Abstract
Traditionally, astrocytes have been considered less susceptible to injury than neurons. Yet, we have recently shown that astrocyte death precedes neuronal death in a rat model of traumatic brain injury (TBI) (Zhao et al.: Glia 44:140–152, 2003). A main mechanism hypothesized to contribute to cellular injury and death after TBI is elevated intracellular calcium ([Ca2+]i). Since calcium regulation is also influenced by regulation of intracellular sodium ([Na+]i), we used an in vitro model of strain-induced traumatic injury and live-cell fluorescent digital imaging to investigate alterations in [Na+]i in cortical astrocytes after injury. Changes in [Na+]i, or [Ca2+]i were monitored after mechanical injury or L-glutamate exposure by ratiometric imaging of sodium-binding benzofuran isophthalate (SBFI-AM), or Fura-2-AM, respectively. Mechanical strain injury or exogenous glutamate application produced increases in [Na+]i that were dependent on the severity of injury or concentration. Injury-induced increases in [Na+]i were significantly reduced, but not completely eliminated, by inhibition of glutamate uptake by DL-threo-β-benzyloxyaspartate (TBOA). Blockade of sodium-dependent calcium influx through the sodium-calcium exchanger with 2-[2-[4-(4-Nitrobenzyloxy)-phenyl]ethyl]isothiourea mesylate (KB-R7943) reduced [Ca2+]i after injury. KB-R7943 also reduced astrocyte death after injury. These findings suggest that in astrocytes subjected to mechanical injury or glutamate excitotoxicity, increases in intracellular Na+ may be a critical component in the injury cascade and a therapeutic target for reduction of lasting deficits after traumatic brain injury.
Keywords: traumatic brain injury, Fura-2, calcium, SBFI-AM, KB-R7943, TBOA, glutamate
INTRODUCTION
A widely accepted hypothesis of CNS injury is that excessive glutamate released from the injury site precipitates an excitotoxic cascade and pathological increase in intracellular calcium that initiates cell death (for review, see Choi, 1988; Young, 1992; Katayama et al., 1995; for exception, see Obrenovitch and Urenjak, 1997). The evidence for increased cellular calcium after traumatic brain injury (TBI) is compelling. Experimental TBI resulted in increased total cell calcium (Hovda et al., 1992; Fineman et al., 1993), increased calcium in axonal injury (Maxwell et al., 1997; Povlishock et al., 1999), and pathologic activation of calcium-dependent proteases (Pike et al., 1998; Newcomb et al., 1999). Similarly, cell culture models of mechanical injury have shown increased intracellular free calcium ([Ca2+]i) (LaPlaca et al., 1997; Weber et al., 1999; Iwata et al., 2004), activated calcium-related phospholipases and kinases (Lamb et al., 1997; Floyd et al., 2001; Neary et al., 2003), and cell damage/death (Ellis et al., 1995; Geddes et al., 2003). Much research in TBI has centered on protecting neurons from excitotoxicity (e.g., Bullock et al., 1999; Narayan et al., 2002).
An important caveat to neuroprotection is that neurons cannot survive in the brain without astrocytes (Aschner and Kimelberg, 1991; Chen and Swanson, 2003), which suggests that protecting astrocytes from excitotoxic injury may also be critical. Astrocyte damage or loss could exacerbate neuronal excitotoxicity by cessation of glutamate clearance (Rosenberg and Aizenman, 1989; Rothstein et al., 1996) or even astroglial efflux of glutamate (Kimelberg et al., 1990; Duan et al., 2003). Astrocytes have classically been considered more resistant to excitotoxic injury than neurons (Goldberg and Choi, 1993); however, after ischemia, astrocyte death precedes neuronal death (Liu et al., 1999; Martin et al., 1997). We have previously shown that astrocyte loss precedes neuronal loss after experimental TBI in rats (Zhao et al., 2003). Thus, early astrocyte protection from excitotoxicity may likely be a component of neuroprotection, yet the mechanisms of astrocyte death are not fully elucidated.
Calcium can enter astrocytes directly through activated voltage-gated calcium channels (MacVicar, 1984; Barres et al., 1990) calcium-sensitive AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) channels (Condorelli et al., 1993; Shelton and McCarthy, 1999); or influx via the reverse mode of the Na+/Ca2+ exchanger (NCX) (Goldman et al., 1994). In uninjured astrocytes, elevated intracellular free calcium is rapidly stored in the endoplasmic reticulum by a specific ATP-dependent Ca2+ pump (Seidler et al., 1989). However, mechanical strain injury not only reduces astrocyte ATP levels (Ahmed et al., 2000), but also releases calcium from intracellular stores (Rzigalinski et al., 1998; Floyd et al., 2001) rendering calcium extrusion mechanisms a potentially critical component in reducing [Ca2+]i. Energy-independent extrusion of intracellular calcium is mainly accomplished by the NCX operating in the forward or calcium-efflux mode (Goldman et al., 1994). The magnitude and direction of calcium flux by the NCX depend in part on the Na+ electrochemical gradient. Mechanical injury to astrocytes may also elevate intracellular sodium ([Na+]i), possibly by astrocyte Na-dependent glutamate uptake (for review, see Hansson et al., 1997; Anderson and Swanson, 2000). Elevated [Na+]i could thereby influence the direction of the NCX after mechanical injury and therefore may be critical to cell survival. In uninjured astrocytes, manipulations which raise intracellular sodium have been shown to increase [Ca2+]i by reversal of the NCX (Goldman et al., 1994).
We hypothesized that after mechanical injury, sodium-dependent glutamate uptake would increase [Na+]i and possibly overwhelm astrocyte sodium extrusion mechanisms. Elevated [Na+]i, coupled with membrane depolarization, could reverse normal Na+/Ca2+ exchange, thereby increasing [Ca2+]i from sodium-dependent calcium influx and contributing to astrocyte damage and death. We evaluated this hypothesis in astrocyte cultures by examining the effects of mechanical strain injury on [Na+]i and [Ca2+]i, using quantitative fluorescent imaging. Injury-induced alterations in intracellular sodium and calcium were compared with ionic perturbations caused by extracellular glutamate application. Glutamate experiments were carried out in this study as parallel investigations, using identical imaging parameters, which permit comparisons with previous studies (for review, see Araque et al., 1999; Scemes, 2000). Inhibition of glutamate transport and reversal of Na+/Ca2+ exchange were examined to determine their contributions to ionic flux and cell damage/death.
MATERIALS AND METHODS
Materials
The inhibitor of glutamate uptake, DL-threo-β-benzyloxyaspartate (TBOA) (Shimamoto et al., 1998; Waage-petersen et al., 2001), and the selective inhibiter of sodium-dependent calcium entry from the sodium-calcium exchanger 2-[2-[4-(4-Nitrobenzyloxy)phenyl]ethyl]i-sothiourea mesylate (KB-R7943) mesylate (KB-R7943) (Iwamoto et al., 1996), were purchased from Tocris Cookson (Ellisville, MO). Stock solutions of TBOA and KB-R7943 were dissolved in distilled water and diluted in HEPES buffered saline to final concentrations of 100 nM TBOA and 10 μM KB-R7943. L-Glutamate and all other chemicals were purchased from Sigma (St. Louis, MO) unless otherwise noted. A stock solution of L-glutamic acid was dissolved in distilled water and diluted in HEPES buffered saline to either 1 mM or 100 μM final concentration.
