached to the glass substrate containing the array as previously described [17].
Measurement of the release energy for microstructure release
The arrayed microstructures were released using a laser-based method described previously [17–21, 24]. A single pulse (5 ns, 532 nm) from a focused Nd:YAG laser (ACL-1, New Wave Research, Fremont, CA) was focused at the interface of the glass substrate and base of each micropallet or microtable leg. “Release energy” was defined as the minimal laser energy required to release ten consecutive microtables using only a single pulse at each leg in three independent experiments. The minimal release energy for pallets was similarly defined as the lowest energy required to release ten consecutive pallets in three experiments.
Quantitative measurement of virtual air wall stability
Arrays composed of micropallets or microtables were fabricated, silanized, and placed in a 60-mm diameter Petri dish. A volume of 10 mL of an alcohol:water mixture (see Results) was then added to the dish. The arrays were inspected using an inverted microscope (TE300, Nikon). The amount of Cassie-Baxter wetting was defined as the total percentage of the area of the array containing air trapped between the microstructures.
Cell culture on micropallet and microtable arrays
HeLa cells, a human cervical carcinoma cell line, stably expressing a fusion gene composed of enhanced green fluorescent protein (eGFP) with H1-histone were cultured at 37 °C in a humidified 5% CO2 atmosphere in DMEM supplemented with FBS (10%), and L-glutamine (584 mg/L). Penicillin (100 units/mL) and streptomycin (100 mg/mL) were added to the media to inhibit bacterial growth. The micropallet and microtable arrays were sterilized by immersion in ethanol (75%) for 10 min and then dried under sterile conditions prior to cell plating and culture. Before use, fibronectin (25 μg/mL) was added to the samples for 2 h to coat the top surface of the micropallets or microtables. Coated arrays were rinsed ×5 with sterile deionized water and cell culture media prior to cell culture experiments. The number and density of cells plated on the array (3750 cells in 0.5 mL media) were arrived at empirically to provide a majority of microtables containing ≤1 cell per pallet.
Microscopic imaging of arrays
Brightfield and fluorescence images were obtained using a 10× objective and inverted epifluorescence microscope (NIKON TE200-U, Melville, NY), and a cooled charge-coupled device (CCD) camera (Photometrics CoolSNAP HQ2, Tucson, AZ). Confocal images were obtained in the Michael Hooker Microscopy Facility at University of North Carolina, Chapel Hill using an inverted laser scanning microscope (Ziess 510, Thornwood, NY). Images were obtained using two-channel laser excitation (488 nm and 543 nm) and a 40×, 0.8NA, Acroplan, water immersion objective.
Collection of microstructures from the array
An open collection vesicle that mated with the microwell array was constructed from PDMS on a glass coverslip [17]. Before use, the chamber was rinsed with ethanol and then ×5 with phosphate buffered saline (PBS). The chamber was then coated with 1 mL of 20 μg/mL fibronectin in PBS overnight immediately prior to use. Prior to laser release, the microarray was rinsed with fresh culture medium ×5 to remove nonadherent cells, and then 1 mL of cell culture medium was added to the microarray chamber. The collection chamber was rinsed with fresh culture medium, and placed directly above the pallet chamber in a sterile environment with the two O-rings of the collection chamber and the microarray opposed. This assembly was placed on the microscope stage and selected cells on microtables/micropallets were released as described above. The assembly was then inverted to transfer the media and released pallets into the collection chamber as described previously [17]. The collection chamber and microarray assembly were separated under sterile conditions and the collection chamber was covered and transferred into a tissue culture incubator. The growth of collected cells on released pallets was then observed over time by microscopy.
