Abstract
Tea ranks second only to water as a major component of fluid intake worldwide and has been considered a health-promoting beverage since ancient times. For the past two decades, we and others have been investigating the potential cancer preventive and therapeutic effects of green tea and its polyphenolic mixture termed GTP. It has become clear that much of these effects of GTP are mediated by its most abundant catechin, epigallocatechin gallate (EGCG). Large amount of encouraging data from in vitro and animal models have emerged making clear that green tea is a nature's gift molecule endowed with anticancer effects. Epidemiological and geographical observations suggest that these laboratory data may be applicable to human population. Clinical trials of GTP, especially in prostate cancer patients have yielded encouraging results. This article briefly reviews properties of GTP, especially EGCG with reference to multitargeted therapy of cancer.
Keywords: Cancer, EGCG, Green tea polyphenols, Signal transduction
1. Introduction
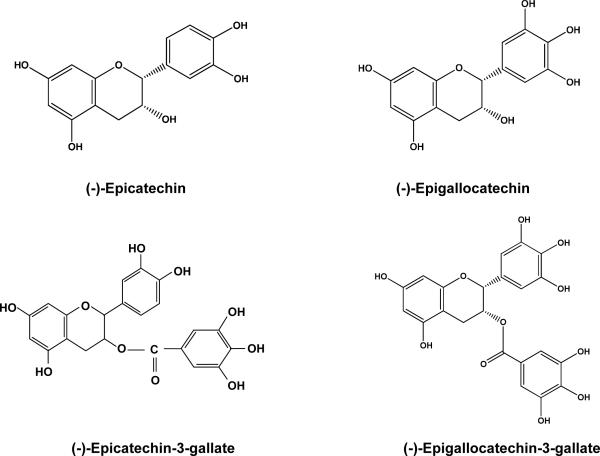
The history of tea began over 5,000 years ago in ancient China. Currently, tea is the most popular beverage consumed by two-thirds of the world's population. Green tea, black tea, and Oolong tea are all derived from the leaves of Camellia sinensis plant and contain an assortment of compounds, the most significant components of which are catechins or polyphenols. To produce green tea, freshly harvested leaves are steamed to prevent fermentation, yielding a dry, stable product. To produce black tea, the fresh leaves are allowed to wither, and then rolled and crushed, initiating fermentation of polyphenols. Oolong tea is produced by a partial oxidation of the leaf, intermediate between the process for green and black tea. Among all teas consumed in the world, green tea is best studied for its health benefits, including chemopreventive and cancer treatment efficacy. It is noteworthy that tea polyphenols are considered to contribute to the prevention of various other degenerative diseases like cardiovascular diseases, arthritis and diabetes. Tea polyphenols, known as catechins, account for 30–42% of the dry weight of the solids in brewed green tea. [1]. Catechins contain a benzopyran skeleton with a phenyl group substituted at the 2-position and a hydroxyl (or ester) function at the 3-position. Variations to the catechin structure include the stereochemistry of the 2,3-substituents and the number of hydroxyl groups in the B- and D-ring. Belonging to the flavan-3-ol class of flavonoids, major catechins found in tea leaves are (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG) and (−)-epicatechin (EC) and their structures are shown in Fig. 1. Catechin, gallocatechin, epigallocatechin digallates, epicatechin digallate, 3-O-methyl EC and EGC, catechin gallate, and gallocatechin gallate are present in smaller quantities. Flavonols, including quercetin, kaempferol, myricitin and their glycosides are also present in tea. A typical tea beverage, prepared in a proportion of 1 g leaf to100 ml water in a 3-min brew, usually contains 250–350 mg tea solids, comprised of 30–42% catechins and 3–6% caffeine [2].
Figure 1.
Chemical structures of major green tea polyphenols.
In our initial work, we prepared a polyphenolic mixture from green tea leaves and termed it green tea polyphenols (GTP) [3]. Since that time, GTP and especially the most abundant catechin present therein, epigallocatechin gallate (EGCG) has been the subjects of intense research. In recent years, the number of studies investigating the roles of green tea and EGCG has risen dramatically. Laboratory research during the last decade has characterized the cancer-preventive and treatment properties and bioavailability of tea constituents. EGCG makes up about 10–50% of the total catechin content and appears to be the most powerful of all the catechins with an antioxidant activity about 25–100 times more potent than that of vitamins C and E. EGCG has both antimatrix metalloproteinase and antiangiogenesis activities [4]. The aim of this article is to review the existing scientific evidence for the biotransformation and bioavailability of GTP, especially EGCG and effects on pathways of signal transduction and clinical trials.
2. Biotransformation and bioavailability
The major metabolic pathways for tea catechins include glucuronidation, sulfation, and methylation. There are species and tissue-specific differences in EGCG and EGC glucuronidation, with humans and mice being more similar than humans and rats. Methylated catechins have been observed in the rat including 3′ and 4′-O-methyl EC, 4′-O-methyl EGC, and 4″-O-methyl EGC and EGCG. The major metabolite detected in the bile of the rat following oral EGCG administration was 4′, 4″di-O-methyl-EGCG [5].
The greatest catalytic efficiency for glucuronidation is in mouse intestinal microsomes followed in decreasing order by mouse liver, human liver, rat liver and rat small intestine. EC undergoes sulfation catalyzed by human and rat intestinal and liver cytosol with the human liver being the most efficient [6]. EGC is detected mainly as the glucuronidated form or sulfated form with only a small amount present as the free form in humans [7]. Tea catechins undergo metabolism in the gut to form the ring fission products 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4), 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (M6) and 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone (M6′) [8]. These metabolic intermediates are further broken down by gut flora to phenylacetic and phenylpropionoic acids. M6 was previously shown to form during anaerobic incubation of ECG and EC with human intestinal bacteria [9].