Cell Culture
Cortical astrocytes were prepared from 1–2-day-old neonatal Sprague-Dawley rat pups (Harlan, Indianapolis, IN) as previously described (Amruthesh et al., 1993) and in accordance with National Institutes of Health (NIH) guidelines for care and use of laboratory animals. Briefly, cortices were isolated and cleaned of white matter and meninges and then minced and papain digested for 30 min. Cell suspensions were diluted with DMEM supplemented with 10% heat-inactivated fetal calf serum and 2 mM glutamine + 100 U/ml penicillin and 100 μg/ml streptomycin sulfate (all from Gibco, Grand Island, NY). Cells were seeded into 75-cm2 flasks at an initial density of 2 × 106 cells per flask. Astrocytes were cultured to confluence (14 days) in a 5% CO2 incubator at 37°C with media changes every 2–3 days. Upon confluency, cells were lifted from the flask surface using 0.25% trypsin/0.02% EDTA. Astrocytes were then washed and plated onto collagen-coated 25-mm diameter Flex Plate (Flexcell International, Hillsborough, NC) wells at 2 × 105 cells per well and cultured to confluency, which took approximately 1–2 weeks. Primary astrocytes were used for experiments after 3–4 weeks in vitro. Astrocyte cultures were characterized for purity as previously described and found to be 98% pure as assessed by the presence of glial fibrillary acidic protein (GFAP) (Amruthesh et al., 1993).
In Vitro Strain Mechanical Injury
Astrocytes were mechanically strain-injured (formerly referred to as stretch or rapid-stretch injury) using a model 94A Cell Injury Controller, as previously described (Bioengineering Facility, VA Commonwealth University, Richmond, VA) (Ellis et al., 1995). Briefly, the 2-mm-thick Silastic membrane of the Flex Plate was rapidly and transiently deformed by a 50-ms pulse of compressed gas, which deformed the Silastic membrane and adherent cells to varying degrees controlled by the pulse pressure. The extent of cell injury, as measured by uptake of propidium iodide (PrI) and by release of lactate dehydrogenase (LDH), has previously been shown to be dependent on the degree of Silastic membrane deformation under the action of applied force from a pulse of compressed gas, or tensile strain (Ellis et al., 1995). Based on previous work, we utilized three levels of cell injury. Mild, moderate, and severe injuries were produced by deforming the Silastic membrane on which the cells are grown by 5.5, 6.5, and 7.5 mm, respectively. These degrees of membrane deformation result in a corresponding biaxial strain of 31%, 38%, and 54% (Ellis et al., 1995). Using gel-filled human skulls, this range of cell strain has been shown to be relevant to what would occur in humans after rotational acceleration/deceleration injury (Margulies et al., 1990; Meaney et al., 1995).
In Vitro Intracellular Imaging of Na+, and Ca2+
Imaging experiments were performed in HEPES buffered saline containing (in mM): 126.5 NaCl, 3 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 10 glucose, 25 HEPES titrated to a pH of 7.2 with KOH. The fluorescent dyes for intracellular measurements were all in the acetoxy-methylester (-AM) form and included SBFI (sodium-binding benzofuran isophthalate) for Na+, and Fura-2 for Ca2+ (all from Molecular Probes, Eugene, OR).
Dye loading
Cells were rinsed twice and then loaded in HEPES buffered saline with either 5 μM Fura-2-AM (60 min) or 20 μM SBFI-AM + 0.1% pluronic acid (90 min) at room temperature (20–22°C). Cells were rinsed before imaging. During the experiments, cells were perfused (flow rate 5 ml/min) with 37°C HEPES buffered saline and bath temperature monitored and maintained with a Peltier-controlled open incubation system (Harvard Apparatus, Holliston, MA).
Measurement and calibration of dyes
Imaging experiments were performed on an upright Nikon E-600 epifluorescence microscope equipped with a 40× dipping objective. Cells were excited every 0.3 s by a Polychrome monochromator (TILL Photonics, Grafelfing, Germany) at 340 and 380 nm for Ca2+ or Na+ (total excitation time 0.6 s). Fluorescent emission was collected at >510 nm by an OrcaII-ER CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan). Background fluorescence was negligible (less than 1% of signal) and was subtracted from all final calculations by selecting a cell-free region of interest (ROI) in each image. Data were collected and quantified with Simple PCI imaging software (C-Imaging by Compix, Cranberry Township, PA), using the Windows XP operating system.
Calibration of SBFI to intracellular Na+ was done by a three-point calibration technique (Rose and Ransom, 1996). Cells were perfused with HEPES buffered saline containing gramicidin (3 μM), ouabain (100 μM), and 0, 30, and then 50 mM Na+. Data were normalized and fitted to a monotonic calibration curve for Na+ that was determined in a separate set of cells perfused with the ionophores and HEPES buffered saline containing Na+ concentrations of 0, 10, 30, 50, 70, and 100 mM Na+ (equimolar cations by adjusting with K+). Calibration of fura-2-AM for calcium concentration was performed according to the method originally described by Grynkiewicz et al. (1985). Briefly, Rmin values were obtained by perfusing cells with calcium-free HEPES buffered saline with ionomycin (10 μM) and EGTA (10 mM). Rmax values were obtained by changing perfusion solution to HEPES buffered saline containing 5 mM Ca2+ and ionomycin. Kd of intracellular Fura-2 was determined to be 227 ± 2 nM at 37°C in the pH range of 6.0–7.4 in a separate set of cells.
Assessment of In Vitro Cell Death
At each specific experimental time-point, unfixed cells were incubated with 10 μl/ml propidium iodide (PrI; Molecular Probes) and fluorescence visualized on a Nikon E-600 at 200×. Five randomly chosen fields (each field area = 0.2 mm2) were manually counted in each dish and averaged to obtain cell death. Triton × (10%) was added to lyse all cells. After 15-min incubation with PrI, five additional random fields were counted and averaged to obtain a total cell count. Cell death was expressed as percentage of total cells.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was set at P < 0.05. In vitro ionic measurement data were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc analysis conducted on SigmaStat version 3.0 for Windows. For in vitro ionic measurements, individual cell values from a microscope field (4–7 cells/field) were averaged for each experimental condition. Each experimental condition was repeated 3 times totaling 12–21 cells/condition (n = 3).
RESULTS
Mechanical Injury or L-Glutamate Increases Intracellular Sodium
We hypothesized that mechanical strain injury causes influx of Na+ into astrocytes, which may result in persistent elevation of [Na+]i. We evaluated this hypothesis by cellular imaging of the Na+ indicator SBFI before and immediately after strain injury in cortical astrocytes (Fig. 1A). Baseline [Na+]i was 13.7 ± 1.6 mM. [Na+]i increased significantly after mild, moderate, and severe strain injury in a strain-dependent manner. Injury-induced elevations in [Na+]i were transient after mild and moderate injury, but persisted throughout the experiment after severe injury (Fig. 2A).