RESULTS AND DISCUSSION
Release of large micropallets
For the purpose of sorting large-sized cells or creating clonal colonies on the microarrays, micropallets of sufficient size are sometimes desired to support colonies composed of more than a few cells. When large micropallets (150 × 150 μm) were targeted for release, only 11 of 30 could be released and these micropallets required a laser release energy of 212 ± 8 μJ. This high energy resulted in significant damage to the structures (Fig. 1A,B). It was possible to release single micropallets with repeated 10 μJ pulses, but this approach required 21 ± 5 pulses (n = 30) positioned across multiple locations of each micropallet, a slow and tedious procedure (Fig. 1C). To determine the influence of the high energy laser pulses on cell health, HeLa cells were plated and cultured for 24 h on micropallet arrays (200 × 200 μm, 40 μm gap). Individual micropallets (n = 20 per array, 3 arrays) were released with a single laser pulse (energy >150 μJ). After the laser pulse, the colonies remained adherent to the micropallets and were collected and placed in culture for another 24 h to assess the viability of isolated cells. After 24 h, the cell morphology was spherical with many of the cells detached from the micropallets. The cells were also unable to exclude trypan blue suggesting that the majority of cells on the micropallets were nonviable. At 72 h, none of the collected cells (n > 60) had divided. Since HeLa cells possess a doubling time of 18 h, each cell should have divided to create 16 cells. The high laser energies and resultant mechanical forces used to detach the pallets were the most likely cause of cell death.
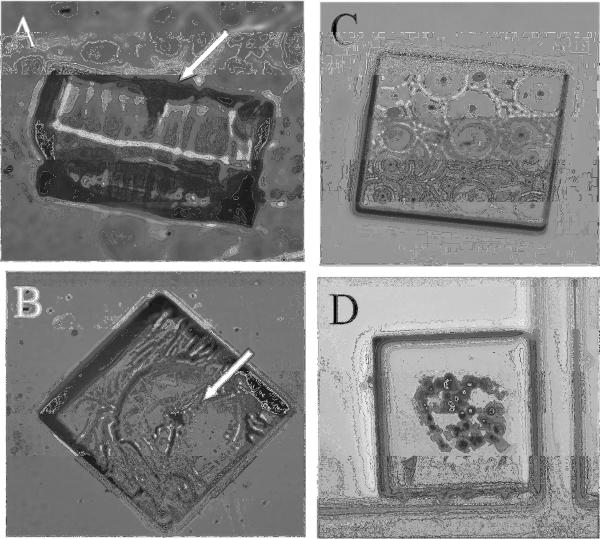
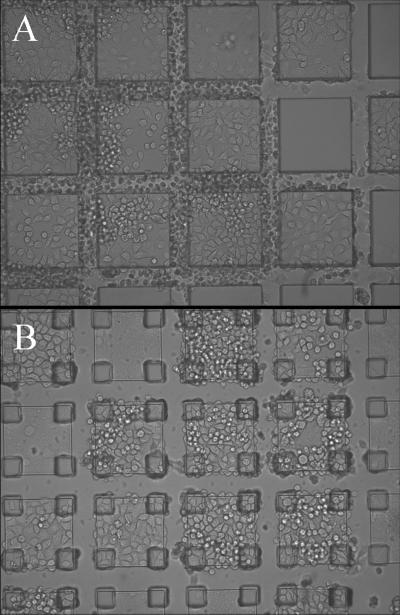
Figure 1.
Release of large micropallets. A, B) A micropallet (150 × 150 μm) released using a single 212 μJ laser pulse: (A) side view (B) bottom view. Note the deep fissure created by the focused laser pulse (arrow). C) A micropallet (200 × 200 μm) released using multiple 10 μJ laser pulses (30 pulses): the patterning on the undersurface of the micropallet is due to local damage to the 1002F surface by each laser pulse. (D) Brightfield image of a colony of HeLa cells on a 200 × 200 μm micropallet 24 h after release by a single laser pulse. All cells adherent to the micropallet have taken up trypan blue indicating that they are nonviable.
