Abstract
The signaling network of innate immunity in Drosophila is constructed by multiple evolutionarily conserved pathways, including the Toll- or Imd-regulated NF-κB and JNK pathways. The p38 MAPK pathway is evolutionarily conserved in stress responses, but its role in Drosophila host defense is not fully understood. Here we show that the p38 pathway also participates in Drosophila host defense. In comparison with wild-type flies, the sensitivity to microbial infection was slightly higher in the p38a mutant, significantly higher in the p38b mutant, but unchanged in the p38c mutant. The p38b;p38a double-mutant flies were hypersensitive to septic injury. The immunodeficiency of p38b;p38a mutant flies was also demonstrated by hindgut melanization and larvae stage lethality that were induced by microbes naturally presented in fly food. A canonical MAP3K-MKK cascade was found to mediate p38 activation in response to infection in flies. However, neither Toll nor Imd was required for microbe-induced p38 activation. We found that p38-activated heat-shock factor and suppressed JNK collectively contributed to host defense against infection. Together, our data demonstrate that the p38 pathway-mediated stress response contribute to Drosophila host defense against microbial infection.
Innate immunity is essential for multicellular organisms to defend themselves against microbial infection. Studies in Drosophila melanogaster revealed several signaling pathways that play essential roles in innate immunity (1). The Toll pathway mediates resistance to pathogenic fungi and Gram-positive bacteria by activating two NF-κB proteins, Dorsal and Dorsal-related immunity factor (1, 2). The Imd pathway activates Relish, the third NF-κB protein, in response to Gram-negative bacteria (3, 4). The NF-κB family of transcription factors regulates the expression of a distinct set of genes coding for antimicrobial peptides (AMP) against bacteria and fungi (5). Recently the JNK and JAK-STAT pathways were shown to control additional targets induced by microbial agents or damage (6–10). Because the above pathways are also important in mammalian host defense, the innate immune responses appear to be largely regulated by evolutionarily conserved signaling pathways.
The p38 MAPK pathway is an evolutionarily conserved signaling pathway that responds to a variety of stresses (11). The p38 pathway can be activated by pathogens in animals, and is known to play a pivotal role in inflammatory responses in mammals (12). Mpk1, a p38 ortholog in Caenorhabditis elegans, is essential for innate immunity by participating in regulating antimicrobial peptides (13). The D. melanogaster genome encodes three p38 orthologs, designated as p38a (CG5475), p38b (CG7393, also known as mpk2), and p38c (CG3338) (14). Orthologs p38a and p38b have almost the same level of sequence similarity with mammalian p38α or p38β, thus whether p38a or p38b corresponds to p38α cannot be determined. The ortholog p38c is unique for Drosophila because it does not contain dual regulation sites, the hallmark of MAPK members. Both p38a and p38b can be activated by bacterial stimuli in cultured cells (14). However, p38a mutant flies do not show a significant change in their susceptibility to bacterial infection (15). Likely because of the redundant functions of these three p38 orthologs in flies, whether the p38 pathway has any role in fly innate immunity still needs further investigation.
In the present study, we generated fly mutants for all three p38 orthologs and studied the role of the p38 pathway in host defense. We found that p38a and p38b, but not p38c, were involved in host defense, and p38b was functionally more important than p38a. Activation of p38 in flies is mediated by a MAP3K-MKK cascade, but not controlled by the Toll and Imd pathways. In addition, p38a and p38b suppress JNK activity, which contributes to the host-defense function of p38. Activation of heat-shock factor (Hsf) by p38 is an important part of antimicrobial reactions in flies. Taken together, our data indicate that the p38 pathway-mediated stress response is part of the innate immunity of flies.
Results
Generation of Mutants for p38 MAPKs.