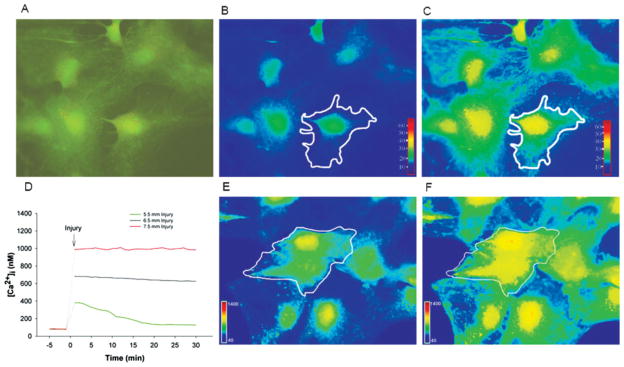
Fig. 1.
Ratiometric live-cell imaging of SBFI-AM and Fura-2-AM. A–C: Ratiometric live cell fluorescent imaging of SFBI-AM in cultured astrocytes. A: 340/380 ratio image before mechanical strain injury, B: Pseudo-colored imaged with white outline cell (region of interest) indicating intracellular Na+ concentration before and after (C) mild (5.5-mm) mechanical strain injury. D: Representative tracings from mild, moderate, and severely strain injured astrocytes where intracellular calcium concentration ([Ca2+]i) values were obtained via ratiometric live cell fluorescent imaging of Fura-2-AM. Note that the dotted line indicates 60-s lapse in recording where cells were removed from microscope to be injured. Imaging resumes 30 s after mechanical stretch injury. E,F: Pseudo-colored image with white outlined cell (region of interest) indicating [Ca2+]i before and after (F) moderate (6.5-mm) mechanical strain injury.
Fig. 2.
Effect of injury or L-glutamate on [Na+]i. Intracellular sodium ([Na+]i) was measured with ratiometric live cell fluorescent imaging of SBFI-AM in cultured astrocytes. A: Effect of mild (5.5-mm), moderate (6.5-mm), or severe (7.5-mm) mechanical strain injury on [Na+]i. Pre-injury baseline [Na+]i was 13.7 ± 1.6 mM. B: Effect of 5-min application of L-glutamate (10, 100, or 1,000 μM) on [Na+]i. Base-line [Na+]i was 11.7 ± 1.8 mM. *, [Na+]i significantly increased over baseline value (time 0) at time-point specified; +, [Na+]i significantly increased over baseline at all time-points measured (n = 3 separate experiments).
Increases in [Na+]i after strain injury were compared with astrocytes exposed to L-glutamate for 5 min. Base-line [Na+]i was 11.7 + 1.8 mM. Bath application of 100 μM or 1 mM glutamate also increased [Na+]i in a dose-dependent manner (Fig. 2B). The 100 μM and 1 mM glutamate concentrations were selected to simulate “signaling” and toxic concentrations, respectively. The 100 μM glutamate application yielded a transient increase in [Na+]i which was similar in magnitude and duration to elevations produced by mild strain injury. Also, the 1 mM glutamate application elevated [Na+]i to a magnitude similar to that produced by severe strain injury.
Mechanical Injury or L-Glutamate-Produced Cell Death
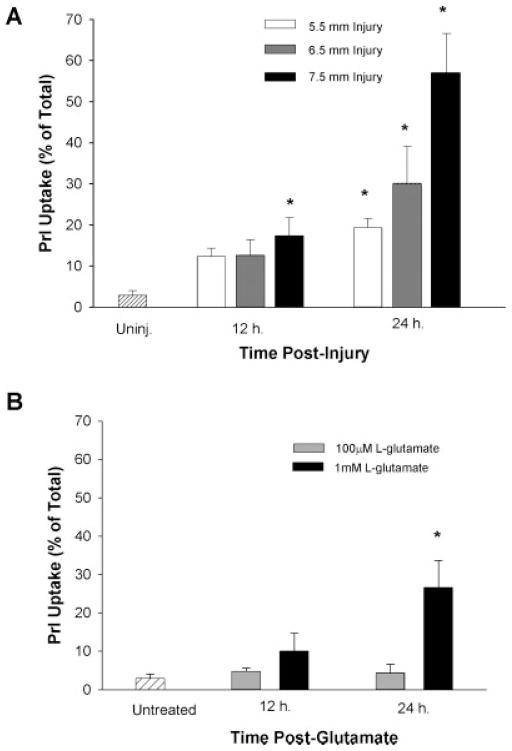
We compared the astrocyte cell death produced by mechanical strain injury or excitotoxic L- glutamate exposure. Astrocyte death was quantified by PrI uptake as a percentage of total cells. At 12 h post-injury, severe (7.5-mm) strain injury significantly increased PrI uptake (17.4 ± 1.4%) as compared with uninjured control values (2.93 ± 1.1; Fig. 3A). At 24 h post-injury, PrI uptake was significantly increased after mild (19.4 ± 2.2), moderate (30.1 ± 9.0), and severe (57.1 ± 9.5) injury with increased PrI uptake dependent on the severity of the strain injury (Fig. 3A). Exposure to 100 μM L-glutamate did not produce significant cell death. However, exposure to 1 mM glutamate produced significant cell death (26.6 ± 6.9) 24 h later (Fig. 3B).
Fig. 3.
Effect of mechanical strain injury or L-glutamate on cell death. Either mechanical strain injury (A) or L-glutamate application (B) was administered to cultured astrocytes. Cell death was indicated by propidium iodide (PrI) uptake expressed as percentage of total PrI uptake. *, PrI was significantly increased over control (uninjured or untreated) values at time-point specified (n = 3 separate experiments).
Inhibition of Glutamate Uptake Reduces [Na+]i
To evaluate the contribution of sodium-dependent glutamate uptake in injury-induced increases in [Na+]i, we used the glutamate uptake inhibitor TBOA (Shimamoto et al., 1998). TBOA (100 nM) was bath perfused before injury or L-glutamate application. Injury-induced increases in [Na+]i were significantly reduced, but not completely eliminated by pre-injury application of TBOA after mild (Fig. 4A), moderate (Fig. 4B), or severe (Fig. 4C) mechanical strain injury. Application of TBOA before injury reduced cell death only after severe strain injury at 24 h post-injury (Fig. 4D).
Fig. 4.
Effect of TBOA on [Na+]i and cell death after mechanical injury. Sodium-dependent glutamate uptake was inhibited with 100 nM TBOA before strain injury. [Na+]i was measured with ratio-metric live cell fluorescent imaging of SBFI-AM in cultured astrocytes. Pre-injury application of TBOA reduced injury-induced increases in [Na+]i after mild (5.5 mm; A), moderate (6.5 mm; B) or severe (7.5 mm; C) injury. Cell death (D) was indicated by PrI uptake and expressed as percentage of total PrI uptake after lysing cells with Triton X. TBOA reduced cell death in the severe (7.5-mm) strain injury group only (*). A–C: *, TBOA application significantly reduced [Na+]i versus injury at time-point specified; +, [Na+]i significantly reduced over injury at all time-points measured (n = 3 separate experiments). Data in non-TBOA conditions are from Fig. 1.