In rats, detailed pharmacokinetic and biotransformation studies of the tea catechins have been conducted [10]. The plasma levels of EGCG, EGC and EC following intragastric administration of decaffeinated green tea to rats were fit to a two-compartment model with elimination half-lives of 165, 66 and 67 min, respectively. The absolute bioavailability of EGCG, EGC and EC after intragastric administration of decaffeinated green tea is 0.1, 14 and 31%, respectively. After oral administration of 100 mg of EGCG, in studies with bile duct-cannulated rats, 3.28% of the dose is recovered in the bile as EGCG (2.65%), 4”-O-methyl-EGCG (0.25%), 3”-O-methyl-EGCG (0.11%), 4'-O-methyl-EGCG (0.11%), 3'-O-methyl-EGCG (0.10%) and 4',4”-di-O-methyl-EGCG (0.06%) [11]. It has been shown that treatment of rats with a green tea polyphenol (GTP) preparation in the drinking fluid result in increasing plasma levels over a 14-d period with levels of EGC and EC being higher than those of EGCG. Plasma levels then decrease over the subsequent 14 days suggesting an adaptive effect. The levels of EGCG were found to be highest in the rat esophagus, intestine and colon, which have direct contact with tea catechins, whereas EGCG levels are lower in the bladder, kidney, colon, lung and prostate. The EGCG levels in the plasma, lung and liver are much higher than in rats when the same polyphenol preparation is given to mice. These levels appear to peak on day 4 and then decrease to <20% of the peak values on days 8–10 [12].
The oral administration of 20 mg green tea solids/kg body weight results in Cmax in the plasma for EGC, EC and EGCG of 223, 124 and 77.9 μg/L, respectively. Tmax was found to range from 1.3 to 1.6 h with t1/2β of 3.4, 1.7 and 2 h for EGCG, EGC and EC, respectively. Plasma EC and EGC are present mainly in the conjugated form whereas 77% of the EGCG was in the free form. The plasma EC is largely in the sulfated form with less glucuronide. EGC but not EC is also methylated (4'-O-methyl-EGC) in humans. EGCG has also been shown to undergo methylation. The maximum plasma concentration of 4',4”-di-O-methyl-EGCG is 20% that of EGCG but the cumulative excretion of 4',4”-di-O-methyl-EGCG is 10-fold higher than that of EGCG over 24 h [10].
3. Green tea polyphenols and cancer
Various studies have demonstrated that GTP, especially EGCG can inhibit carcinogenesis and also the growth of established cancers at various organ sites. For a detailed discussion, readers are advised to refer our recently published reviews [2, 4].
4. Green tea polyphenols and effects on signal transduction pathways
GTP has been known to modify the activities of various receptor tyrosine kinases and particular pathways of signal transduction, thereby altering the expression of genes involved in cell proliferation, angiogenesis, and apoptosis. Here, we discuss the effects of GTP on signal transduction pathways that are related to cancer chemoprevention. Fig. 2 shows the effect of EGCG on various pathways of signal transduction.
Figure 2.
Effect of green tea polyphenol, EGCG on the modulation of various signal transduction pathways.
4.1. Inhibition of mitogen activated protein (MAP) kinases and activator protein-1 (AP-1)
MAPK has received increasing attention as a target molecule for cancer prevention and therapy. Activation of the MAPK pathways may cause the induction of phase II detoxifying enzymes, and its inhibition may inhibit AP-1-mediated gene expression. Once activated, MAPKs (ERK, JNK, and p38) can activate a variety of transcription factors, including ELK and c-Jun, a component of AP-1, thus leading to changes in the expression of genes that play critical roles in cell proliferation, migration and apoptosis. It has been shown that treatment of H2O2 resulted in phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), JNK, and p38 in human epidermal keratinocytes. H2O2-induced phosphorylation of ERK1/2, JNK, and p38 was found to be significantly inhibited when these cells were pretreated with EGCG. These findings demonstrate that EGCG has the potential to inhibit oxidative stress-mediated phosphorylation of MAPK signaling pathways [13]. EGCG inhibited the phosphorylation of ERK1/2, and suppressed p38 MAPK activity in human fibrosarcoma HT1080 cells [14]. In breast cancer cell line T47D, catechin containing approximately 53% of EGCG has been shown to phosphorylate JNK and p38. The phosphorylated JNK and p38 inhibited the phosphorylation of cdc2, and regulated the expression of cyclin A, cyclin B1 and cdk proteins, thereby causing G2 arrest [15]. It has been shown recently that EGCG rapidly and substantially hampered UV-B irradiation-induced activation of ASK-1 and phosphorylation of ERK1/2, JNK and p38 in dermal fibroblasts. These results demonstrate that EGCG has abilities to hamper UV-B-induced collagenolytic MMP production via interfering with the MAPK-responsive pathways [16]. We have shown that topically application of GTP decreased UVB-induced phosphorylation of ERK1/2 and JNK and p38 proteins in SKH-1 mice [17]. In SKH-1 mice, it has also been reported that GTP when applied in topical hydrophilic ointment or through drinking water, prevented UVB-induced depletion of antioxidant enzymes in skin. GTP treatment also decreased UVB-induced phosphorylation of ERK1/2, JNK and p38 proteins [18]. In normal human epidermal keratinocytes (NHEK), low concentrations of EGCG increased cell proliferation and inhibited UV-induced apoptosis, possibly through activating ERK and Akt pathways and changing the Bax/Bcl-2 balance [19].
