Abstract
Lewis base catalyzed bromo- and iodolactonization reactions have been developed and the effects of catalyst structure on rate and cyclization selectivity have been systematically explored. The effects of substrate structure on halolactonization reactions and the interaction of those effects with the effects of catalyst structure have been investigated, leading to synthetically useful improvements in cyclization selectivity. The knowledge acquired was applied to the development of Lewis base catalyzed bromo- and iodocycloetherification reactions. The ability of some of the surveyed catalysts to influence the cyclization selectivity of halolactonization reactions demonstrates their presence in the transition structure of the product-determining cyclization step. This observation implies that chiral derivatives of these catalysts have the potential to provide enantioenriched products regardless of the rates or mechanisms of halonium ion racemization.
Keywords: halocyclofunctionalization, halogenation
Electrophilic halocyclizations of olefins, in which electrophilic halonium ions are generated from olefins and opened intramolecularly by nucleophilic functional groups (Fig. 1), are versatile synthetic transformations with proven applications to the synthesis of biologically relevant molecules (1–6). The development of catalytic enantioselective halocyclization methods is a topic of increasing interest in synthetic organic chemistry, one that presents unique challenges and opportunities for various paradigms of catalysis in addition to the obvious importance and utility of this transformation. To date, only a few notable successes have been reported; these include the use of chiral Ti-salen complexes in iodoetherification (7) and cinchonidinium phase-transfer catalysts in iodolactonization (8). Recently, modified Cinchona alkaloids have been successfully employed in catalytic enantioselective chlorolactonization (9), as well as a catalytic enantioselective bromolactonization of 1,3-eneynes via conjugate opening of achiral bromonium ions (10–15*).
Fig. 1.
General scheme of halocyclization reactions.
Careful mechanistic studies by Brown and coworkers and from these laboratories identified a serious obstacle to the development of catalytic enantioselective iodination and bromination methods, namely the propensity of iodonium and bromonium ions (but not chloronium ions) to undergo degenerate halogen exchange with olefins (Fig. 2) (16–19). This process racemizes the halonium ions at rates that can compete with nucleophilic capture. Chiral Lewis base catalysts have the potential to prevent this or other racemization processes, if they remain bound to the halonium ion until the newly created stereocenters are irreversibly set, thus maintaining a chiral environment regardless of exchange. Lewis base catalysis of halogenation has been reported in several different contexts (20–23), as have enantioselective halocyclization reactions promoted by stoichiometric amounts of chiral Lewis bases (13–15), but the paucity of catalytic, enantioselective Lewis base catalyzed halogenations suggests that some of the aforementioned systems do not preserve the stereochemical integrity of intermediates as described here. Given the significant effort required for the preparation of new chiral Lewis bases, and the structural and functional diversity of the reported achiral Lewis base catalysts, a systematic means of inferring the properties of chiral Lewis base catalysts from those of readily available achiral analogs is highly desirable.
Fig. 2.
The racemization of halonium ions by degenerate exchange; nondegeneracy induced by the presence of a chiral Lewis base.
Halolactonization and cycloetherification reactions can produce either of two constitutional isomers, arising from cyclization in an exo or endo fashion (24). The ratio of the two isomers is influenced largely by the substrate, the identity of the halogen, and the choice of reaction conditions. Under conditions wherein the halonium ion is undergoing rapid exchange, the product-determining step must also be the stereochemistry-determining step. Therefore, the ratio of constitutional isomers produced in the presence of an achiral Lewis base catalyst serves as an indicator of the presence of the catalyst in the stereo-determining transition structure. This knowledge would allow the search for an enantioselective catalyst to focus on classes of compound whose presence in and capacity to influence the relevant transition state structure has been demonstrated.
In this paper we report a systematic investigation into the influence of Lewis base catalysts on the rate and constitutional site selectivity in bromo- and iodo- lactonization reactions. The changes in the ratio of 6-endo to 5-exo cyclization products induced by the presence of a wide range of achiral Lewis base catalysts provides evidence for the presence of the Lewis base in the site-selectivity-determining step, whereas in situ IR monitoring provides comparative rate data. These findings are then applied to the development of Lewis base catalysis of bromo- and iodo-etherification reactions.
Results
The program to evaluate the feasibility of Lewis base catalysis of halofunctionalization of isolated double bonds would involve electrophilic bromine and iodine sources (halosuccinimides) in conjunction with unsaturated carboxylic acids and alcohols. For the initial survey of Lewis bases, 5-phenyl-4-pentenoic acid (1a) was chosen as the test substrate and a standard experimental procedure was adopted. All reactions were run in dichloromethane at 0.15 M in substrate with 1.2 equivalent (equiv) of electrophile and 0.05 equiv of Lewis base. The temperature was adjusted such that the background (uncatalyzed) reaction was negligible. A broad selection of Lewis bases was evaluated for kinetic competence. The optimal catalyst was then employed in a brief survey of olefinic substrates with varying double bond geometries and substituents.
Bromolactonization.
The influence of Lewis bases on the rate and cyclization selectivity in the bromolactonization of 1a with N-bromosuccinimide (NBS) was evaluated first. Reaction progress was measured by the disappearance of the IR band at 967 cm-1. The yields and ratios of cyclization products 2aa and 3aa were assayed by 1H NMR integration against an internal standard.
The results from the Lewis base survey are compiled in Table 1 and are organized by donor heteroatom. Under the standard reaction protocol, the mixture was homogenous and the rate of the background reaction in the absence of catalyst was insignificant (entry 1). The oxygen donors  , n-Bu3P = O, and (Me2N)3P = O (entries 2–4) were only marginally faster than the background rate and were still incomplete after 3 h. On the other hand DMSO (entry 5), which could function as either an oxygen or sulfur donor, led to complete reaction after only 1 h. Gratifyingly, very high rate acceleration was observed with many Lewis bases bearing sulfur, selenium, and phosphorus donor atoms. The thiono containing bases (thiourea, phosphine sulfides, or a thiophosphoramide, entries 6–10) were all indistinguishably rapid (t1/2 < 35 sec) and provided good yields of the cyclization products. Interestingly, other divalent sulfur donors behaved quite differently. Whereas tetrahydrothiophene (entry 11) was equipotent with the thiono bases, reaction using dimethyl sulfide (entry 12) was somewhat slower and diphenyl disulfide (entry 13) was completely ineffective. All of the selenium- (entries 14–16) and phosphorus- (entries 17 and 18) based donors were powerful catalysts and within the resolution of this measurement, indistinguishable. In a final control experiment, bromine, a common impurity in NBS, was shown to be an effective catalyst (entry 19), most likely due to formation of HBr by rapid uncatalyzed bromolactonization.