We obtained several Drosophila lines with P-element insertions that are closely located in the loci of p38 MAPKs from the Gene Disrupt Project database (http://flypush.imgen.bcm.tmc.edu/pscreen/) (Fig. S1). P-element-mediated imprecise excisions were carried out to obtain mutants for the all three p38 orthologs, following the method as described previously (16) (Fig. S1). Flies with single mutations of p38a and p38c or double mutations of p38a and p38c did not have any visible morphological or developmental abnormalities. The p38b mutant flies appeared to be morphologically normal, but compared with wild-type they required approximately two additional days to develop from egg deposition to eclosion at 22 °C. The p38b;p38a double-mutant flies typically died at larval and pupal stages under standard culture conditions, with only a few escapers. The mutants lacking all three p38 orthologs (p38b; p38a,p38c) died at early larval stages and rarely proceeded to third instar larvae. The above results indicate that the p38 homologs could compensate for each other to some extent.
p38 MAPKs Protect Drosophila from Infections by Microbes in Food.
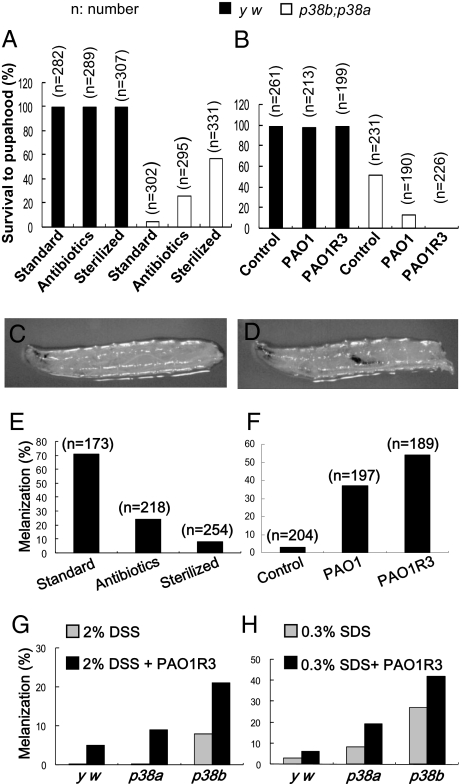
The lethality of p38b;p38a and p38b;p38a,p38c mutant flies could be a result of developmental defects or impairment of host defense. We studied p38b;p38a double mutants to address this issue. Because Drosophila encounters many microorganisms when cultured with standard food in laboratories, we cultured p38b;p38a mutant flies in either near-sterile conditions (Materials and Methods) or with standard food containing antibiotics and observed a significant increase in survival rate under both culture conditions (Fig. 1A). Therefore, microbial infection was a major cause of lethality in p38b;p38a mutant flies cultured with standard methods. To confirm that microbial pathogens were the cause of lethality in p38b;p38a mutant flies, we introduced Pseudomonas aeruginosa into sterilized food and found an elevated death rate (Fig. 1B). Including nonpathogenic bacterium Escherichia coli or yeast Saccharomyces cerevisiae in sterilized food had no effect on survival, suggesting an increased immune susceptibility for pathogens like P. aeruginosa. The notion that microbes in the standard culture food could kill p38b;p38a double-mutant but not wild-type flies was further supported by the fact that over 30% of the p38b;p38a double mutants could reach adulthood, with a mean lifespan of almost 4 wk, under near-sterile conditions (Fig. S2A). These results suggest that the immunity to microbial infection was weakened in p38b;p38a mutant flies compared with wild-type counterparts.
Fig. 1.