AP-1 is a dimeric complex that comprises members of the Jun and Fos families. Binding of the AP-1 complex to the TRE sequence present in the promoter region of several genes is induced by growth factors, cytokines and oncoproteins, which play roles in cell proliferation, survival, differentiation and transformation. Indeed, the functional activation of AP-1 is associated with both tumor promotion and malignant transformation [20]. EGCG has been shown to exert anti-invasive effect in gastric cancer by controlling MMP expression through the suppression of MAPK and AP-1 activation [21]. EGCG inhibited TPA or epidermal growth factor (EGF)-induced transformation of mouse epidermal cell line JB6, and the activity was closely related to the inhibition of activation of the transcription factor AP-1 [22]. Tea polyphenols, EGCG and theaflavins, have been shown to effectively block arsenite-induced apoptosis of JB6 cells and inhibited arsenite-induced AP-1 transcription activity and AP-1 DNA binding activity. EGCG and theaflavins also potently inhibited arsenite-induced ERK1/2 activity, but not p38 kinase activity [23].
4.2. Inhibition of Nuclear factor-κB (NF-κB) signaling pathway
NF-κB is a sequence specific inflammatory and innate immune responses. It is sequestered in the cytoplasm in an inactive form through interaction with IκB. Phosphorylation of IβB by IβB kinase (IKK) causes ubiquitination and degradation of IβB, thus releasing NF-βB which then translocates to the nucleus, where it binds to specific κB binding sites in the promoter regions of several genes. The MAPKKK protein MEKK1, which plays a role in the JNK-mediated signaling pathway, also activates NF-κB via activation of IKKβ. Dysregulation of the NF-κB pathway plays an important role in the development of various types of cancer [24, 25]. EGCG inhibited TPA-induced DNA binding of NF-κB and CREB in mouse skin in vivo. EGCG also suppressed TPA-induced phosphorylation and subsequent degradation of IκBα, and prevented nuclear translocation of p65 [26]. EGCG has been reported to exert an anti-inflammatory effect in endothelial cells by controlling monocyte chemotactic protein-1 expression, at least in part, mediated through the suppression of p38 and NF-κB activation [27]. EGCG has been shown to be helpful in regulating mast-cell-mediated allergic inflammatory response by inhibiting the production of TNF-α, IL-6 and IL-8 through the inhibition of the intracellular Ca(2+) level, ERK1/2 and NF-κB activation [28]. EGCG markedly inhibited IL-1β-mediated IL-1β-receptor-associated kinase (IRAK) degradation and the signaling events downstream from IRAK degradation such as IKK activation, IκBα degradation, and NF-κB activation. In addition, EGCG inhibited phosphorylation of the p65 subunit of NF-κB. The functional consequence of this inhibition was evident by inhibition of IL-8 gene expression [29]. We have reported that EGCG-induced apoptosis in human prostate carcinoma LNCaP cells by negative regulation of NF-κB activity, thereby decreasing the expression of the proapoptotic protein Bcl-2 [30].
We have shown that treatment of EGCG dose and time-dependently increased IκB level, and inhibited NF-κB nuclear translocation in A431 epidermoid carcinoma cells [31]. It has also been shown that UVB irradiation-induced NFκB activation in NHEK was associated with increased IκB phosphorylation and degradation and EGCG was shown to block NFκB activation and nuclear translocation [32]. Topical application of GTP to UVB-irradiated SKH-1 hairless skin decreased phosphorylation and degradation of IκB and the subsequent activation of NF-κB [17]. Pretreatment of NHBE cells with EGCG suppressed cigarette smoke condensate (CSC)-induced phosphorylation of IκBα and activation and nuclear translocation of NF-κB/p65. NHBE cells transfected with a luciferase reporter plasmid containing an NF-κB-inducible promoter sequence showed an increased reporter activity after CSC exposure that was specifically inhibited by EGCG pretreatment [33].