, n-Bu3P = O, and (Me2N)3P = O (entries 2–4) were only marginally faster than the background rate and were still incomplete after 3 h. On the other hand DMSO (entry 5), which could function as either an oxygen or sulfur donor, led to complete reaction after only 1 h. Gratifyingly, very high rate acceleration was observed with many Lewis bases bearing sulfur, selenium, and phosphorus donor atoms. The thiono containing bases (thiourea, phosphine sulfides, or a thiophosphoramide, entries 6–10) were all indistinguishably rapid (t1/2 < 35 sec) and provided good yields of the cyclization products. Interestingly, other divalent sulfur donors behaved quite differently. Whereas tetrahydrothiophene (entry 11) was equipotent with the thiono bases, reaction using dimethyl sulfide (entry 12) was somewhat slower and diphenyl disulfide (entry 13) was completely ineffective. All of the selenium- (entries 14–16) and phosphorus- (entries 17 and 18) based donors were powerful catalysts and within the resolution of this measurement, indistinguishable. In a final control experiment, bromine, a common impurity in NBS, was shown to be an effective catalyst (entry 19), most likely due to formation of HBr by rapid uncatalyzed bromolactonization.
Table 1.
Catalyst survey for Lewis base catalyzed bromolactonization
| Entry | Catalyst | t1/2 (min)* | Reaction time (min)† | Yield (%)‡ | 2aa∶3aa§ |
| 1 | None | > 180 | 180 | 13 | 25∶1 |
| 2 |  |
> 180 | 180 | 36 | 23∶1 |
| 3 | n-Bu3P = O | > 180 | 180 | 47 | 51∶1 |
| 4 | (Me2N)3P = O | > 180 | 180 | 15 | 50∶1 |
| 5 | Me2S = O | 25 | 60 | 93 | 23∶1 |
| 6 | (Me2N)2C = S | < 0.5 | 8 | 71 | 7.3∶1 |
| 7 |  |
< 0.5 | 8 | 82 | 91∶1 |
| 8 | n-Bu3P = S | < 0.5 | 8 | 89 | 75∶1 |
| 9 | Cy3P = S | < 0.5 | 8 | 78 | 25∶1 |
| 10 | (Me2N)3P = S | < 0.5 | 8 | 87 | 3.4∶1 |
| 11 | (CH2)4S | < 0.5 | 8 | 89 | 27∶1 |
| 12 | Me2S | 6 | 20 | 94 | 19∶1 |
| 13 | (PhS)2 | > 180 | 180 | 8 | N/D |
| 14 | n-Bu3P = Se | < 0.5 | 8 | 78 | 85∶1 |
| 15 | (Me2N)3P = Se | < 0.5 | 8 | 88 | 8.1∶1 |
| 16 | (PhSe)2 | 0.5–1 | 8 | 84 | 94∶1 |
| 17 | n-Bu3P | < 0.5 | 5 | 75 | 38∶1 |
| 18 | (Me2N)3P | < 0.5 | 8 | 86 | 6.7∶1 |
| 19 | Br2 | < 0.5 | 8 | 64 | 400∶1 |
*Determined by React-IR monitoring, reactions performed on 0.2 mmol of 1a, in 1.5 mL of CH2Cl2
†Time elapsed before quenching
‡Determined by integration of 1H NMR signals for H-6 against  internal standard
internal standard
§Determined by integration of 1H NMR signals for H-6
In addition to dramatic differences in the rates of the catalyzed reactions, the steric course of the reaction was also significantly influenced by the action of the different catalysts. Although substrate 1a is intrinsically biased toward endocyclic closure (entry 1), the endo/exo selectivity in the catalyzed reactions ranged from 3.4∶1–94∶1. Highly reactive catalysts that also lead to increases in the already high endo/exo selectivity included  (entry 7), n-Bu3P = S (entry 8), n-Bu3P = Se (entry 14), (PhSe)2 (entry 16), and n-Bu3P (entry 17). Of the Lewis base catalysts surveyed, the highest endo selectivity was observed in the presence of
(entry 7), n-Bu3P = S (entry 8), n-Bu3P = Se (entry 14), (PhSe)2 (entry 16), and n-Bu3P (entry 17). Of the Lewis base catalysts surveyed, the highest endo selectivity was observed in the presence of  and (PhSe)2, but the highest overall endo selectivity was observed in the control experiment with a catalytic quantity of Br2 (entry 19). Substantial reductions in the ratio of 2aa to 3aa were observed in the presence of (Me2N)2C = S, (Me2N)3P = S, (Me2N)3P = Se, and (Me2N)3P, and (entries 6, 10, 15, 18, resp.). Among the thiono donors an apparent correlation of increasing steric bulk with decreasing endo/exo selectivity was noted (entries 6–10).
and (PhSe)2, but the highest overall endo selectivity was observed in the control experiment with a catalytic quantity of Br2 (entry 19). Substantial reductions in the ratio of 2aa to 3aa were observed in the presence of (Me2N)2C = S, (Me2N)3P = S, (Me2N)3P = Se, and (Me2N)3P, and (entries 6, 10, 15, 18, resp.). Among the thiono donors an apparent correlation of increasing steric bulk with decreasing endo/exo selectivity was noted (entries 6–10).