TBOA also significantly reduced, but did not completely eliminate, L-glutamate-induced increases in [Na+]i after 100 μM (Fig. 5A) or 1 mM L-glutamate (Fig. 5B) stimulation. Data in non-TBOA conditions are also shown in Figure 1. TBOA did not alter [Na+]i in uninjured astrocytes (data not shown). TBOA also produced a modest, but significant, reduction in cell death after 1 mM L-glutamate application as indicated by PrI uptake at 24 h post-injury (Fig. 5C).
Fig. 5.
Effect of TBOA on [Na+]i and cell death after L-glutamate. Sodium-dependent glutamate uptake was inhibited with 100 nM TBOA before and during L-glutamate application. [Na+]i was measured with ratiometric live cell fluorescent imaging of SBFI-AM in cultured astrocytes. Pre-glutamate application of TBOA reduced glutamate-induced increases in [Na+]i after 100 μM (A) and 1 mM (B). TBOA significantly reduced cell death in the 1 mM condition (C). A,B: *, TBOA application significantly reduced [Na+]i versus glutamate level at time-point specified; +, [Na+]i significantly reduced over glutamate level at all time-points measured (n = 3 separate experiments). Data in non-TBOA conditions are from Fig. 1.
Pharmacological Inhibition of the Reversed Mode of the Na+/Ca+ Exchanger (NCX) Reduced Injury-Induced Increases in [Ca+]i
Increased [Ca2+]i after mechanical strain injury was previously reported in astrocytes (Rzigalinski et al., 1998) and neurons (Weber et al., 2001). We also observed that mechanical strain injury increased [Ca2+]i in astrocytes, which corresponded with the severity of strain injury (Fig. 1A,B). We hypothesized that the elevated [Na+]i produced by mechanical injury may be sufficient to reverse Na+/Ca2+ exchange, which could contribute significantly to the increased [Ca2+]i observed after mechanical injury to astrocytes. Thus, we evaluated the effect of KB-R7943, a selective inhibitor of sodium-dependent calcium influx by NCX (reversed mode) (Iwamoto et al., 1996) on strain injury-induced increases in [Ca2+]i (Fig. 6A–C). Pre-injury application of KB-R7943 did not reduce injury-induced increases in [Ca2+]i after mild injury (Fig. 6A), but did modestly reduce [Ca2+]i elevation after moderate injury (Fig. 6B). Pre-injury application of KB-R7943 produced a pronounced reduction in injury-induced increases in [Ca2+]i after severe injury (Fig. 6C). Corresponding with the calcium data, pre-injury administration of KB-R79743 yielded no, modest, and pronounced cell protection after mild, moderate, and severe injuries, respectively (Fig. 6D).
Fig. 6.
Effect of mechanical strain injury on [Ca2+]i and Na+/Ca2+ exchange. Intracellular calcium ([Ca2+]i) was measured with ratiometric live cell fluorescent imaging of Fura-2-AM in cultured astrocytes. The sodium-calcium exchanger (NCX) was inhibited in the reverse direction with 100 nM KBR-7943 before strain injury. Pre-injury application of KB-R7943 did not change [Ca2+]i after mild injury (5.5 mm; A), but modestly reduced [Ca2+]i after moderate strain injury (6.5 mm; B), and more robustly reduced injury-induced [Ca2+]i after severe injury (7.5 mm; C). Pre-administration of KB-R7943 also reduced propidium iodide (PrI) uptake in moderate or severely injured astrocytes measured 24 h post-injury (D, *). A–C: *, KB-R7943 application significantly reduced [Ca2+]i versus injury level at time-point specified; +, [Ca2+]i significantly reduced over injury level at all time-points measured (n = 3 separate experiments).
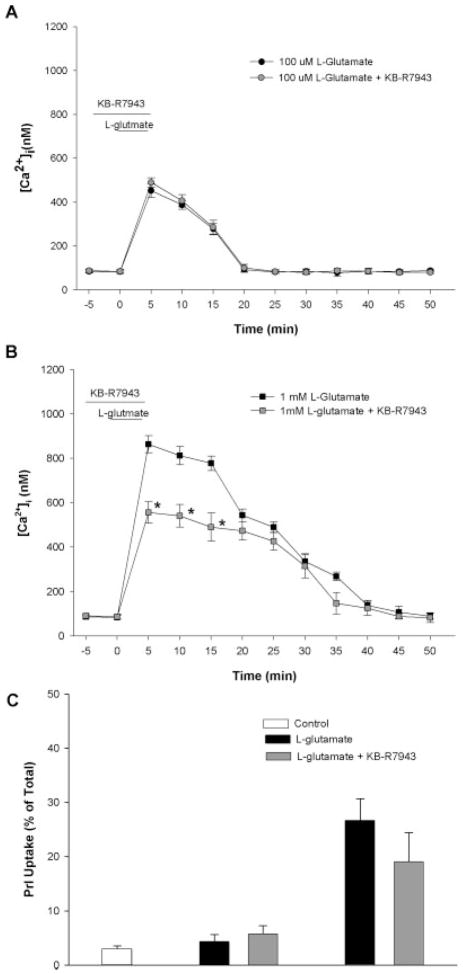
Similar to the strain injury experiments, KB-R7943 was administered before and during L-glutamate exposure to evaluate the role of NCX-mediated calcium entry. In the absence of KB-R7943, L-glutamate produces a rapid and dose-dependent increase in intracellular calcium (Fig. 7A,B). KB-R7943 did not reduce increases in [Ca2+]i after 100 μM L-glutamate exposure (Fig. 7A), but did produce a significant attenuation of increased [Ca2+]i after 1 mM L-glutamate exposure (Fig. 7B). KB-R7943 did not significantly reduce cell death at 24 h after either 100 μM or 1 mM L-glutamate exposure; however, there was a non-significant trend toward decrease in PrI uptake after the 1 mM L-glutamate exposure (P = 0.052).
Fig. 7.
Effect of L-glutamate on [Ca2+]i and Na+/Ca2+ exchange. Intracellular calcium ([Ca2+]i) was measured with ratiometric live cell fluorescent imaging of Fura-2-AM in cultured astrocytes. The sodium-calcium exchanger (NCX) was inhibited in the reverse direction with 100 nM KB-R7943 before L-glutamate application. Pre-glutamate application of KB-R7943 did not reduce [Ca2+]i in 100 μM condition. After 1 mM L-glutamate application, KB-R7943 reduced [Ca2+]i at some time-points (*). Pre-administration of KB-R7943 did not significantly reduce propidium iodide (PrI) uptake in either condition, although there was a nonsignificant trend (P = 0.052) toward reduction after 1 mM glutamate application. (n = 3 separate experiments).