4.3. Inhibition of Epidermal growth factor receptor (EGFR)-mediated pathways
EGFR is a transmembrane glycoprotein with intrinsic tyrosine kinase activity that regulates cell proliferation and differentiation. Four EGFR family members have been identified in mammals, erbB1 (also termed EGFR), erbB2, erbB3, and erbB4. Six different ligands, including epidermal growth factor (EGF) and TGF-α are also known to exist. EGFR is expressed by many types of cells, particularly epithelial cells that commence cell cycle progression upon binding to a ligand [34]. Thus, interactions between EGFR and its ligands are thought to play an important role in the regeneration of injured epithelium. EGFR is also expressed by various epithelial tumors and its high levels of expression in these cells indicate a poor prognosis or a late stage of disease. Therefore, EGFR has become a novel molecular target of cancer therapies. EGCG was found to inhibit EGFR autophosphorylation in YCU-N861 and YCU-H891 head and neck carcinoma cells and MDA-MB-231 breast carcinoma cells [35, 36]. EGCG was shown to inhibit the activation of the EGFR, HER2 and multiple downstream signaling pathways in colon cancer cell lines [37]. It has been reported that EGCG binds to a specific metastasis associated 67-kDa laminin receptor that is expressed on a variety of tumor cells. It was shown using a subtraction cloning strategy involving cDNA libraries constructed from cells treated or untreated with all trans-retinoic acid that the anticancer action of EGCG is mediated by LR and it allows EGCG to bind to the cell surface [38]. We have reported that GTP resulted in substantial reduction in the levels of IGF-I and significant increase in the levels of IGFBP-3 in TRAMP mice [39]. GTP inhibited the activation of platelet-derived growth factor β (PDGF-β) receptor in A431 cells, mouse NIH3T3 fibroblast cells, and A172 human glioblastoma [40]. It has been shown using in vitro kinase assays that EGCG potently inhibited the kinase activity of EGFR, PDGFR, and fibroblast growth factor-R. In A431 human epidermoid carcinoma cells, pretreatment with EGCG completely abolished ligand-induced autophosphorylation. Binding studies with 132I-EGF showed that pretreatment with EGCG dose-dependently inhibited binding of EGFR [41]. Recently, it has been shown that EGCG inhibits the binding of EGF to the EGFR and the subsequent dimerization and activation of the EGFR by altering membrane organization [42]. EGCG has also been reported to suppress gene expression of EGFR in rat activated hepatic stellate cells in vitro mediated by reducing the trans-activation activity of Egr-1 [43]. EGCG has been shown to suppress the phosphorylation of EGFR in esophageal squamous cell carcinoma KYSE 150 cells and epidermoid squamous cell carcinoma A431 cells [44]. EGCG inhibited EGF-dependent activation of EGFR, and EGFR-dependent activation of ERK1/2 and AKT activity in cervical cells [45]. EGCG has also been found to inhibit activation of the HER-2 receptor in human head and neck squamous cell carcinoma and breast carcinoma cell lines that display constitutive activation of HER-2. This was associated with inhibition of Stat-3 activation, inhibition of c-fos and cyclin D1 promoter activity, and decreased cellular levels of the cyclin D1 and Bcl-xL proteins [46].
4.4. Inhibition of Insulin-like growth factor (IGF)-I mediated signal transduction pathway
The IGF pathway comprises a complex system of molecules involved in regulation of a diverse array of normal and pathological biological functions. The IGF is a complex system of peptide hormones, cell surface receptors and circulating binding proteins. IGF-1 and -2 are mitogens that play a role in regulating cell proliferation, differentiation and apoptosis. Their effects are mediated through the IGF-R1 which initiates signaling cascades that result in regulation of a number of biological responses. IGF-R2, together with IGF-BPs is involved in binding, internalization and degradation of IGF-2. The overexpression of IGF-1 receptor (IGF-1R) has been linked to neoplastic development and has a role in regulating proliferation and differentiation. A final important determinant of IGF activity is through a family of at least six distinct IGF-binding proteins (IGFBP) that modulate bioavailability of IGFs in the circulation. Low circulating levels of IGFBPs favor an increased IGF mitogenic activity. IGF could be an appropriate target for cancer chemoprevention as increasing levels of IGF-I are associated with an increased risk of cancer [47]. We have shown that GTP resulted in substantial reduction in the levels of IGF-I and significant increase in the levels of IGFBP-3 in TRAMP mice [39]. EGCG has been reported to abrogate anchorage-independent growth induced by IGF-IR overexpression and also prevented human breast and cervical cancer cell phenotype expression through inhibition of IGF-IR downstream signaling [48]. We have also shown recently that combination treatment with GTP and celecoxib resulted in enhanced tumor growth inhibition by lowering of IGF-1 levels and increase in circulating levels of serum IGFBP-3 compared with results of single-agent treatment [49]. EGCG also caused inhibition of activation of IGF-1R, a decrease in the IGF-1 and an increase in the IGFBP-3 proteins. EGCG also caused a decrease in the levels of mRNAs that encode MMPs-7 and -9, proteins that proteolyze IGFBP-3 and a transient increase in the expression of TGF-β2, an inducer of IGFBP-3 expression in the HT29 human colon cancer cells [50]. We have earlier shown that 0.1% GTP (wt/vol) provided as the sole source of drinking fluid to TRAMP mice from 8 to 32 weeks of age resulted in significant inhibition in serum IGF-1 and restoration of IGFBP-3 levels in the prostate compared with water-fed TRAMP mice [51].
4.5. Inhibition of proteasome activities
The proteasome is a multicatalytic enzyme found in all eukaryotic cells and is now recognized as a novel and promising target for chemotherapy. Over the past decade, the proteasome has become increasingly appreciated as an important component of a complex pathway that targets and destroys intracellular proteins. Numerous substrates of the proteasome have been identified including cyclins, cyclin-dependent kinase inhibitors, p53, Bcl-2, and IκB among others. These proteins have various functions like regulation of the cell cycle, protection from apoptosis, and transcriptional regulation. The proteasome regulates cell growth, survival, and metastasis of cancer cells makes it an attractive target for new treatments [52]. It has been reported that EGCG and ECG, but not EGC and EC, potently inhibited the chymotryptic activity of the 20s proteasome both in cell-free systems and in tumor cell lines at the concentrations found in the serum of green tea drinkers. This inhibition of the proteasome by EGCG in several tumor and transformed cell lines results in the accumulation of two natural proteasome substrates, p27(Kip1) and IκBα, followed by growth arrest in the G(1) phase of the cell cycle [53]. EGCG analogs with acetyl protected -OH groups were reported to be much more potent than natural EGCG in inhibiting the proteasome in cultured tumor cells. Consistently, these protected analogs showed much higher potency than EGCG to inhibit proliferation and transforming activity and to induce apoptosis in human leukemic, prostate, breast, and simian virus 40-transformed cells [54].