To evaluate the preparative utility of this bromolactonization, the reaction of test substrate 1a was run on a 1.0 mmol scale and the cyclization products were isolated in 81% yield and a 90∶1 ratio of isomers favoring 2aa (Fig. 3). Next, unsaturated acids 1b and 1c, representing the effects of olefin geometry and conjugation, were examined. Under the optimized reaction conditions, both 1b and 1c afforded mixtures of constitutional isomers now weakly favoring exo-cyclization product 3. Interestingly, the use of (MeN)3P = S, which previously displayed the lowest endo selectivity (Table 1, entry 6), produced 3ba with high exo selectivity. In the cyclization of 1c, the exo selectivity increased with the use of (Me2N)3P = S, but only marginally.
Fig. 3.
Scope of Lewis base catalyzed bromolactonization.
Bromocycloetherification.
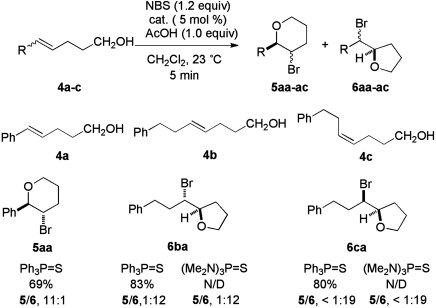
The preparative utility of the catalytic bromofunctionalization was next extended to include Lewis base catalyzed bromocycloetherification. Unsaturated alcohols 4a–c were chosen to demonstrate the effects of conjugation and alkene geometry. In situ FTIR monitoring of the cyclization of 4a under the previously optimized reaction conditions (5 mol% of  ), revealed that only ca. 50% of 4a was consumed before the reaction stalled. A previous report from these laboratories on selenocyclization revealed a similar trend for unsaturated acids and alcohols which could be ameliorated by the addition of a weak Brønsted acid. Accordingly, bromocycloetherification of 4a was carried out in the presence of 1.0 equiv of AcOH with the intention of producing a level of acidity similar to that present during bromolactonization. Gratifyingly, this simple remedy rescued the reactivity of 4a and allowed the reaction to proceed rapidly to completion in good yield and with good endo selectivity (Fig. 4).
), revealed that only ca. 50% of 4a was consumed before the reaction stalled. A previous report from these laboratories on selenocyclization revealed a similar trend for unsaturated acids and alcohols which could be ameliorated by the addition of a weak Brønsted acid. Accordingly, bromocycloetherification of 4a was carried out in the presence of 1.0 equiv of AcOH with the intention of producing a level of acidity similar to that present during bromolactonization. Gratifyingly, this simple remedy rescued the reactivity of 4a and allowed the reaction to proceed rapidly to completion in good yield and with good endo selectivity (Fig. 4).
Fig. 4.
Scope of Lewis base catalyzed bromocycloetherification.
Bromocycloetherification of 4b in the presence of  and AcOH afforded the product of exo cyclization, 6ba in high yield and with good selectivity. In the hope of obtaining even higher exo selectivity, this reaction was attempted in the presence of (Me2N)3P = S, however no alteration in the isomer ratio was observed. Similarly, the (Z)-alkenol 4c produced 6ca in good yield and with high exo selectivity.
and AcOH afforded the product of exo cyclization, 6ba in high yield and with good selectivity. In the hope of obtaining even higher exo selectivity, this reaction was attempted in the presence of (Me2N)3P = S, however no alteration in the isomer ratio was observed. Similarly, the (Z)-alkenol 4c produced 6ca in good yield and with high exo selectivity.
Iodolactonization.
Iodolactonization is an extensively studied cyclofunctionalization process and often provides different, sometimes complementary selectivity compared to bromolactonization (25), typically producing a greater proportion of 5-exo cyclization. Given the successful application of Lewis base catalysis to bromolactonization and the range of selectivities observed, catalysis of iodolactonization presented an attractive target. As before, 1a was selected as a representative substrate and was subjected to the action of N-iodosuccinimide (NIS) in the presence of a wide variety of Lewis bases (Table 2). As above, the reaction progress was monitored by in situ FTIR analysis and the yield and product ratios were obtained from 1H NMR spectra.
Table 2.
Catalyst survey for Lewis base catalyzed iodolactonization
| Entry | Catalyst | T1/2 (min)* | Reaction time (h)† | Yield (%)‡ |
 § §
|
| 1 | None | > 180 | 2 | 4 | 9.5∶1 |
| 2 |  |
> 180 | 3 | 10 | 20∶1 |
| 3 | n-Bu3P = O | > 180 | 3 | 14 | 16∶1 |
| 4 | (Me2N)3P = O | > 180 | 3 | 33 | 20∶1 |
| 5 | Me2SO | > 180 | 3 | 4 | N/D |
| 6 | (Me2N)2C = S | 4 | 1 | 91 | 4.7∶1 |
| 7 |  |
35 | 1 | 92 | 5.5∶1 |
| 8 | n-Bu3P = S | 7 | 1 | 72 | 6.1∶1 |
| 9 | Cy3P = S | 8 | 1 | 93 | 1.7∶1 |
| 10 | (Me2N)3P = S | 4 | 1 | 97 | 2.5∶1 |
| 11 | (CH2)4S | 10 | 1 | 97 | 5.8∶1 |
| 12 | Me2S | > 180 | 3 | 45 | 5.8∶1 |
| 13 | (PhS)2 | > 180 | 3 | 2 | N/D |
| 14 | n-Bu3P = Se | 9 | 1 | 77 | 8.5∶1 |
| 15 | (Me2N)3P = Se | 4 | 1 | 95 | 4.6∶1 |
| 16 | (PhSe)2 | 15 | 1 | 82 | 10∶1 |
| 17 | n-Bu3P | 52 | 2 | 94 | 5.1∶1 |
| 18 | (Me2N)3P | 40 | 2 | 53 | 1.4∶1 |
| 19 | I2 | > 180 | 3 | 10 | 24∶1 |
| 20 | TFA | > 180 | 3 | 0 | N/D |
*Determined by React-IR monitoring, all reactions run on 0.2 mmol of 1a
†Time elapsed before quenching
‡Determined by integration of 1H NMR signals for H-6 against PhMe6 internal standard
§Determined by integration of 1H NMR signals for H-6
Unsurprisingly, iodolactonization is much faster than bromolactonization such that suppressing the uncatalyzed reaction of 1a with NIS in dichloromethane required executing the experiments at -40 °C (Table 2, entry 1). Consequently, the solubility of NIS in dichloromethane, which is only modest at room temperature, was quite low.