Fabrication of microtable arrays
To prevent cell damage and enhance cell viability, the total energy used to release larger microstructures needed to be decreased. A new microstructure was designed and fabricated that possessed support legs at each corner of a square top surface resembling a table. The reduced surface area in contact with the underlying substrate might then permit release of the microstructures at much lower laser energies. Fabrication of these structures was simplified by taking advantage of previous findings which showed that the UV energy required to crosslink 1002F was much higher than that for SU-8 [16]. In the current studies, the amount of photoinitiator in the 1002F photoresist formulation was reduced to 1/3 of the optimum used in prior MEMS applications in order to provide a large gap in the energy required to crosslink 1002F vs. SU-8. To make the table legs, an initial layer of 1002F was spin-coated onto a glass surface and exposed to UV light through a photomask. The energy required to crosslink the 1002F layer in creating the legs was 1200 mJ/cm2. A second layer composed of SU-8 was spin-coated onto the photo-exposed (but undeveloped 1002F layer). The SU-8 layer was then exposed to 150 mJ/cm2 of UV light through a photomask. The 150 mJ/cm2 required to crosslink the SU-8 layer did not polymerize the underlying 1002F layer. This strategy was used to fabricate microtable arrays with legs of 40 × 40 μm, 50 × 50 μm or 60 × 60 μm in cross section, and with tabletop dimensions of 150 × 150 μm, 200 × 200 μm or 250 × 250 μm (Fig. 2). In all arrays, the height of the support legs was 68 ± 3.7 μm and the total height of the microstructures was 84 ± 3.5 μm (n = 5). The success rate for creating the microtable arrays was 82% for 3 batches of 7 arrays. Survey of individual arrays (n = 21) revealed that 95 ± 5% of microtables were properly formed on the arrays.
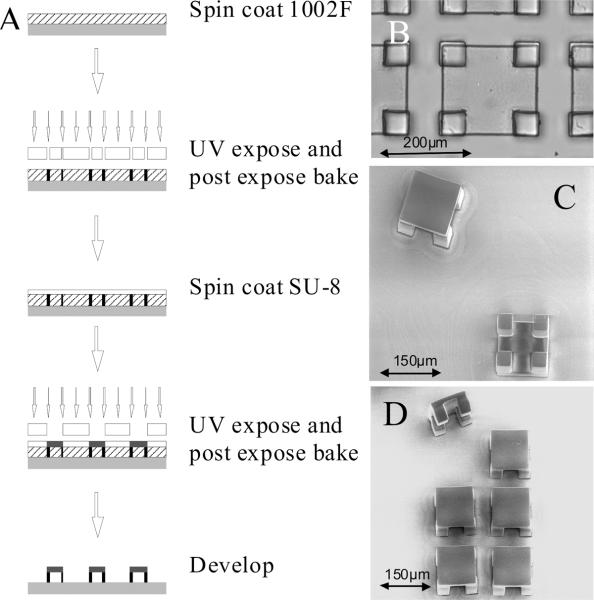
Figure 2.
(A) Microtable fabrication process. (B) Brightfield image of a microtable. (C) SEM of microtables released from the array showing top and bottom views of the structure. (D) SEM of a 2 × 3 portion of an array. One microtable has been released and is shown in side view.
Release of individual microstructures from the array
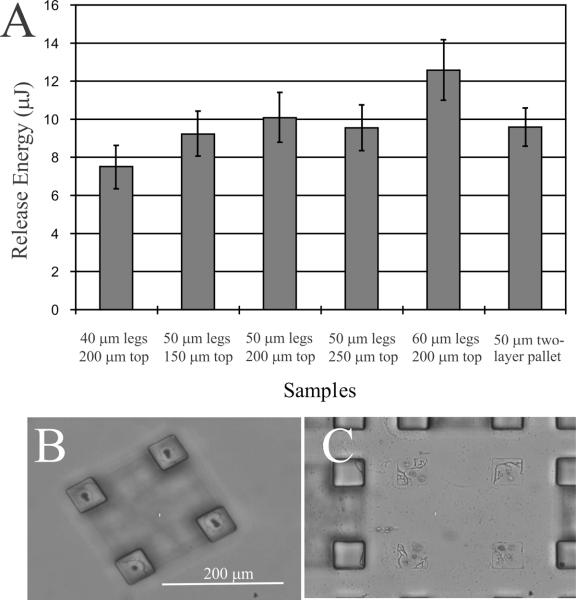
A series of experiments comparing laser-based release of microtables to micropallets of varying dimensions was performed. The microtables were successfully released by directing a single, focused laser pulse at the base of each leg (Fig. 3A). The release energy was dependent on the area of the microtable leg in contact with the surface [20]. The release energy required to detach individual legs increased from 7.5 ± 1.1 μJ for a surface area of 1600 μm2 to 12.6 ± 1.6 μJ for an area of 3600 μm2. The minimum release energy needed to dislodge each leg was very similar to that required to release individual micropallets manufactured in the same manner and with the same surface area in contact with the glass substrate. For example, micropallets of 2500 μm2 required a minimum release energy of 9.6 ± 1.0 μJ. To release a table, four pulses were required to dislodge each leg from the surface. While a 150 μm micropallet required 212 μJ, a 150 μm table top with 50-μm legs required 36 μJ (4 × 9 μJ), a nearly 6-fold decrease in release energy. More importantly, the cell(s) growing on the surface of a microtable were exposed to a much lower peak mechanical impulse since the energy was delivered as a series of 4 pulses rather than a single very large shock [19].