4.6. Inhibition of Matrix metalloproteinases (MMPs)
The matrix metalloproteinase (MMP) family consists of more than 25 structurally related, zinc-dependent, endopeptidases that are capable of degrading various components of the extracellular matrix (ECM). They occupy a pivotal role in the ECM remodelling that accompanies several physiologic processes and pathological conditions including inflammatory, vascular, autoimmune disorders and carcinogenesis. Tumor invasion and metastasis is a multistep process that involves proteolytic degradation of the ECM, alteration of the cell-cell and cell-ECM interactions, and migration of the cancer cell through the basement membrane. Several in vivo and in vitro studies have demonstrated the important role of MMPs in these processes. MMPs promote angiogenesis, stimulate tumor growth, regulate innate immunity and exhibit antiapoptotic properties. Overexpression of MMPs in animal models induces cellular hyperproliferation and increases the susceptibility to tumorigenesis after exposure to chemical carcinogens or activation of oncogenes. Although some of these activities can be attributed to the matrix-degrading abilities of the MMPs, the majority of these biologic effects are mediated primarily by the proteolytic activity of MMPs on several non-matrix substrates such as chemokines, adhesion molecules, growth factors, growth factor receptors, proapoptotic and antiapoptotic molecules [55]. EGCG reduced the expression of MMP-2 mRNA and protein in rat hepatic stellate cells and concanavalin A-induced activation of secreted MMP-2 and reduced membrane-type 1-MMP activity in a dose-dependent manner [56].
EGCG was also found to inhibit G(1) to S-phase cell cycle progression and MMP-9 expression through the transcription factors NF-κB and AP-1 in TNF-α induced vascular smooth muscle cells [57]. EGCG, ECG, theaflavin and TFdiG inhibit collagenase activity in vitro [58]. EGCG also inhibited the invasion of Lewis lung cancer cells through a Matrigel basement membrane in vitro. EGCG has been shown to inhibit the activity of secreted MMP-2 and MMP9 [59]. We have earlier shown that orally administered GTP (0.1% in drinking water) caused marked inhibition of MMP-2 and MMP-9 in the prostate in TRAMP mice [38]. EGCG has also been reported to inhibit the invasion and migration of human oral cancer cells and that the effects may partially because of the decreased productions of MMP-2, MMP-9, and uPA [60].
4.7. Inhibition of urokinase-plasminogen activator (uPA)
uPA is primarily associated with the degradation and regeneration of the basement membrane and extracellular matrix that leads to metastasis. It also aids in anti-thrombolytic activities to remove blood clots and helps stimulate angiogenesis in tumor cells. It also catalyzes the activation of plasminogen into plasmin by cleaving the arginine-valine bond. In turn, plasmin facilitates the release of several proteolytic enzymes, including gelatinase, fibronectin, fibrin, laminin and latent forms of collagenases and stromelysins [61]. It has been reported that EGCG inhibited the activity of uPA. With the use of molecular modeling, it was shown that EGCG binds to urokinase, blocking His 57 and Ser 195 of the urokinase catalytic triad and extending toward Arg 35 from a positively charged loop of urokinase [62].
EGCG was found to decrease the expressions of MMP-2, MMP-9 and uPA in a concentration-dependent manner in human oral cancer cell line [60]. GTP inhibited constitutively active transcription factors AP-1 and NF-κB, which further suppressed secretion of uPA from breast cancer cells [63]. We have reported that GTP infusion to TRAMP mice resulted in marked inhibition of uPA in the dorso-lateral prostate [39]. Recently, we have shown that EGCG sensitizes TRAIL-resistant LNCaP cells to TRAIL-mediated apoptosis in part, through inhibition in the protein expression of VEGF, uPA and angiopoietin 1 and 2 in prostate cancer cells [64].
4.8. Induction of apoptosis and cell cycle arrest
We have shown for the very first time that EGCG induces apoptosis and cell cycle arrest in many cancer cells without affecting normal cells [65]. This observation has been verified by many subsequent studies in several cell types including lung, colon, pancreas, skin and prostate [66]. We have reported that in EGCG-treated LNCaP cells, p53 protein was stabilized, and EGCG inhibited NFκ transcription activity. The balance between pro- and anti-apoptotic Bcl-2 family proteins favored apoptosis and LNCaP cells were arrested in the G0/G1 phase [67]. A study with NMR spectroscopy showed the direct binding of tea polyphenols to the BH3 pocket of anti-apoptotic Bcl-2 family proteins, suggesting a mechanism for EGCG to inhibit the anti-apoptotic function of Bcl-2 proteins. The BH3 domain was recognized as one of the binding sites of tea polyphenols [68]. In SKH-1 mice, an increase in the number of apoptotic epidermal cells was observed when green tea was administered in drinking fluid prior to UVB exposure [69]. Topical application of EGCG to the skin of SKH-1 mice also caused increase in the number of caspase-3 positive apoptotic tumor cells which were induced by prior irradiation with UVB [70]. In the A/J mouse model, green tea in drinking fluid increased the apoptosis index in lung adenoma in chemically induced lung tumors [71]. EGCG induced expression of p21 and p27, inhibited the activity of CDK2 and CDK4 and caused Rb hypophosphorylation. In prostate cancer cells, EGCG increased the expression of p16, p18, p21, and p53, which are associated with negative regulation of cell cycle progression [72, 73]. It has also been shown that treatment of MCF7 breast cancer cells with EGCG resulted in G0/G1 phase cell cycle arrest [74].