The results from the survey of Lewis bases are compiled in Table 2 in the same order as was presented in Table 1. Here as well, the oxygen-based donors  , n-Bu3P = O, and (Me2N)3P = O, (entries 2–4)) provided at best only modest rate acceleration. However, the complete lack of reactivity of DMSO (entry 5) diverged markedly from its behavior in bromolactonization. As was the case in bromolactonization, the highest rates were observed in the presence of thiono- ((Me2N)2C = S, n-Bu3P = S, Cy3P = S, (Me2N)3P = S, entries 6, 8–10) and seleno-based donors (n-Bu3P = S and (Me2N)3P = Se (entries 14, 15), though
, n-Bu3P = O, and (Me2N)3P = O, (entries 2–4)) provided at best only modest rate acceleration. However, the complete lack of reactivity of DMSO (entry 5) diverged markedly from its behavior in bromolactonization. As was the case in bromolactonization, the highest rates were observed in the presence of thiono- ((Me2N)2C = S, n-Bu3P = S, Cy3P = S, (Me2N)3P = S, entries 6, 8–10) and seleno-based donors (n-Bu3P = S and (Me2N)3P = Se (entries 14, 15), though  was less effective (entry 7). Similarly, the divalent chalcogen donors tetrahydrothiophene (entry 11) and (PhSe)2 (entry 16) also gave rise to high reaction rates. On the other hand, dimethyl sulfide (entry 12) was ca. 20 times less reactive than tetrahydrothiophene, the pnictogens (Me2N)3P and n-Bu3P (entries 17, 18) were substantially less reactive, and (Ph2S)2 (entries 13) was completely ineffective. In contrast to bromolactonization, the addition of 5 mol% of I2 had a negligible impact on the rate of iodolactonization, although the endo/exo ratio increased (entry 19).
was less effective (entry 7). Similarly, the divalent chalcogen donors tetrahydrothiophene (entry 11) and (PhSe)2 (entry 16) also gave rise to high reaction rates. On the other hand, dimethyl sulfide (entry 12) was ca. 20 times less reactive than tetrahydrothiophene, the pnictogens (Me2N)3P and n-Bu3P (entries 17, 18) were substantially less reactive, and (Ph2S)2 (entries 13) was completely ineffective. In contrast to bromolactonization, the addition of 5 mol% of I2 had a negligible impact on the rate of iodolactonization, although the endo/exo ratio increased (entry 19).
The intrinsic preference for endocyclization of 1a persisted, but with attenuated magnitude ranging from 1.4∶1–20∶1 ratios for 5aa/6aa. In fact, only the oxygen-based donors (entries 2–4) afforded improved selectivities compared to background reaction. Moreover, substantial reductions in the endo/exo selectivity were observed in the presence of Cy3P = S, (Me2N)3P = S, and (Me2N)3P (entries 9, 10, 18). Diphenyl diselenide had no effect on the product ratio (entry 16), and the other Lewis bases tested reduced the exo selectivity modestly.
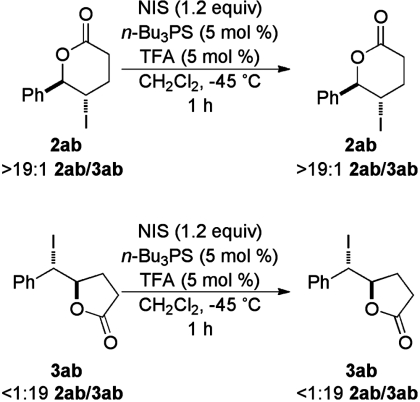
These results were both disappointing and also perplexing. In preliminary experiments, n-Bu3P = S displayed a greatly increased endo selectivity compared to the result shown in entry 8. After several unsuccessful attempts to reproduce the original, more promising result, it was hypothesized that some component of the original reaction mixture had been contaminated in some way. Among several potential contaminants examined, a trace amount of TFA, in combination with n-Bu3P = S resulted in the almost exclusive formation of 2ab (Fig. 5). To exclude the possibility that this outcome was the result of thermodynamic control of the product ratio, 2ab and 3ab, the latter produced under established conditions of thermodynamic control (26, 27), were submitted to the reaction conditions as single purified isomers (Fig. 6). Almost no isomerization was observed, thus demonstrating that the product ratio was the result of kinetically controlled selectivity.
Fig. 5.
Scope of Lewis base catalyzed iodolactonization.
Fig. 6.
Stability of isomeric iodolactones to iodolactonization conditions.
Substrates 1b and 1c were again chosen to explore the scope of this iodolactonization and to demonstrate the effects of olefin geometry and conjugation. Iodolactonization of 1b in the presence of (Me2N)3P = S afforded 3bb in good yield and exo selectivity (Fig. 5). The iodolactonization of 1c was attempted in the presence of  and Cy3P = S separately. As was seen previously, one of the least endo selective catalysts, Cy3P = S, provided slightly higher exo selectivity, allowing 3cb to be isolated in good yield.
and Cy3P = S separately. As was seen previously, one of the least endo selective catalysts, Cy3P = S, provided slightly higher exo selectivity, allowing 3cb to be isolated in good yield.
4. Iodocycloetherification.
To further explore the scope of Lewis base catalysis of iodocyclization, the insights gained from the development of iodolactonization were applied to the development of iodocycloetherification. As was done for bromocycloetherification, the conjugated (E)-alkenol 4a and the unconjugated (E)- and (Z)-alkenols 4b and 4c were chosen to represent the effects of conjugation and olefin geometry. The conditions that were previously optimized for the iodolactonization of 1a were applied to the iodocycloetherification of 4a (5 mol% of n-Bu3P = S, 5 mol% TFA, -45 °C), and afforded the product of endo cyclization, 5ab, in a good yield and high selectivity (Fig. 7). Aside from extending the reaction time, no further changes to the reaction conditions were required in this case. The aliphatic alkenols 4b and 4c were both cyclized in good yield and high 5-exo selectivity. No measurable effect on the endo/exo ratio was observed for the more exo selective catalyst (Me2N)3P = S.