Modeling the Driving Force for Reversal of NCX After Mechanical Strain Injury
Since the transmembrane gradients of Na+, Ca2+ and membrane potential determine the direction of Ca2+ (influx or efflux) transported by NCX, we compared the observed effect of pharmacological inhibition of sodium-dependent calcium influx (reversed) mode of the NCX with KB-R7943 with a mathematical model of the NCX reversal potentials. The driving force for the NCX as a function of membrane potential at each severity of injury was modeled using the following equation (Blaustein and Lederer, 1999) that defines the difference between the membrane potential (Vm) and the reversal potential of the exchanger (ENCX). If the reversal potential of the exchanger is less (i.e., more negative) than the membrane potential, sodium-dependent calcium influx (reversed mode) will occur. With a coupling ratio of 3, the ENCX = 3ENa−2ECa where the equilibrium potentials (in mV) for sodium (ENa) and calcium (ECa) are
and
where R, the gas constant, equals 1.987 cal/mol/deg;T, the absolute temperature, equals 310 K at 37°C; and F, Faraday’s constant, equals 23,062 cal/V/mol. Subscripts o and i indicate extracellular and intracellular concentration. Modeled ENCX in mV at post-injury time-points are listed in Table 1. When the NCX reversal potential is considerably lower (more negative) than the estimated membrane potential it is likely for NCX to be operating in the calcium influx mode (reversal). These modeled ENCX reversal potential values are bolded in Table 1. If a physiologically relevant membrane potential of approximately Em ≥−85 mV is assumed (Somjen, 1995), intracellular sodium and calcium values that were measured after mild injury are sufficient to produce reversal of NCX for only the first 10 min post-injury. Also, moderate strain injury increased [Na+]i and [Ca2+]i to levels that this model predicts could reverse the NCX for approximately 15 min post-injury. However, severe strain injury increased [Na+]i and [Ca2+]i to levels that are predicted to reverse the NCX throughout the duration of the recording time (50 min). These model calculations are consistent with the pharmacological reduction in [Ca2+]i and astrocyte protection observed in severe injury by application of KB-R7943 (Fig. 7C).
TABLE 1.
Modeled Reversal Potentials for NCX Following Strain Injury*
| Injury (mm) | Time post-injury (mini) |
||||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 30 | 40 | 50 | |
| 5.5 | −108 | −103 | −85 | −78 | −67 | −70 | −74 |
| 6.5 | −129 | −117 | −96 | −66 | −47 | −46 | −49 |
| 7.5 | −137 | −132 | −122 | −122 | −115 | −116 | −110 |
NCX; Na+/Ca2+ exchanges.
Reversal potential of the NCX (ENCX) as calculated with the following equation: ENCX = 3ENa − 2ECa, where the equilibrium potentials (in mV) for sodium (ENa) and calcium (ECa) are ENa = (RT/F) × ln([Na+]o/[Na+]i) and Eca = (RT/2F) × ln([Ca2+]o/[Ca2+]i]), where R is the gas constant, T is the absolute temperature, and F is Faraday’s constant. Subscripts o and i indicate extracellular and intracellular concentration with the mean value measured post-injury used. When the ENCX is less (more negative) than the estimated membrane potential (Em), NCX will operate in the calcium influx (reversed) mode. The value of the membrane potential (Vm) taken from relevant literature and cited accordingly in the text is −85 mV. Bold ENCX values represent modeled sodium-mediated calcium entry (reversed mode). Measured mean values for [Na+]i and [Ca2+]i are from data in figs. 2 and 6, respectively. Sodium-dependent calcium influx is predicted for the 7.5-mm strain injury in which this model shows conditions consistent with a sustained reversal of the NCX. In contrast, the 5.5- and 6.5-mm strain injuries show possible conditions for a transient, but less likely, NCX reversal.
DISCUSSION
Our results indicate that mechanical injury to astrocytes results in a rapid increase in [Na+]i, which is similar in magnitude to the [Na+]i increase induced by a brief (5-min) exogenous glutamate application. Injury- or glutamate-induced increases in [Na+]i were dependent on the stimulus strength with 100 μM glutamate or mild rapid strain injury producing a transient elevation in [Na+]i while high glutamate (1 mM) and severe strain injury produced a substantial and protracted elevation in [Na+]i. The direct measurement of intracellular Na+ in mechanically injured astrocytes has not been previously reported, and the increased [Na+]i is similar to that previously reported in astrocytes after exogenous glutamate application (Rose and Ransom, 1996) or ischemia (Rose et al., 1998; Bondarenko and Chesler 2001a,b). Injury-induced Na+ influx also has been implicated as a mechanism of neuronal injury including mechanical stretch injury to axons (Wolf et al., 2001), glutamate toxicity to dendrites (Hasbani et al., 1998), and neuronal ischemic injury (for review, see Fern and Ransom, 1997). Moreover, astrocyte swelling after severe CNS injuries is likely related to sodium-dependent glutamate uptake (Kimelberg et al., 1995; Hansson et al., 1997).
We attribute the injury-induced increases in [Na+]i mainly to sodium-dependent glutamate uptake as injury-induced increases closely resembled glutamate induced-increases in [Na+]i and inhibition of glutamate uptake by TBOA attenuated approximately 75% of post- injury [Na+]i accumulation. Our findings are similar to the report that TBOA inhibited nearly 70% of the elevation in [Na+]i caused by 200 μM of L-glutamate applied to uninjured mouse astrocytes (Chatton et al., 2001). However, it is important to emphasize that although we found that glutamate uptake provides a main component of injury-induced increases in [Na+]i, not all the increased sodium after injury was due to sodium-dependent glutamate uptake. An additional likely source of sodium may be the AMPA receptor. Previous work by Goforth et al. (2004) suggests that mechanical strain injury to neurons potentates agonist-stimulated AMPA current by producing a lasting reduction of AMPA receptor desensitization. Additionally, voltage-gated sodium channels (MacVicar, 1984) or other sodium transporters, such as Na+-K+-ATPase (Hertz et al., 1998), may be involved, but have not been evaluated after mechanical injury. Interestingly, the magnitude and duration of elevated [Na+]i was similar between mild mechanical strain (5.5-mm) injury and low glutamate (100 μM) exposure, as well as between severe mechanical strain (7.5 mm) and high glutamate (1 mM) exposure. Yet, the degree of cell death in the mechanical injuries was consistently 2-fold greater than the corresponding glutamate exposure conditions. In this study, cell death measured by PrI uptake may have been greater in the mechanical injury conditions due to physical perturbations of cell membranes or other mechanical disruption of cell components that may trigger cell death pathway not initiated by elevated [Na+]i.
A question arises in our neuron-free cultures as to the source of glutamate for the proposed sodium-dependent glutamate uptake producing the elevated [Na+]i. Strain-injured astrocytes release both glutamate and ATP (Ahmed et al., 2000; Neary et al., 2003), although the mechanism of release after injury has not been clearly established. In uninjured astrocytes, ligands such as ATP, prostaglandins, and bradykinin induce calcium-dependent release of glutamate (Jeftinija et al., 1996; Parpura and Haydon, 2000; Jeremic et al., 2001). Calcium-independent release of glutamate by uninjured astrocytes can result from reversal of glutamate uptake (Nicholls and Attwell, 1990) opening of volume-sensitive anion channels (Longuemare et al., 1999) ATP activation of P2X7 (P2Z) receptors (Duan et al., 2003), and from release by unopposed gap junction hemichannels (Ye et al., 2003). Thus after mechanical strain injury in astrocyte cultures, glutamate could come from multiple sources.