5. Relevance to humans
Green tea catechins given in the form of capsules when given to men with high-grade prostate intraepithelial neoplasia (PIN) demonstrated cancer preventive activity by inhibiting the conversion of high grade PIN lesions to cancer. There was 3% incidence of prostate cancer in men given green tea catechins capsules, whereas 30% incidence in placebo-treated men after one year [75]. Evidence from a case-control study conducted in south-east China assessing 130 patients with histologically confirmed incidental prostate cancer and 274 patients without cancer matched by age, showed that the prostate cancer risk declined with increasing frequency, duration and quantity of green tea consumed. This reduction was statistically significant, suggesting that green tea protects against prostate cancer [76]. In a case control study, conducted in southeast China in 2004–2005 on 1009 female patients aged 20–87 years with histologically confirmed breast cancer, green tea consumption was found to be associated with a reduced risk of developed breast cancer [77]. A prospective cohort study over 10 years in Japan showed that the daily consumption of green tea delayed the onset of cancer in both smokers and nonsmokers [78]. In the Japan Public Health Center-based Prospective Study, 49,920 men aged 40–69 years completed a questionnaire that included their green tea consumption habit. Green tea was not associated with localized prostate cancer. However, consumption was associated with a dose-dependent decrease in the risk of advanced prostate cancer [79]. A recent meta-analysis of 13 papers on breast cancer showed that the combined results of green tea consumption from four studies showed a reduced risk of breast cancer for highest versus lowest intake groups [80]. It has been reported that plasma estrone levels were significantly lower in regular green tea drinkers than in non- or irregular green tea drinkers. Tea was found to be more protective against breast cancers in individuals with low catechol O-methyltransferase activity alleles compared those with high activity alleles in one study, but such an association was not found in another [81]. A study in Shanghai demonstrated that daily consumption of the equivalent of two or three cups of green tea reduced the risk for esophageal cancer among non-smokers/non-drinkers of alcoholic beverages [82]. On the other hand, studies in Japan usually suggest that ten cups of green tea is cancer-preventive [83]. In patients with androgen independent prostate carcinoma, the antineoplastic effects of green tea have been reported [84]. Daily consumption of green tea in a prospective cohort study with over 8000 individuals resulted in delayed cancer onset, and a follow-up study of breast cancer patients found that stages I and II breast cancer patients experienced a lower recurrence rate and longer disease-free period [85]. EGCG delivered in the form of capsule (200 mg orally for 12 weeks) has been reported to be effective in the patients with human papilloma virus (HPV) infected cervical lesions [86].
6. Conclusion and future prospects
Tea is now moving from a traditional beverage to a healthy drink, a source of pharmacologically active molecules, an important member of the antioxidant food group, and a functional food endowed with beneficial health properties. Nowadays, green tea is considered one of the most promising dietary agents for the prevention and treatment of many diseases and consequently, it is being studied extensively worldwide. The amount of experimental evidence documenting the properties of tea and its constituents continues to increase. Numerous studies in a variety of experimental animal models have demonstrated that GTP possesses antioxidant, antimutagenic, antidiabetic, anti-inflammatory, antibacterial and anticancer properties. Better understanding of the fundamental mechanism(s) of action of the tea constituents and their bioavailability is needed to more effectively determine the potential usefulness of tea as a cancer preventive agent. Integration of new molecular findings into clinical practice is a major challenge of cancer prevention. Therefore, identification of more molecular targets and biomarkers for tea polyphenols is essential for improving the design of clinical trials and will greatly assist in better understanding of the mechanisms underlying its anti-cancer activity. A fundamental understanding in this area is important for the rational design of future human intervention trials and cohort studies to elucidate the relationship between tea consumption and cancer.