Fig. 7.
Scope of Lewis base catalyzed iodocycloetherification.
Discussion
The development of Lewis base catalyzed bromo- and iodolactonization described above has led to insights into the effects of catalyst structure on the rate and selectivity of cyclization, highlighted the effects of substrate structure and the identity of the halogen on cyclization selectivity, and produced synthetically useful improvements in cyclization selectivity which will be discussed along with their implications for the development of enantioselective catalysts.
Rates of Iodolactonization.
The first readily apparent trend in the rates of catalyzed iodolactonization is that the donor atom has a significant influence as follows:  , (Table 2). The ordering of the chalcogen derivatives parallels the differences in softness between the isolated atoms (ionization potentials (IP): Se, 9.75 eV; S, 10.36 eV; P, 10.49 eV; O, 13.61 eV) as well as the differences in ionization potentials for the ((Me2N)3P = X chalcogenides which Bruno et. al. have estimated from the charge transfer band of their I2 complexes (IP (Me2N)3P = O - (Me2N)3P = S = 1.5 eV; (Me2N)3P = S - (Me2N)3P = Se = 0.18 eV) (28).
, (Table 2). The ordering of the chalcogen derivatives parallels the differences in softness between the isolated atoms (ionization potentials (IP): Se, 9.75 eV; S, 10.36 eV; P, 10.49 eV; O, 13.61 eV) as well as the differences in ionization potentials for the ((Me2N)3P = X chalcogenides which Bruno et. al. have estimated from the charge transfer band of their I2 complexes (IP (Me2N)3P = O - (Me2N)3P = S = 1.5 eV; (Me2N)3P = S - (Me2N)3P = Se = 0.18 eV) (28).
The trend in rate with variation in the substituents on the P(III) and P(V) donors also follows the order  . This order is the same as that of their enthalpies of I2 complexation (29).
. This order is the same as that of their enthalpies of I2 complexation (29).
Rates of Bromolactonization.
The most readily apparent trend in the rates of catalyzed bromolactonization parallels that of iodolactonization, namely: (Me2N)2C = S ≈ R3P = Se ≈ R3P = S ≈ R3P≫R3P = O, with any rate difference between the sulfur and selenium homologs and their parent phosphines obscured by the rates of the data acquisition (Table 1). Although this ordering is similar to the trend that was observed in iodolactonization, there are discernable differences in the relative reactivities of some Lewis bases. Diphenyldiselenide is slightly slower than the phosphine sulfide and selenide donors, where as in iodolactonization it was faster than Ph3PS. In iodolactonization the reactivity of (Me2N)3P = O is greater than n-Bu3P = O and  while in bromolactonization it is the least active of the three (Table 1, entries 16–18; Table 2, entries 15–17).
while in bromolactonization it is the least active of the three (Table 1, entries 16–18; Table 2, entries 15–17).
Substrate Effects on Selectivity.
The preference for 5-exo cyclization over 6-endo cyclization observed with substrates 1c and 4b–c, which lack a conjugating substituent on the alkene is typical for a variety of halocyclization reactions (2–4). Substrates 1a and 4a favor 6-endo cyclization as a result of the greater stability of positive charge localized on benzylic carbons. The greater proportion of 6-endo cyclization observed in bromolactonization and bromocycloetherification compared to their iodine counterparts has ample precedent and appears to be a general phenomenon, although a satisfactory explanation has yet to be proposed (25). The superposition of these factors frequently leads to modest selectivity in bromolactonizations, such as was observed in the case of 1c.
Alkene geometry can have a strong influence on site selectivity as well; the preference of 1b for 5-exo cyclization despite the directing effect of the phenyl group has been observed previously (30), and a less dramatic effect was seen in bromocycloetherification of 4c.
Catalyst Effects on Selectivity.
The least endo selective catalysts for 1a and most exo selective catalysts for 1b and 1c share two important structural features: (i) they possess two symmetry unique points of branching, either dimethylamino groups or cyclohexyl groups, and (ii) donate a nonbonding pair from an element softer than oxygen. Tetramethylthiourea and (Me2N)3P, as well as the latter's sulfide and selenide, are exo selective in both iodo- and bromolactonization. In contrast, Cy3P = S, which lacks electron donating dimethylamino groups, is less electron rich but still very sterically demanding, promotes exo iodolactonization but not exo bromolactonization of 1b and 1c. Such exceptional behavior of Cy3P = S may be a result of the differences in reaction conditions rather than differences in the halogen. The iodolactonizations were conducted at lower temperatures, which should entropically favor ligand association and under conditions of low NIS solubility, which should limit competitive binding of the catalyst to free NIS. These factors should allow somewhat weaker binding ligands to have noticeable effects on the outcome of the reaction.
Comparing the catalyst effects on bromo- and iodo- lactonization of 1c, which lacks a conjugating substituent, illuminates the roles played by the steric and electronic properties of the Lewis bases. In the bromolactonization of 1c the magnitude of the catalyst effect on cyclization selectivity is small (Fig. 3) although the reduction in endo/exo ratio using (Me2N)3P = S is consistent with the direction of the effect observed in the bromolactonization of 1a (Table 1). Similarly, the endo/exo ratio observed in the iodolactonization of 1c (Fig. 5) was only modestly reduced by the use of Cy3P = S as the catalyst, although the greater preference for exo cyclization is in the same direction as that observed in the iodolactonization of 1a. In contrast the endo/exo ratios in the bromolactonizations of 1a–b, which possess conjugating substituents, were both greatly reduced by the use of (Me2N)3P = S compared to  (Table 1, Fig. 3) despite the inversion of overall selectivity. This selectivity suggests that the effect of (Me2N)3P = S on the bromolactonizations of 1a–b predominately arises from modulation of the directing ability of the phenyl group, possibly by reducing the positive charge localized on the electrophilic carbons.