Figure 3.
(A) Minimum release energy for microtables of various dimensions and for two-layer micropallets with 50 μm sides. (B) Brightfield image of a released microtable. The focal plane is at the base of the legs. Small defects in each leg base show the site of the laser pulsed used to dislodge the leg from the glass substrate. (C) Brightfield image of the substrate surface after microtable release. Remnants of 1002F polymer are present where each leg was attached to the substrate.
The energy to release the table legs was greater than that for a standard one-layer, 1002F micropallet of equivalent dimensions. For example, 1002F micropallets with a surface area of 2500 μm2 (50 × 50 μm) were released at 4.0 ± 0.4 μJ, whereas 2500 μm2 microtable legs possessed a release energy 9 μJ. When micropallets were fabricated using the two-layer process for the microtables (a layer of 1002F overlaid with SU-8), the release energy of the two-layer micropallet was similar to that of a table leg of the same dimensions (Fig. 3A). The difference in energy between a standard micropallet and the table leg or two-layer pallet was likely due to differences in the fabrication process. For microtable fabrication, the leg structures were exposed to two additional baking steps of 10 min at 95 °C and 3 min at 120 °C during the fabrication of the tabletop due to the addition of the second layer. The additional baking steps likely led to an increase in adhesion of the 1002F layer to the glass substrate contributing to the higher release energy. Supporting this postulation, if the post-exposure bake of the SU-8 layer was reduced to 10 min at 95 °C, the minimum release energy of the 2500 μm2 table legs was reduced to 6.6 ± 0.9 μJ, although the quality and repeatability of the fabricated microtable structures were diminished.
Virtual air wall stability
The virtual air wall used to form a barrier between the microstructures or cell culture sites is required to direct cells onto the array sites and block access to the canyons between the microstructures. The air wall is created by Cassie-Baxter wetting and its stability depends on the dimensions of the arrayed microstructures, particularly the gap between the structures, and the surface tension of the wetting liquid, among other factors [14]. To evaluate whether arrays of microtables displayed different characteristics in air wall stability compared with micropallet arrays, a series of experiments were carried out varying the dimensions of the gap between the microstructures and the surface tension of the wetting fluid. Virtual wall stability was assessed by measuring the percentage of the array over which air was entrapped between the microstructures under the various conditions [14]. Arrays composed of 36 (6 × 6) micropallets or microtables were fabricated. The two-layer micropallet and the microtable top surface dimensions were 200 × 200 μm. The microtable legs were 50 × 50 μm. The gap between the microstructures (table tops or pallets) was varied between 50 and 200 μm. The surface tension of the wetting liquid was also varied by mixing water (surface tension 72.7 mM/m) with ethanol (surface tension 22.4 mM/m) at varying ratios. Immediately after immersion in the water/ethanol mixtures, the arrays were assessed for the presence of the virtual air wall.