Acknowledgement
The original work from the author's (HM) laboratory outlined in this review was supported by United States Public Health Service Grants RO1 CA 78809, RO1 CA 101039, RO1 CA 120451 and P50 DK065303.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol. Appl. Pharmacol. 1999;158:207–210. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- [2].Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khan WA, Wang ZY, Athar M, Bickers DR, Mukhtar H. Inhibition of the skin tumorigenicity of (+/−)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene by tannic acid, green tea polyphenols and quercetin in Sencar mice. Cancer Lett. 1988;42:7–12. doi: 10.1016/0304-3835(88)90232-7. [DOI] [PubMed] [Google Scholar]
- [4].Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: Progress and Promise. Antioxid Redox Signal. 2007;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- [5].Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat. Res. 2003;523–524:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- [6].Vaidyanathan JB, Walle T. Glucuronidation and sulfation of the tea flavonoid (−)-epicatechin by the human and rat enzymes. Drug Metab. Dispos. 2002;30:897–903. doi: 10.1124/dmd.30.8.897. [DOI] [PubMed] [Google Scholar]
- [7].Lee MJ, Wang ZY, Li H, Chen L, Sun Y, Gobbo S, et al. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomarkers Prev. 1995;4:393–399. [PubMed] [Google Scholar]
- [8].Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem. Res. Toxicol. 2000;13:177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- [9].Meselhy MR, Nakamura N, Hattori M. Biotransformation of (−)-epicatechin 3-O-gallate by human intestinal bacteria. Chem. Pharm. Bull. (Tokyo) 1997;45:888–893. doi: 10.1248/cpb.45.888. [DOI] [PubMed] [Google Scholar]
- [10].Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- [11].Okushio K, Suzuki M, Matsumoto N, Nanjo F, Hara Y. Identification of (−)-epicatechin metabolites and their metabolic fate in the rat. Drug Metab. Dispos. 1999;27:309–316. [PubMed] [Google Scholar]
- [12].Kim S, Lee MJ, Hong J, Li C, Smith TJ, Yang GY, et al. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- [13].Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- [14].Maeda-Yamamoto M, Suzuki N, Sawai Y, Miyase T, Sano M, Hashimoto-Ohta A, Isemura M. Association of suppression of extracellular signal-regulated kinase phosphorylation by epigallocatechin gallate with the reduction of matrix metalloproteinase activities in human fibrosarcoma HT1080 cells. J. Agric. Food Chem. 2003;51:1858–1863. doi: 10.1021/jf021039l. [DOI] [PubMed] [Google Scholar]
- [15].Deguchi H, Fujii T, Nakagawa S, Koga T, Shirouzu K. Analysis of cell growth inhibitory effects of catechin through MAPK in human breast cancer cell line T47D. Int. J. Oncol. 2002;21:1301–1305. [PubMed] [Google Scholar]
- [16].Bae JY, Choi JS, Choi YJ, Shin SY, Kang SW, Han SJ, et al. (−)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food Chem. Toxicol. 2007 doi: 10.1016/j.fct.2007.09.112. In press. [DOI] [PubMed] [Google Scholar]
- [17].Afaq F, Ahmad N, Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22:9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- [18].Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- [19].Chung H, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, et al. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003;17:1913–1915. doi: 10.1096/fj.02-0914fje. [DOI] [PubMed] [Google Scholar]
- [20].Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- [21].Kim HS, Kim MH, Jeong M, Hwang YS, Lim SH, Shin BA, et al. EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells. Anticancer Res. 2004;24:747–753. [PubMed] [Google Scholar]
- [22].Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–4419. [PubMed] [Google Scholar]
- [23].Chen NY, Ma WY, Yang CS, Dong Z. Inhibition of arsenite-induced apoptosis and AP-1 activity by epigallocatechin-3-gallate and theaflavins. J. Environ. Pathol. Toxicol. Oncol. 2000;19:287–295. [PubMed] [Google Scholar]
- [24].Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann. N. Y. Acad. Sci. 2004;1030:434–441. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- [26].Kundu JK, Surh YJ. Epigallocatechin gallate inhibits phorbol ester-induced activation of NF-kappa B and CREB in mouse skin: role of p38 MAPK. Ann. N. Y. Acad. Sci. 2007;1095:504–512. doi: 10.1196/annals.1397.054. [DOI] [PubMed] [Google Scholar]
- [27].Hong MH, Kim MH, Chang HJ, Kim NH, Shin BA, Ahn BW, Jung YD. (−)-Epigallocatechin-3-gallate inhibits monocyte chemotactic protein-1 expression in endothelial cells via blocking NF-kappaB signaling. Life Sci. 2007;80:1957–1965. doi: 10.1016/j.lfs.2007.02.024. [DOI] [PubMed] [Google Scholar]
- [28].Shin HY, Kim SH, Jeong HJ, Kim SY, Shin TY, Um JY. Epigallocatechin-3-gallate inhibits secretion of TNF-alpha, IL-6 and IL-8 through the attenuation of ERK and NF-kappaB in HMC-1 cell. Int. Arch. Allergy Immunol. 2007;142:335–344. doi: 10.1159/000097503. [DOI] [PubMed] [Google Scholar]
- [29].Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cell. J. Nutr. 2004;134:1039–1044. doi: 10.1093/jn/134.5.1039. [DOI] [PubMed] [Google Scholar]
- [30].Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- [31].Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004;23:2507–2522. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- [32].Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- [33].Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia N, Spiegelman VS. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–682. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- [34].Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- [35].Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin. Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- [36].Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- [37].Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- [38].Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- [39].Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- [40].Sachinidis A, Seul C, Seewald S, Ahn H, Ko Y, Vetter H. Green tea compounds inhibit tyrosine phosphorylation of PDGF beta-receptor and transformation of A172 human glioblastoma. FEBS Lett. 2000;471:51–55. doi: 10.1016/s0014-5793(00)01360-0. [DOI] [PubMed] [Google Scholar]
- [41].Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J. Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [42].Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, et al. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- [43].Fu Y, Chen A. The phyto-chemical (−)-epigallocatechin gallate suppresses gene expression of epidermal growth factor receptor in rat hepatic stellate cells in vitro by reducing the activity of Egr-1. Biochem. Pharmacol. 2006;72:227–238. doi: 10.1016/j.bcp.2006.04.026. [DOI] [PubMed] [Google Scholar]