(Table 1, Fig. 3) despite the inversion of overall selectivity. This selectivity suggests that the effect of (Me2N)3P = S on the bromolactonizations of 1a–b predominately arises from modulation of the directing ability of the phenyl group, possibly by reducing the positive charge localized on the electrophilic carbons.
The stability of the isomers 2ab and 3ab to the reaction conditions under which 2ab was formed (n-Bu3P = S/TFA, 1∶1; 5 mol%, NIS, -40 °C, Fig. 7) demonstrates that the inclusion of a cocatalytic amount of TFA does not result in thermodynamic control of the endo/exo ratio. Therefore, because the ratio is under kinetic control, the same consideration applies to TFA as applies to Lewis bases, namely that to affect the endo/exo ratio the agent must be present in the product-determining transition state structure. The acidity of TFA naturally leads to the hypothesis that this is due to proton transfer from TFA, perhaps by causing a change in the counter ion, protonation state, or the timing of proton transfer. This hypothesis is reinforced by the extremely high endo selectivity observed in the bromolactonization of 1a in the presence of Br2, which likely produces HBr through uncatalyzed bromolactonization. Two plausible mechanisms of halolactonization can be envisioned that differ by the rate and timing of proton transfer (Fig. 8), one in which the nucleophile is deprotonated prior to (or during) irreversible cyclization (Fig. 8, path A), and one in which the nucleophile is deprotonated after cyclization (path B). The former path should be favored by the presence of base, such as the succinimidate anion generated during the reaction, while the latter should be favored under conditions with added TFA. Proton transfer from any of the intermediates depicted in Fig. 8 to succinimidate anion (pKa of succinimide 9.6 (31); pKa of TFA 0.26; pKa of acetic acid 4.76 (32); pKBH+MeOAc-3.9 (33) is thermodynamically favorable, whereas transfer to trifluoroacetate is only favorable from the protonated lactones.
Fig. 8.
Proposed role of timing of proton transfer on the mechanism of halolactonization.
The most critical conclusion from all of these experiments is that those catalysts that influenced the endo/exo ratio in halolactonization must have been present in the product-determining transition structure. This conclusion implies that chiral analogs of these catalysts have the potential to catalyze enantioselective halocyclization reactions. This conclusion does not mean that catalysts that did not measurably influence the endo/exo ratio must not have been present in the product-determining transition structure, simply that the experiments described in this paper do not provide evidence that they were. Similarly, the absence of an observable catalyst effect on the endo/exo ratios of halocycloetherification does not prove that the catalysts are not present in this product-determining transition structure either. It is possible that the conditions or substrates chosen were simply not conducive to observing such an effect.
Conclusions
A systematic examination of the affect of a wide range of Lewis bases on the rate and constitutional site selectivity in halofunctionalization reactions has been conducted. Cyclization reactions of unsaturated acids and alcohols with NBS and NIS are dramatically accelerated by Lewis bases that contain sulfur, selenium, and phosphorus donor atoms. The mode of cyclization (exo vs. endo) is primarily controlled by the structure of the substrate such that conjugated (E)-alkenes undergo highly endo selective cyclization whereas conjugated (Z)-alkene and alkenes of either geometry bearing aliphatic substituents undergo exo selective cyclization. In both cases the cyclization diastereoselectivities are perfect. Lewis bases significantly influence the constitutional site selectivity in all halolactonizations indicating that the Lewis base must be present in the stereochemistry-determining transition structure. This observation implies that chiral derivatives of these catalysts have the potential to provide enantioenriched products regardless of the rates or mechanisms of halonium ion racemization.
Materials and Methods
Bromolactonization of 1a. Preparation of rel-(5R,6S)-5-Bromotetrahydro-6-phenyl-2H-pyran-2-one (2aa) (34).
To a solution of NBS (213 mg, 1.2 mmol, 1.2 equiv) in CH2Cl2 (5.0 mL) under Ar in the dark was added a solution of 1a (176.1 mg, 1.0 mmol, 1.0 equiv) in CH2Cl2 (2.0 mL) by cannula, followed by a solution of  in CH2Cl2 (0.5 mL, 0.05 equiv, 0.05 mmol). The solution was stirred at 23 C for 5 min, quenched (sat. (saturated) aq. Na2S2O3 solution (5 mL)), diluted with H2O, and extracted with CH2Cl2. The combined organic extracts were dried over MgSO4, filtered, concentrated in vacuo, and purified by column chromatography (silica gel, CH2Cl2) to provide 207 mg (81%) of 2aa as a white solid. Melting point (mp) 104–106 ° C.
in CH2Cl2 (0.5 mL, 0.05 equiv, 0.05 mmol). The solution was stirred at 23 C for 5 min, quenched (sat. (saturated) aq. Na2S2O3 solution (5 mL)), diluted with H2O, and extracted with CH2Cl2. The combined organic extracts were dried over MgSO4, filtered, concentrated in vacuo, and purified by column chromatography (silica gel, CH2Cl2) to provide 207 mg (81%) of 2aa as a white solid. Melting point (mp) 104–106 ° C.
Bromocycloetherification of 4a. Preparation of rel-(2R,3S)-3-Bromotetrahydro-2-phenyl-2H-pyran (5aa) (35).
To a solution of NBS (213 mg, 1.2 mmol, 1.2 equiv) in CH2Cl2 (5.0 mL) under Ar in the dark was added a solution of 4a (162 mg, 1.0 mmol, 1.0 equiv) and AcOH (57 μL, 1.0 mmol, 1.0 equiv) in CH2Cl2 (2.0 mL) by cannula, followed by a solution of  in CH3Cl2 (0.5 mL, 0.05 equiv, 0.05 mmol). The solution was stirred at 23 C for 5 min, quenched (sat. aq. Na2S2O3 solution (5 mL), sat. aq. NaHCO3 solution (5 mL)), diluted with H2O, and extracted with EtOAc. The combined organic extracts were dried over MgSO4, filtered, concentrated in vacuo, and purified by column chromatography (silica gel, hexane/EtOAc, 95∶5) to provide 165.5 mg (69%) of 5aa as colorless needles. Melting point 41–42 °C.
in CH3Cl2 (0.5 mL, 0.05 equiv, 0.05 mmol). The solution was stirred at 23 C for 5 min, quenched (sat. aq. Na2S2O3 solution (5 mL), sat. aq. NaHCO3 solution (5 mL)), diluted with H2O, and extracted with EtOAc. The combined organic extracts were dried over MgSO4, filtered, concentrated in vacuo, and purified by column chromatography (silica gel, hexane/EtOAc, 95∶5) to provide 165.5 mg (69%) of 5aa as colorless needles. Melting point 41–42 °C.