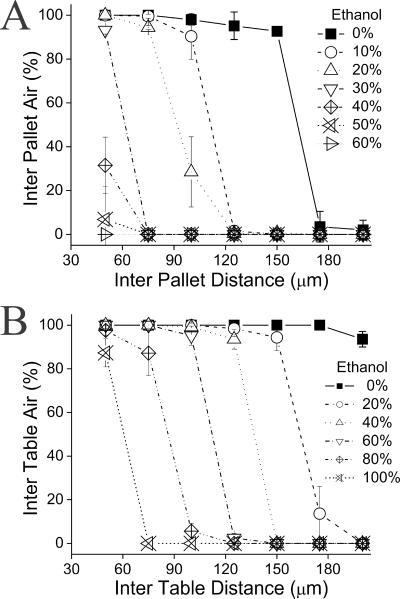
For the micropallet array in 100% water, the virtual wall remained stable at a gap of 75 μm with only a minor loss of air at greater gaps until a threshold was reached at 150 μm. At gaps ≥150 μm the air wall broke down almost entirely with only 3.5 ± 7% of the array canyons covered with air (Fig. 4A). In contrast, the virtual wall on the microtable array remained stable until the gap was greater than 175 μm and was only slightly reduced at a gap of 200 μm with 94 ± 4% of the array canyons covered with air (Fig. 4B). The stability of the air wall was also greater at each gap size in the presence of a reduced surface tension of the wetting solution. In 50% ethanol, only 7 ± 15% of the virtual air wall on the micropallet array was present at the minimal gap studied (50 μm). In contrast, at this same concentration of ethanol, the air wall stability of the microtable array was not diminished at gap sizes less than 100 μm. This enhanced stability suggests that the microtable array will enable greater gap sizes to be employed between the microstructures used for cell culture, thus reducing the likelihood of cells migrating between culture sites. The greater stability of air walls on the microtable arrays was most likely due to the hydrophobic cavity beneath the table top and between the legs in which air was trapped after the array was wetted.
Figure 4.
The stability of the virtual air wall in solutions of varying concentrations of ethanol on (A) micropallet arrays and (B) microtable arrays. Shown on the x-axes are the distances between individual microstructures on the arrays. The y-axes display the fraction of the surface area between the microstructures covered with air. The error bars represent the standard deviation of the data points.
Cell culture on microtable arrays
In the absence of cells, the virtual air walls remain stable for periods of up to one month under the conditions used for cell culture [14]. However, when cells are cultured on the micropallet arrays, breakdown of the virtual wall typically occurs over time, possibly due to the deposition of extracellular matrix at the leading edge of the hydrophobic regions as the cells propagate in culture. The breakdown of the virtual wall enables cells on the microstructures to migrate off of the culture sites, into the canyons, and admix with neighboring colonies. Breakdown of the virtual wall limits the time for colony expansion and the colony size that can be achieved on the arrays. To compare the microtable and micropallet arrays for cell culture, arrays of 2-layer (SU-8/1002F) micropallets (200 × 200 μm, 84 μm height, 50 μm gap) and arrays of microtables (200 × 200 μm top surface, 50 × 50 μm leg surface, 84 μm height, 50 μm gap) were fabricated. A suspension of HeLa cells stably expressing a GFP fusion protein was cultured on the arrays and then the cells were observed daily over a one week period. Loss of the virtual air wall in regions of the micropallet arrays occurred at 72 h, and the wall was completely lost by 110 h. In contrast, at 72 h the virtual wall of the microtable arrays was 100% intact, although by 110 h regions were present with wall breakdown and by 144 h, the virtual wall was no longer present. After loss of the surrounding air wall, cells on the micropallets rapidly migrated to the glass substrate with admixing of cells from neighboring micropallets by 120 hours (Fig. 5A). In contrast, at 144 h despite loss of the virtual walls, colonies on the microtables remained segregated (Fig. 5B).
Figure 5.
Brightfield images of HeLa cells cultured for 144 h on (A) arrays of micropallets (200 × 200 μm) and (B) arrays of microtables (200 × 200 μm). The virtual walls were absent on both arrays in the area imaged. Cells migrated across the inter-pallet region on the micropallet array, but remain localized to the culture sites on the microtable array.
To understand why the microtables segregated adjacent colonies for a longer period, cells growing on microtable arrays were imaged by brightfield, fluorescence and confocal microscopy in a region where the virtual air wall was lost (Fig. 6). At 72 h, imaging in different focal planes showed that as the cells propagated on the microtables, they grew down the sides of the tabletop and migrated along the undersurface of the tabletop. This effectively doubled the culture area of the microstructure compared to the micropallet. By 144 h, cells reached the glass substrate by migrating along the microtable legs. The legs also presented a limited surface region for the cell migration that may help slow migration to neighboring culture sites. Thus, colonies remained segregated for longer periods on arrays of microtables compared with micropallets due to prolonged stability of the virtual air wall, increased effective culture area, and restricted regions for migration off the microstructure.