- [44].Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, et al. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- [45].Sah JF, Balasubramanian S, Eckert RL, Rorke EA. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway: Evidence for direct inhibition of ERK1/2 and AKT kinases. J. Biol. Chem. 2004;279:12755–12762. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- [46].Masuda M, Suzui M, Lim JT, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin. Cancer Res. 2003;9:3486–3491. [PubMed] [Google Scholar]
- [47].Adhami VM, Afaq F, Mukhtar H. Insulin-like growth factor-I axis as a pathway for cancer chemoprevention. Clin. Cancer Res. 2006;12:5611–5614. doi: 10.1158/1078-0432.CCR-06-1564. [DOI] [PubMed] [Google Scholar]
- [48].Li M, He Z, Ermakova S, Zheng D, Tang F, Cho YY, et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol. Biomarkers Prev. 2007;16:598–605. doi: 10.1158/1055-9965.EPI-06-0892. [DOI] [PubMed] [Google Scholar]
- [49].Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin. Cancer Res. 2007;13:1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- [50].Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005;334:947–953. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- [51].Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl. Acad. Sci. U S A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Montagut C, Rovira A, Albanell J. The proteasome: a novel target for anticancer therapy. Clin. Transl. Oncol. 2006;8:313–317. doi: 10.1007/s12094-006-0176-8. [DOI] [PubMed] [Google Scholar]
- [53].Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J. Biol. Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- [54].Kuhn D, Lam WH, Kazi A, Daniel KG, Song S, Chow LM, et al. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front. Biosci. 2005;10:1010–1023. doi: 10.2741/1595. [DOI] [PubMed] [Google Scholar]
- [55].Konstantinopoulos PA, Karamouzis MV, Papatsoris AG, Papavassiliou AG. Matrix metalloproteinase inhibitors as anticancer agents. Int. J. Biochem. Cell Biol. 2007 doi: 10.1016/j.biocel.2007.11.007. In Press. [DOI] [PubMed] [Google Scholar]
- [56].Zhen MC, Huang XH, Wang Q, Sun K, Liu YJ, Li W, et al. Green tea polyphenol epigallocatechin-3-gallate suppresses rat hepatic stellate cell invasion by inhibition of MMP-2 expression and its activation. Acta Pharmacol. Sin. 2006;27:1600–1607. doi: 10.1111/j.1745-7254.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- [57].Kim CH, Moon SK. Epigallocatechin-3-gallate causes the p21/WAF1-mediated G(1)-phase arrest of cell cycle and inhibits matrix metalloproteinase-9 expression in TNF-alpha-induced vascular smooth muscle cells. Arch. Biochem. Biophys. 2005;435:264–272. doi: 10.1016/j.abb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- [58].Isemura M, Saeki K, Minami T, Hayakawa S, Kimura T, Shoji Y, et al. Inhibition of matrix metalloproteinases by tea catechins and related polyphenols. Ann. N Y Acad. Sci. 1999;878:629–631. doi: 10.1111/j.1749-6632.1999.tb07746.x. [DOI] [PubMed] [Google Scholar]
- [59].Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer. 2001;91:822–832. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [60].Ho YC, Yang SF, Peng CY, Chou MY, Chang YC. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J. Oral Pathol. Med. 2007;36:588–593. doi: 10.1111/j.1600-0714.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- [61].Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2007 doi: 10.1016/j.ctrv.2007.10.005. In Press. [DOI] [PubMed] [Google Scholar]
- [62].Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- [63].Slivova V, Zaloga G, DeMichele SJ, Mukerji P, Huang YS, Siddiqui R, et al. Green tea polyphenols modulate secretion of urokinase plasminogen activator (uPA) and inhibit invasive behavior of breast cancer cells. Nutr. Cancer. 2005;52:66–73. doi: 10.1207/s15327914nc5201_9. [DOI] [PubMed] [Google Scholar]
- [64].Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2007 doi: 10.1038/sj.onc.1210840. In Press. [DOI] [PubMed] [Google Scholar]
- [65].Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- [66].Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- [67].Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- [68].Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- [69].Lu YP, Lou YR, Li XH, Xie JG, Brash D, Huang MT, et al. Stimulatory effect of oral administration of green tea or caffeine on ultraviolet light-induced increases in epidermal wild-type p53, p21(WAF1/CIP1), and apoptotic sunburn cells in SKH-1 mice. Cancer Res. 2000;60:4785–4791. [PubMed] [Google Scholar]
- [70].Lu YP, Lou YR, Xie JG, Peng QY, Liao J, Yang CS, et al. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. U S A. 2002;99:12455–12460. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, et al. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr. Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- [72].Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch. Biochem. Biophys. 2003;410:177–185. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- [73].Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- [74].Liang YC, Lin-Shiau SY, Chen CF, Lin JK. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate. J. Cell Biochem. 1999;75:1–12. [PubMed] [Google Scholar]
- [75].Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- [76].Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int. J. Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- [77].Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis. 2007;28:1074–1078. doi: 10.1093/carcin/bgl252. [DOI] [PubMed] [Google Scholar]
- [78].Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors. 2000;13:49–54. doi: 10.1002/biof.5520130109. [DOI] [PubMed] [Google Scholar]
- [79].Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am. J. Epidemiol. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- [80].Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis. 2006;27:1301–1309. doi: 10.1093/carcin/bgl024. [DOI] [PubMed] [Google Scholar]
- [81].Wu AH, Yu MC. Tea, hormone-related cancers and endogenous hormone levels. Mol. Nutr. Food Res. 2006;50:160–169. doi: 10.1002/mnfr.200500142. [DOI] [PubMed] [Google Scholar]
- [82].Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr. Reduced risk of esophageal cancer associated with green tea consumption. J. Natl. Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- [83].Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev. Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- [84].Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–1446. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- [85].Fujiki H. Two stages of cancer prevention with green tea. J. Cancer Res. Clin. Oncol. 1999;125:589–597. doi: 10.1007/s004320050321. [DOI] [PubMed] [Google Scholar]
- [86].Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003;12:383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]