Iodolactonization of 1a. Preparation of rel-(5R,6S)-5-iodotetrahydro-6-phenyl-2H-pyran-2-one (2ab) (8).
To a -45 °C suspension of NIS (270 mg, 1.2 mmol, 1.2 equiv) in CH2Cl2 (5.0 mL) under Ar in the dark was added a solution of 1a (176.1 mg, 1.0 mmol, 1.0 equiv) and TFA (3.8 μL, 0.05 mmol, 0.05 equiv) in CH2Cl2 (2.0 mL) by cannula, followed by a solution of  in CH2Cl2 (0.5 mL, 0.05 equiv, 0.05 mmol). The solution was stirred for 2 h, quenched (butyl vinyl ether in EtOH (2.5 mL, 1.2 M)), sat. aq. Na2S2O3 solution (5 mL), sat. aq. NaHCO3 solution (5 mL)), diluted with H2O, and extracted with EtOAc. The combined organic extracts were dried over MgSO4, filtered, concentrated in vacuo, and purified by column chromatography (silica gel, hexane/EtOAc, 80∶20) to provide 183.4 mg (61%) of 2ab as a white solid. Melting point 68–76 °C (decomposes).
in CH2Cl2 (0.5 mL, 0.05 equiv, 0.05 mmol). The solution was stirred for 2 h, quenched (butyl vinyl ether in EtOH (2.5 mL, 1.2 M)), sat. aq. Na2S2O3 solution (5 mL), sat. aq. NaHCO3 solution (5 mL)), diluted with H2O, and extracted with EtOAc. The combined organic extracts were dried over MgSO4, filtered, concentrated in vacuo, and purified by column chromatography (silica gel, hexane/EtOAc, 80∶20) to provide 183.4 mg (61%) of 2ab as a white solid. Melting point 68–76 °C (decomposes).
Iodocycloetherification of 4a. Preparation of rel-(2R,3S)-3-Iodotetrahydro-2-phenyl-2H-pyran (5ab).
To a -45 °C suspension of NIS (270 mg, 1.2 mmol, 1.2 equiv) in CH2Cl2 (5.0 mL) under Ar in the dark was added a solution of 4a (162.2 mg, 1.0 mmol, 1.0 equiv) and TFA (3.8 μL, 0.05 mmol, 0.05 equiv) in CH2Cl2 (2.0 mL) by cannula, followed by a solution of n-Bu3P = S in CH2Cl2 (0.5 mL, 0.05 equiv, 0.05 mmol). The solution was stirred for 2 h, quenched (butyl vinyl ether in EtOH (2.5 mL, 1.2 M), sat. aq. Na2S2O3 solution (5 mL), sat. aq. NaHCO3 solution (5 mL)), diluted with H2O, and extracted with EtOAc. The combined organic extracts were washed with sat. NaHCO3 solution, dried over MgSO4, filtered, concentrated in vacuo, and purified twice by column chromatography (silica gel, hexane/EtOAc, 95∶5) to provide 215.7 mg (75%) of 5ab as a colorless oil. TLC∶Rf 0.19 (hexanes/EtOAc, 19∶1) [UV].
Supporting Information Available.
Procedures for the preparation, characterization, and cyclofunctionalization of all substrates along with full characterization of halofunctionalization products see SI Appendix.
Supplementary Material
Acknowledgments.
Peter Yao is thanked for preliminary experiments and helpful discussions. We are grateful to the National Science Foundation (NSF CHE-0717989) and National Institutes of Health (GM R01-085235) for generous financial support. M.T.B. thanks Abbott Laboratories for a Graduate Fellowship in Synthetic Organic Chemistry.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005296107/-/DCSupplemental.
References
- 1.Ranganathan S, Muraleedharan KM, Vaish NK, Jayaraman N. Halo- and selenolactonization: the two major strategies for cyclofunctionalization. Tetrahedron. 2004;60:5273–5308. [Google Scholar]
- 2.Laya MS, Banerjee AK, Cabrera EV. Iodolactonization: past and present examples. Curr Org Chem. 2009;13:720–730. [Google Scholar]
- 3.Cardillo G, Orena M. Sterocontrolled cyclofunctionalizations of double bonds through heterocyclic intermediates. Tetrahedron. 1990;46:3321–3408. [Google Scholar]
- 4.Dowle MD, Davies DI. Synthesis and synthetic utility of halolactones. Chem Soc Rev. 1979;8:171–197. [Google Scholar]
- 5.French AN, Bissmire S, Wirth T. Iodine electrophiles in stereoselective reactions: recent developments and synthetic applications. Chem Soc Rev. 2004;33:354–362. doi: 10.1039/b310389g. [DOI] [PubMed] [Google Scholar]
- 6.Montana AM, Batalla C, Barcia JA. Intramolecular haloetherification and transannular hydroxycyclization of alkenes. A synthetic methodology to obtain polycyclic ethers and amines. Curr Org Chem. 2009;13:919–938. [Google Scholar]
- 7.Kwon HY, Park CM, Lee SB, Youn J-H, Kang SH. Asymmetric iodocyclization catalyzed by salen-CrIIICl: its synthetic application to swainsonine. Chemistry—A European Journal. 2008;14:1023–1028. doi: 10.1002/chem.200701199. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, et al. Enantioselective iodolactonization catalyzed by chiral quaternary ammonium salts derived from cinchonidine. J Org Chem. 2004;69:2874–2876. doi: 10.1021/jo035719e. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead DC, Yousefi R, Jaganathan A, Borhan B. An organocatalytic asymmetric chlorolactonization. J Am Chem Soc. 2010;132:3298–3300. doi: 10.1021/ja100502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, et al. Enantioselective bromolactonization of conjugated (Z)-enynes. J Am Chem Soc. 2010;132:3664–3665. doi: 10.1021/ja100173w. [DOI] [PubMed] [Google Scholar]