Inhibition of the sodium-dependent calcium entry by the NCX (reversed mode) with KB-R7943 diminished, but did not completely abolish, injury-induced increases in [Ca2+]i and subsequent astrocyte cell death. Our data indicate that mild (5.5-mm) mechanical strain injury increased [Na+]i about 3-fold (to 30 mM) and that KB-R7943 did not significantly reduce injury-induced increased [Ca2+]i. Moderate and severe mechanical injury, accompanied by larger 4- and 5- fold increases in [Na+]i respectively, activate sodium-dependent calcium influx in a graded and time-dependent fashion, based on the reduction in [Ca2+]i seen with KB-R7943. This finding suggests that a portion of the deleterious rise in [Ca2+]i after injury can be accounted for by calcium influx from the NCX, which likely combines with other injury-induced increases in [Ca2+]i, such as voltage-gated calcium channel influx (MacVicar, 1984), activation of metabotropic glutamate receptors (Floyd et al., 2004), and release from intracellular stores (Rzigalinski et al., 1998; Floyd et al., 2001). Similar injury-dependent sodium-induced calcium influx has been documented in neurons (Czyz and Kiedrowski, 2002). Other investigators have shown that NCX reversed mode inhibition with KB-R7943 is protective after spinal cord injury (Li et al., 2000; Tomes and Agrawal, 2002); oxygen and glucose deprivation (MacGregor et al., 2003); in ischemic injury in brain (Pilitsis et al., 2001) and hippocampal organotypic brain slices (Schroder et al., 1999; Breder et al., 2000). An exception is that KB-R7943 was not protective when astrocyte [Na+]i was elevated by a mixture of veratridine (Na+ channel opener) and ouabain (Na+/K+ ATPase inhibitor; Takahashi et al., 2000). It is also possible that KB-R7943 could be acting on other modes of calcium entry, such as store-operated calcium entry (Arakawa et al., 2000) or nicotinic acetylcholine receptors (Pintado et al., 2000). Our pharmacological data and most previous reports suggest that blocking sodium-dependent calcium influx of NCX (reversal) may be effective in reducing cell injury. Importantly, blocking only the reversed mode of the NCX would allow calcium extrusion under conditions favoring the forward direction such as high [Ca2+]i and lower [Na+]i.
The modeled driving force for NCX reversal after mechanical strain injury (Table 1) was generally consistent with the pharmacological data indicating that with high magnitude strain injury, sodium-dependent calcium influx (reversal) persisted throughout the period of measurement. In contrast, after mild or moderate strain injury, the values for the ENCX predict a return to a calcium extrusion mode (forward) by 10 min after injury. There are a few caveats about predicting NCX influx or efflux using calcium and sodium values obtained from fluorescent imaging. It is important to emphasize that in our use of the model for driving force of the NCX, we incorporated only measured changes in intracellular sodium and calcium and did not incorporate changes in membrane potential (i.e., used −85 mV as a constant). Thus, the values in Table 1 are intended to predict trends over time, and not absolute values. Accordingly, we interpreted our reversal potential calculations in conjunction with the pharmacological data, which combine to suggest that severe injury to astrocytes increases [Na+]i to levels that cause persistent reversal of the NCX. Additionally, our calculations assume that the coupling ratio of the NCX in astrocytes is 3 sodium ions: 1 calcium ion as has been previously reported (Blaustein and Lederer, 1999), but a 4:1 coupling ratio may also occur (Dong et al., 2002). Changing the coupling ratio to 4:1 would shift the calculated reversal potential only slightly such that no reversal would be predicted after mild strain injury. The predictions for reversal of the NCX after moderate and severe strain injury would still indicate a brief and prolonged reversal, respectively (data not shown). Furthermore, we evaluated ionic changes in the entire soma of the astrocyte without regard for microdomains. Recent reports suggest that in astrocytes, the NCX may be physically located primarily in a microdomain composed of the endoplasmic reticulum and the plasma membrane. Moreover, the plasma membrane and endoplasmic reticulum may act as a functional unit for the regulation and sequestration of [Ca2+]i with the remaining cytosol having a substantially lower [Ca2+]i than the intervening junctional space between the plasma membrane and endoplasmic reticulum (Blaustein et al., 2002). Thus, our total cell measurements of [Ca2+]i and [Na+]i using cytoplasmic fluorescent indicators may largely underestimate concentrations in the microdomains. Lastly, our experiments were conducted in pure astrocyte cultures; however, culturing astrocytes without neurons may alter the expression of ion channels and exchangers (Eng et al., 1997).
What is the proposed relationship between elevations in intracellular sodium, calcium, and cell death in injured astrocytes? We found that both mechanical strain injury and excitotoxic glutamate application produced significant elevations in intracellular sodium, and that sodium-dependent glutamate uptake is a main component. We also found that with severe injury (i.e., 7.5-mm strain or 1 mM glutamate), intracellular sodium reached levels sufficient to reverse the NCX, contributing to increased [Ca2+]i after injury. However, when the cell death produced by the two modes of injury are compared, our data show that severe mechanical injury (7.5 mm) produces about double the amount of astrocyte death as that observed with 1 mM glutamate. Also, the mild mechanical injury (5.5 mm) produces about 4-fold greater astrocyte death than does 100 mM glutamate. Measuring [Ca2+]i indicated that severe mechanical injury (7.5 mm) produced a maximum [Ca2+]i of ≈1200 nM while the 1 mM glutamate produces a maximum [Ca2+]i of ≈900 nM. Although these differences are not the 2-fold values we observed in cell death between the injury modes, the mechanical injury produces a much longer sustained rise in [Ca2+]i (i.e., >50 min with 7.5-mm strain vs. ≈20 min with 1 mM glutamate). Similarly, mild strain (5.5 mm) and 100 μM glutamate both produce similar peak [Ca2+]i (≈400nm), but strain injury produced a much longer duration of elevated [Ca2+]i. These data suggest that the duration of elevated calcium may be a better predictor of cell death than the maximum elevation and that extended duration may account for differences in observed cell death between the injury models. We speculate that with a longer duration of elevated [Ca2+]i, cellular calcium buffering may be overwhelmed. In support of this hypothesis, other work has shown that ATP-driven calcium sequestration or extrusion mechanisms are damaged by mechanical strain injury in astrocytes (Rzigalinski et al., 1998; Chen et al., 2004) and neurons (Weber et al., 2001).
In summary, we found that mechanical injury increases intracellular Na+, largely due to sodium-dependent glutamate uptake. Increased [Na+]i with severe injury also activated sodium-dependent calcium influx (reverse mode) by the NCX further contributing to injury-induced elevations in [Ca2+]i. Reverse mode (calcium entry) NCX inhibition with KB-R7943 reduced injury-induced increases in [Ca2+]i and was astrocyte protective. The reversal potentials of the NCX calculated from measured values of intracellular sodium and calcium after injury are consistent with the pharamacological data and support the conclusion that sodium-dependent calcium entry may occur briefly at moderate injuries, and persist after severe injury. These findings indicate that in mechanically injured astrocytes, increased intracellular sodium is likely a critical component in the injury cascade, perhaps by increasing the duration of injury-induced elevations in intracellular calcium, and therefore may be a therapeutic target for reduction of lasting deficits after traumatic brain injury.