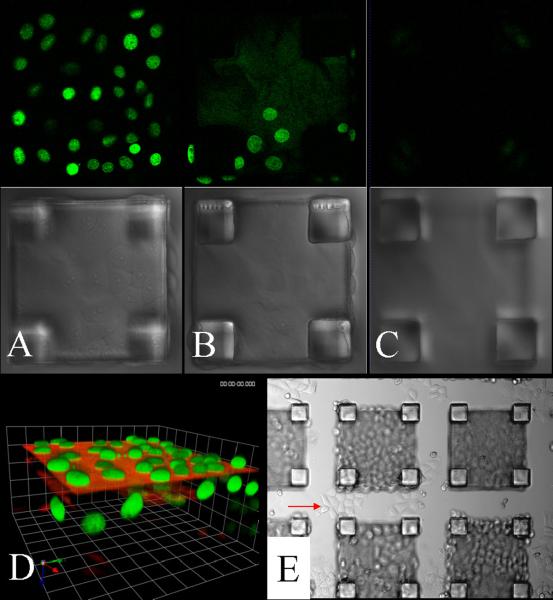
Figure 6.
Confocal images of HeLa cells with fluorescent nuclei cultured on a microtable array for 72 h. Shown is a single microtable at different image planes: (A) top surface of tabletop, (B) undersurface of tabletop, (C) glass substrate, (D) 3-D reconstruction of the series. (E) Brightfield image of a microtable array after 168 h in culture. The cells on the underlying glass surface (arrow) are adjacent to the table legs.
Collection of viable colonies from the microtable array
To determine whether microtables could be used to successfully isolate viable colonies, microtables containing a cell colony were released, collected, and the isolated cells were cultured. HeLa-GFP cells were plated and cultured for 48 h on microtable arrays (200 × 200 μm top surface, 50 × 50 μm legs, 50 μm gap). Individual microtables (n = 10 per array, 3 arrays) were released with a single laser pulse (10 μJ) directed at each leg and the microtables were collected. Both the isolated microtables and the arrays were returned to culture. After 72 h, all colonies remaining on the array that were adjacent to the isolated colonies (n > 20) continued to expand indicating that these cells remained viable. After 3 d in culture, 100% ± 0 of the colonies isolated from the arrays continued to expand in culture demonstrating successful selection and separation of viable colonies using the microtables.
CONCLUSION
Arrays of microtables were developed for cell selection and isolation. This design possessed a number of strengths relative to the micropallets. Unlike many two-layer microfabrication processes, no sacrificial layer was required during fabrication, which simplified creation of the patterned structures. This design overcame the high release energy required to selectively dislodge microstructures whose footprint would otherwise provide too great an adhesive area to readily release with the focused laser beam. The table-like structure also improved the stability of the Cassie-Baxter wetting needed to pattern cells during plating and growth on the array. By virtue of this enhanced air entrapment and the increased surface area for colony expansion, colonies can be maintained at a single array site much longer without admixing with cells on nearby array elements than when cultured on similar sized micropallets. Colonies grown on the array were shown to be readily isolated and expanded with high efficiency. Cells isolated using the microtables were viable while those isolated on large micropallets were nonviable. In addition, the continued viability of cells remaining on the array after the initial isolation suggests that multiple rounds of isolation could be performed on the same array. The microtable design will be a valuable addition to the armamentarium currently available for cell isolation and cloning.
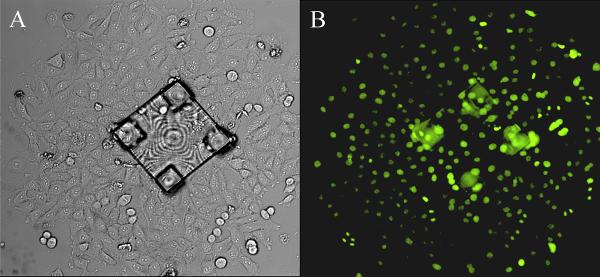
Figure 7.