- 11.El-Qisairi A, Hamed O, Henry PM. A new palladium(II)-catalyzed asymmetric chlorohydrin synthesis. J Org Chem. 1998;63:2790–2791. [Google Scholar]
- 12.El-Qisairi AK, et al. New palladium(II)-catalyzed asymmetric 1,2-dibromo synthesis. Org Lett. 2003;5:439–441. doi: 10.1021/ol0273093. [DOI] [PubMed] [Google Scholar]
- 13.Garnier JM, Robin S, Rousseau G. An approach to enantioselective 5-endo halo-lactonization reactions. Eur J Org Chem. 2007;2007:3281–3291. [Google Scholar]
- 14.Haas J, Bissmire S, Wirth T. Iodine monochloride-amine complexes: an experimental and computational approach to new chiral electrophiles. Chemistry—A European Journal. 2005;11:5777–5785. doi: 10.1002/chem.200500507. [DOI] [PubMed] [Google Scholar]
- 15.Sakakura A, Ukai A, Ishihara K. Enantioselective halocyclization of polyprenoids induced by nucleophilic phosphoramidites. Nature. 2007;445:900–903. doi: 10.1038/nature05553. [DOI] [PubMed] [Google Scholar]
- 16.Brown RS, et al. Stable bromonium and iodonium ions of the hindered olefins adamantylideneadamantane and Bicyclo[3.3.1]nonylidenebicyclo[3.3.1]nonane. X-ray structure, transfer of positive halogens to acceptor olefins, and ab initio studies. J Am Chem Soc. 1994;116:2448–2456. [Google Scholar]
- 17.Neverov AA, Brown RS. Br+ and I+ transfer from the halonium ions of adamantylideneadamantane to acceptor olefins. halocyclization of 1,ω-alkenols and alkenoic acids proceeds via reversibly formed intermediates. J Org Chem. 1996;61:962–968. [Google Scholar]
- 18.Brown RS. Investigation of the early steps in electrophilic bromination through the study of the reaction with sterically encumbered olefins. Acc Chem Res. 1997;30:131–137. [Google Scholar]
- 19.Denmark SE, Burk MT, Hoover AJ. On the absolute configurational stability of bromonium and chloronium ions. J Am Chem Soc. 2010;132:1232–1233. doi: 10.1021/ja909965h. [DOI] [PubMed] [Google Scholar]
- 20.Mellegaard-Waetzig SR, Wang C, Tunge JA. Selenium-catalyzed oxidative halogenation. Tetrahedron. 2006;62:7191–7198. [Google Scholar]
- 21.Ahmad SM, Braddock DC, Cansell G, Hermitage SA. Dimethylformamide, dimethylacetamide, and tetramethylguanidine as nucleophilic organocatalysts for the transfer of electrophilic bromine from N-bromosuccinimide to alkenes. Tetrahedron Lett. 2007;48:915–918. [Google Scholar]
- 22.Castellote I, et al. Reaction of imines with N-iodosuccinimide (NIS): unexpected formation of stable 1∶1 complexes. Chem Commun. 2007;43:1281–1283. doi: 10.1039/b615183c. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Xu H, Xu H, Tang W. DABCO-catalyzed 1,4-bromolactonization of conjugated enynes: highly stereoselective formation of a stereogenic center and an axially chiral allene. J Am Chem Soc. 2009;131:3832–3833. doi: 10.1021/ja8099008. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett PA. Olefin cyclization processes that form carbon-heteroatom bonds. In: Morrison JD, editor. Asymmetric Synthesis. Vol. 3. FL: Academic; 1984. pp. 411–454. [Google Scholar]
- 25.Snider BB, Johnston MI. Regioselectivity of the halolactonization of γ,δ-unsaturated acids. Tetrahedron. 1985;26:5497–5500. [Google Scholar]
- 26.Bartlett PA, Myerson J. Stereoselective epoxidation of acyclic olefinic carboxylic acids via iodolactonization. J Am Chem Soc. 1978;100:3950–3952. [Google Scholar]
- 27.Brown HC, Brewster JH, Shechter H. An interpretation of the chemical behavior of five- and six-membered ring compounds. J Am Chem Soc. 1954;76:467–474. [Google Scholar]
- 28.Bruno P, Caselli M, Fragale C, Magrino S. Charge transfer complexes of hexamethylphosphoramide chalcogenides. J Inorg Nucl Chem. 1977;39:1757–1759. [Google Scholar]
- 29.Glera J, Sobczyk L, Paetroldt R. Correlation between the Dipole moments and thermodynamical data of the iodine complexes with organic oxides, sulfides, and selenides. J Phys Chem. 1980;84:2602–2605. [Google Scholar]
- 30.Julia MM, Guy-Roualt A. Iodolactonization and dihydroxylation of 5-phenyl pentenoic acids (in French) C R Hebd Seances Acad Sci. 1964;258:3728–3730. [Google Scholar]
- 31.Walton H, Schilt A. The ionization constant and rate of hydrolysis of succinimide. J Am Chem Soc. 1952;74:4995–4996. [Google Scholar]
- 32.Goldberg RN. Thermodynamic quantities for the ionization reactions of buffers. J Phys Chem Ref Data. 1999;31:231–370. [Google Scholar]
- 33.Bagno A, Scorrano G. Acid-base properties of organic solvents. J Am Chem Soc. 1988;110:4577–4582. [Google Scholar]
- 34.Crich D, Beckwith ALJ, Filzen GF, Longmore RW. Free radical chemistry of lactones: ring contractions and expansions. J Am Chem Soc. 1996;118:7422–7423. [Google Scholar]
- 35.Galpin DR, Bobbink SR, Ary TE. cis- and trans-3-Methylamino-2-phenyltetrahydropyran. J Pharm Sci. 1972;61:963–964. doi: 10.1002/jps.2600610634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.