Acknowledgments
Grant sponsor: National Institutes of Health (NIH); Grant number: NS 45136; Grant number: NS 29995; Grant number: NS 40489; Grant number: NS 43085; Grant sponsor: UCLA Brain Injury Research Center.
References
- Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74:1951–1960. [PubMed] [Google Scholar]
- Amruthesh SC, Boerschel MF, McKinney JS, Willoughby KA, Ellis EF. Metabolism of arachidonic acid to epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and prostaglandins in cultured rat hippocampal astrocytes. J Neurochem. 1993;61:150–159. doi: 10.1111/j.1471-4159.1993.tb03550.x. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Arakawa N, Sakaue M, Yokoyama I, Hashimoto H, Koyama Y, Baba A, Matsuda T. KB-R7943 inhibits store-operated Ca2+ entry in cultured neurons and astrocytes. Biochem Biophys Res Commun. 2000;279:354–357. doi: 10.1006/bbrc.2000.3968. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol. 1999;77:699–706. [PubMed] [Google Scholar]
- Aschner M, Kimelberg HK. The use of astrocytes in culture as model systems for evaluating neurotoxic-induced-injury. Neurotoxicology. 1991;12:505–517. [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron. 1990;5:527–544. doi: 10.1016/0896-6273(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann NY Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Bondarenko A, Chesler M. Calcium dependence of rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001a;34:143–149. doi: 10.1002/glia.1049. [DOI] [PubMed] [Google Scholar]
- Bondarenko A, Chesler M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001b;34:134–142. doi: 10.1002/glia.1048. [DOI] [PubMed] [Google Scholar]
- Breder J, Sabelhaus CF, Opitz T, Reymann KG, Schroder UH. Inhibition of different pathways influencing Na+ homeostasis protects organotypic hippocampal slice cultures from hypoxic/hypoglycemic injury. Neuropharmacology. 2000;39:1779–1787. doi: 10.1016/s0028-3908(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Bullock MR, Lyeth BG, Muizelaar JP. Current status of neuroprotection trials for traumatic brain injury: lessons from animal models and clinical studies. Neurosurgery. 1999;45:207–217. doi: 10.1097/00006123-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Shimamoto K, Magistretti PJ. Effects of glial glutamate transporter inhibitors on intracellular Na+ in mouse astrocytes. Brain Res. 2001;893:46–52. doi: 10.1016/s0006-8993(00)03286-8. [DOI] [PubMed] [Google Scholar]
- Chen T, Willoughby KA, Ellis EF. Group I metabotropic receptor antagonism blocks depletion of calcium stores and reduces potentiated capacitative calcium entry in strain-injured neurons and astrocytes. J Neurotrauma. 2004;21:271–281. doi: 10.1089/089771504322972068. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Dell’Albani P, Corsaro M, Barresi V, Giuffrida Stella AM. AMPA-selective glutamate receptor subunits in astroglial cultures. J Neurosci Res. 1993;36:344–356. doi: 10.1002/jnr.490360312. [DOI] [PubMed] [Google Scholar]
- Czyz A, Kiedrowski L. In depolarized and glucose-deprived neurons, Na+ influx reverses plasmalemmal K+-dependent and K+-independent Na+/Ca2+ exchangers and contributes to NMDA excitotoxicity. J Neurochem. 2002;83:1321–1328. doi: 10.1046/j.1471-4159.2002.01227.x. [DOI] [PubMed] [Google Scholar]
- Dong H, Dunn J, Lytton J. Electrophysiological studies of the cloned rat cardiac NCX1.1 in transfected HEK cells: a focus on the stoichiometry. Ann NY Acad Sci. 2002;976:159–165. doi: 10.1111/j.1749-6632.2002.tb04737.x. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma. 1995;12:325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- Eng DL, Lee YL, Lal PG. Expression of glutamate uptake transporters after dibutyryl cyclic AMP differentiation and traumatic injury in cultured astrocytes. Brain Res. 1997;778:215–221. doi: 10.1016/s0006-8993(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Fern R, Ransom BR. Ischemic injury of optic nerve axons: the nuts and bolts. Clin Neurosci. 1997;4:246–250. [PubMed] [Google Scholar]
- Fineman I, Hovda DA, Smith M, Yoshino A, Becker DP. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Rzigalinski BA, Weber JT, Sitterding HA, Willoughby KA, Ellis EF. Traumatic injury of cultured astrocytes alters inositol (1, 4,5)-trisphosphate-mediated signaling. Glia. 2001;33:12–23. doi: 10.1002/1098-1136(20010101)33:1<12::aid-glia1002>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Rzigalinski BA, Sitterding HA, Willoughby KA, Ellis EF. Antagonism of group I metabotropic glutamate receptors and PLC attenuates increases in inositol trisphosphate and reduces reactive gliosis in strain-injured astrocytes. J Neurotrauma. 2004;21:205–216. doi: 10.1089/089771504322778668. [DOI] [PubMed] [Google Scholar]
- Geddes DM, Cargill RS, II, LaPlaca MC. Mechanical stretch to neurons results in a strain rate and magnitude-dependent increase in plasma membrane permeability. J Neurotrauma. 2003;20:1039–1049. doi: 10.1089/089771503770195885. [DOI] [PubMed] [Google Scholar]
- Goforth PB, Ellis EF, Satin LS. Mechanical injury modulates AMPA receptor kinetics via an NMDA receptor-dependent pathway. J Neurotrauma. 2004;21:719–732. doi: 10.1089/0897715041269704. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman WF, Yarowsky PJ, Juhaszova M, Krueger BK, Blaustein MP. Sodium/calcium exchange in rat cortical astrocytes. J Neurosci. 1994;14:5834–5843. doi: 10.1523/JNEUROSCI.14-10-05834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hansson E, Blomstrand F, Khatibi S, Olsson T, Ronnback L. Glutamate induced astroglial swelling—methods and mechanisms. Acta Neurochir Suppl (Wien) 1997;70:148–151. doi: 10.1007/978-3-7091-6837-0_45. [DOI] [PubMed] [Google Scholar]
- Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP. Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp Neurol. 1998;154:241–258. doi: 10.1006/exnr.1998.6929. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Lai JC. Functional studies in cultured astrocytes. Methods. 1998;16:293–310. doi: 10.1006/meth.1998.0686. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Becker DP, Katayama Y. Secondary injury and acidosis. J Neurotrauma. 1992;9(suppl 1):S47–60. [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Iwata A, Stys PK, Wolf JA, Chen XH, Taylor AG, Meaney DF, Smith DH. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci. 2004;24:4605–4613. doi: 10.1523/JNEUROSCI.0515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV, Stefanovic G, Liu F. Neuroligand-evoked calcium-dependent release of excitatory amino acids from cultured astrocytes. J Neurochem. 1996;66:676–684. doi: 10.1046/j.1471-4159.1996.66020676.x. [DOI] [PubMed] [Google Scholar]
- Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Maeda T, Koshinaga M, Kawamata T, Tsubokawa T. Role of excitatory amino acid-mediated ionic fluxes in traumatic brain injury. Brain Pathol. 1995;5:427–435. doi: 10.1111/j.1750-3639.1995.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Rutledge E, Goderie S, Charniga C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab. 1995;15:409–416. doi: 10.1038/jcbfm.1995.51. [DOI] [PubMed] [Google Scholar]