(A) Brightfield image and (B) fluorescence image 3 days after a colony of cells on a microtable was released and collected from an array. The colony expanded as the cells divided and grew off the structure.
Acknowledgements
This research was supported by the NIH (EB007612 and HG004843). We gratefully acknowledge the technical support for the confocal analyses provided by Dr. Michael Chua in the Hooker Microscopy Facility, University of North Carolina – Chapel Hill.
REFERENCES
- 1.Patel D. Separating Cells. Springer-Verlag; New York: 2001. [Google Scholar]
- 2.Welm B, Behbod F, Goodell MA, Rosen JM. Cell Proliferation. 2003;36(Suppl 1):17–32. doi: 10.1046/j.1365-2184.36.s.1.3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burridge K, Chrzanowska-Wodnicka M. Ann. Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 4.Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Biochem Cell Biol. 1996;74:737–744. doi: 10.1139/o96-080. [DOI] [PubMed] [Google Scholar]
- 5.Ingber DE. Ann Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 6.Seidl J, Knuechel R, Kunz-Schughart LA. Cytometry. 1999;36:102–111. doi: 10.1002/(sici)1097-0320(19990601)36:2<102::aid-cyto3>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Piercy KT, Donnell RL, Kirkpatrick SS, Mundy BL, Stevens SL, Freeman MB, Goldman MH. J Surg Res. 2001;100:211–216. doi: 10.1006/jsre.2001.6247. [DOI] [PubMed] [Google Scholar]
- 8.Mackie EJ, Pagel CN, Smith R, de Niese MR, Song SJ, Pike RN. IUBMB Life. 2002;53:277–281. doi: 10.1080/15216540213469. [DOI] [PubMed] [Google Scholar]
- 9.Miki M, Nakamura Y, Takahashi A, Nakaya Y, Eguchi H, Masegi T, Yoneda K, Yasouka S, Sone S. J Med Invest. 2003;50:95–107. [PubMed] [Google Scholar]
- 10.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 11.Todd R, Lingen MW, Kuo WP. Expert Rev Mol Diagn. 2002;2:497–507. doi: 10.1586/14737159.2.5.497. [DOI] [PubMed] [Google Scholar]
- 12.Salazar GT, Wang Y, Young G, Bachman M, Sims CE, Li GP, Allbritton NL. Anal Chem. 2007;79:682–687. doi: 10.1021/ac0615706. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Sims CE, Marc P, Bachman M, Li GP, Allbritton NL. Langmuir. 2006;22:8257–8262. doi: 10.1021/la061602k. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Sims CE, Marc P, Bachman M, Li GP, Allbritton NL. Anal Chem. 2007;79:7104–7109. doi: 10.1021/ac070911s. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Young G, Bachman M, Sims CE, Li GP, Allbritton NL. Anal Chem. 2007;79:2359–2366. doi: 10.1021/ac062180m. [DOI] [PubMed] [Google Scholar]
- 16.Pai JH, Wang Y, Salazar GT, Bachman M, Sims CE, Li GP, Allbritton NL. Anal Chem. 2007;79:8774–8780. doi: 10.1021/ac071528q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu W, Sims CE, Allbritton NL. Anal Chem. 2010;82:3161–3167. doi: 10.1021/ac100434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Young G, Aoto PC, Pai JH, Bachman M, Li GP, Sims CE, Allbritton NL. Cytometry Part A. 2007;71:866–874. doi: 10.1002/cyto.a.20424. [DOI] [PubMed] [Google Scholar]
- 19.Quinto-Su PA, Salazar GT, Sims CE, Allbritton NL, Venugopalan V. Anal Chem. 2008;80:4675–4679. doi: 10.1021/ac800129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazar GT, Wang Y, Sims CE, Allbritton NL. J Biomed Optics. 2008;79:682–687. doi: 10.1021/ac0615706. [DOI] [PubMed] [Google Scholar]
- 21.Sum TC, Bettiol AA, Van Kan JA, Watt F, Pun EYB, Tung KK. Applied Physics Letters. 2003;83:1707–1709. [Google Scholar]