- Lamb RG, Harper CC, McKinney JS, Rzigalinski BA, Ellis EF. Alterations in phosphatidylcholine metabolism of stretch-injured cultured rat astrocytes. J Neurochem. 1997;68:1904–1910. doi: 10.1046/j.1471-4159.1997.68051904.x. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Lee VM, Thibault LE. An in vitro model of traumatic neuronal injury: loading rate-dependent changes in acute cytosolic calcium and lactate dehydrogenase release. J Neurotrauma. 1997;14:355–368. doi: 10.1089/neu.1997.14.355. [DOI] [PubMed] [Google Scholar]
- Li S, Jiang Q, Stys PK. Important role of reverse Na+-Ca2+ exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84:1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res Mol Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Longuemare MC, Rose CR, Farrell K, Ransom BR, Waxman SG, Swanson RA. K+-induced reversal of astrocyte glutamate uptake is limited by compensatory changes in intracellular Na+ Neuroscience. 1999;93:285–292. doi: 10.1016/s0306-4522(99)00152-9. [DOI] [PubMed] [Google Scholar]
- MacGregor DG, Avshalumov MV, Rice ME. Brain edema induced by in vitro ischemia: causal factors and neuroprotection. J Neurochem. 2003;85:1402–1411. doi: 10.1046/j.1471-4159.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- MacVicar BA. Voltage-dependent calcium channels in glial cells. Science. 1984;226:1345–1347. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- Margulies SS, Thibault LE, Gennarelli TA. Physical model simulations of brain injury in the primate. J Biomech. 1990;23:823–836. doi: 10.1016/0021-9290(90)90029-3. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traystman RJ. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- Meaney DF, Smith DH, Shreiber DI, Bain AC, Miller RT, Ross DT, Gennarelli TA. Biomechanical analysis of experimental diffuse axonal injury. J Neurotrauma. 1995;12:689–694. doi: 10.1089/neu.1995.12.689. [DOI] [PubMed] [Google Scholar]
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extra-cellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2256. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb JK, Pike BR, Zhao X, Banik NL, Hayes RL. Altered calpastatin protein levels following traumatic brain injury in rat. J Neurotrauma. 1999;16:1–11. doi: 10.1089/neu.1999.16.1. [DOI] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J. Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Prog Neurobiol. 1997;51:39–87. doi: 10.1016/s0301-0082(96)00049-4. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike BR, Zhao X, Newcomb JK, Posmantur RM, Wang KK, Hayes RL. Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. NeuroReport. 1998;9:2437–2442. doi: 10.1097/00001756-199808030-00002. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Diaz FG, O’Regan MH, Phillis JW. Inhibition of Na+/Ca2+ exchange by KB-R7943, a novel selective antagonist, attenuates phosphoethanolamine and free fatty acid efflux in rat cerebral cortex during ischemia-reperfusion injury. Brain Res. 2001;916:192–198. doi: 10.1016/s0006-8993(01)02896-7. [DOI] [PubMed] [Google Scholar]
- Pintado AJ, Herrero CJ, Garcia AG, Montiel C. The novel Na+/Ca2+ exchange inhibitor KB-R7943 also blocks native and expressed neuronal nicotinic receptors. Br J Pharmacol. 2000;130:1893–1902. doi: 10.1038/sj.bjp.0703519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Buki A, Koiziumi H, Stone J, Okonkwo DO. Initiating mechanisms involved in the pathobiology of traumatically induced axonal injury and interventions targeted at blunting their progression. Acta Neurochir Suppl (Wien) 1999;73:15–20. doi: 10.1007/978-3-7091-6391-7_3. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Mechanisms of H+ and Na+ changes induced by glutamate, kainate, and D-aspartate in rat hippocampal astrocytes. J Neurosci. 1996;16:5393–5404. doi: 10.1523/JNEUROSCI.16-17-05393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Waxman SG, Ransom BR. Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci. 1998;18:3554–3562. doi: 10.1523/JNEUROSCI.18-10-03554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103:162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rzigalinski BA, Weber JT, Willoughby KA, Ellis EF. Intracellular free calcium dynamics in stretch-injured astrocytes. J Neurochem. 1998;70:2377–85. doi: 10.1046/j.1471-4159.1998.70062377.x. [DOI] [PubMed] [Google Scholar]
- Scemes E. Components of astrocytic intercellular calcium signaling. Mol Neurobiol. 2000;22:167–179. doi: 10.1385/MN:22:1-3:167. [DOI] [PubMed] [Google Scholar]
- Schroder UH, Breder J, Sabelhaus CF, Reymann KG. The novel Na+/Ca2+ exchange inhibitor KB-R7943 protects CA1 neurons in rat hippocampal slices against hypoxic/hypoglycemic injury. Neuropharmacology. 1999;38:319–321. doi: 10.1016/s0028-3908(98)00198-1. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Shelton MK, McCarthy KD. Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ. Glia. 1999;26:1–11. doi: 10.1002/(sici)1098-1136(199903)26:1<1::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Somjen G. Electrophysiology of mammalian glia cells in situ. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995. pp. 319–331. [Google Scholar]
- Takahashi S, Shibata M, Gotoh J, Fukuuchi Y. Astroglial cell death induced by excessive influx of sodium ions. Eur J Pharmacol. 2000;408:127–135. doi: 10.1016/s0014-2999(00)00790-1. [DOI] [PubMed] [Google Scholar]
- Tomes DJ, Agrawal SK. Role of Na+-Ca2+ exchanger after traumatic or hypoxic/ischemic injury to spinal cord white matter. Spine J. 2002;2:35–40. doi: 10.1016/s1529-9430(01)00151-6. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Shimamoto K, Schousboe A. Comparison of effects of DL-threo-beta-benzyloxyaspartate (DL-TBOA) and L-transpyrrolidine-2, 4-dicarboxylate (t-2,4-PDC) on uptake and release of [3h]D-aspartate in astrocytes and glutamatergic neurons. Neurochem Res. 2001;26:661–666. doi: 10.1023/a:1010939304104. [DOI] [PubMed] [Google Scholar]
- Weber JT, Rzigalinski BA, Willoughby KA, Moore SF, Ellis EF. Alterations in calcium-mediated signal transduction after traumatic injury of cortical neurons. Cell Calcium. 1999;26:289–299. doi: 10.1054/ceca.1999.0082. [DOI] [PubMed] [Google Scholar]
- Weber JT, Rzigalinski BA, Ellis EF. Traumatic injury of cortical neurons causes changes in intracellular calcium stores and capacitative calcium influx. J Biol Chem. 2001;276:1800–1807. doi: 10.1074/jbc.M009209200. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. Role of calcium in central nervous system injuries. J Neurotrauma. 1992;9(suppl 1):S9–25. [PubMed] [Google Scholar]
- Zhao X, Ahram A, Berman RF, Muizelaar JP, Lyeth BG. Early loss of astrocytes after experimental traumatic brain injury. Glia. 2003;44:140–152. doi: 10.1002/glia.10283. [DOI] [PubMed] [Google Scholar]