The p38-deficient flies are more sensitive to microbial pathogens in food. (A) The survival rate to pupahood of the wild-type yellow white strain (y w) and p38ab;p38a double-mutant (p38a13; p38b156A) (Fig. S1) larvae cultured in standard food with or without a mixture of antibiotics (500 μg/mL ampicillin, 50 μg/mL tetracycline, and 200 μg/mL rifamycin), or in sterilized food. (B) The survival rate to pupahood of the wild-type and p38b;p38a larvae cultured in sterilized food with or without adding 105/mL P. aeruginosa (PAO1, wild-type strain; PAOR3, a more virulent mutant strain repressing quorum-sensing controlled genes). (C) Third instar larvae of y w as wild-type control. (D) Hindgut melanization in the p38b;p38a double mutant. (E) The melanization rate in the hindgut of the p38b;p38a larvae cultured in standard food with or without a mixture of antibiotics as in A, or in sterilized food. (F) The melanization rate in the hindgut of the p38b;p38a larvae cultured in sterilized food with or without adding 105/mL P. aeruginosa as in B. (G and H) Melanization in the hindgut can be induced by dextran sodium sulfate (DSS) or SDS with the combination of PAO1R3.
Microbes in Culture Food Causes Hindgut Melanization in p38a/b Mutants.
We found melanization in the hindgut of p38b;p38a double-mutant larvae but not in wild-type, p38a, p38b, p38c, or p38a,p38c mutant larvae (Fig. 1 C and D, and Fig. S2B). Because the Drosophila hindgut encounters many microorganisms when cultured with standard methods in laboratories, and melanization is known to participate in host defense in insects, we sought to determine whether the melanization observed in p38b;p38a double mutant flies is related to microbial infections. A significant reduction in melanization was observed when p38b;p38a mutant flies were cultured in a near-sterile conditions or with standard food containing antibiotics (Fig. 1E), which is correlated with the increase in survival rate (Fig. 1A), indicating that microbial infection was a major cause of melanization in p38b;p38a mutants. In support of this conclusion, introducing P. aeruginosa into sterilized food elevated melanization, correlating with increased death rates (Fig. 1 B and F). Nonpathogenic E. coli or S. cerevisiae had no effect on the melanization of the hindgut. Collectively, our data demonstrated that infection was a cause of melanization in the hindgut of p38b;p38a mutant larvae, and the rate of melanization correlated with the death rate.
The wall of the Drosophila hindgut is composed of a monolayer of epithelial cells mounted outside with a thin layer of circular muscle fibers (17). The melanization in p38b;p38a mutants could be observed as early as in the second instar larvae; it gradually extended and could eventually occupy the whole hindgut (Fig. S3A). The adult flies of p38b;p38a double mutants also showed melanization in the hindgut (Fig. S3B). The histology of paraffin sections showed that the melanization starts inside epithelial layer of hindgut in p38b;p38a mutant larvae (Fig. S3C). Electron microscope analysis detected vacuoles in the epithelial cells in the melanized regions (Fig. S3D). Muscle disruption could also be found after severe melanization (Fig. S3E). Although there were no visible morphological abnormalities in the hindgut of p38b;p38a mutant larvae before melanization, the peritrophic membrane, which is secreted by epithelial cells to cover the surface of the gut lumen and protect the epithelium from digestion and invasion by pathogens, was thinner in the hindgut of p38b;p38a mutants in comparison with wild-type counterparts, as determined by Calcofluor staining (Fig. S3F). This finding suggested that developmental defects in the hindgut in p38b;p38a mutants contributed to the increased susceptibility to infection of gut microbial pathogens.
Dextran sodium sulfate (DSS)-induced colitis is one of the most widely used models of intestinal inflammation in mice. DSS is directly toxic to gut epithelial cells of the basal crypts and affects the integrity of the mucosal barrier. Because melanization of the hindgut in flies is associated with tissue damage, we sought to test whether DSS has an effect on the fly gut. We found that melanization in the fly hindgut could not be induced by oral feeding with DSS in wild-type larvae but was induced in larvae of p38b single mutants (Fig. 1G), suggesting that epithelial injury can facilitate melanization in the hindgut. P. aeruginosa, at an amount that does not cause melanization, significantly enhanced the rates of DSS-induced melanization in a p38b and p38a flies (Fig. 1G), confirming that melanization was associated with infection. In addition, we observed that SDS was more potent than DSS in triggering melanization in the fly hindgut (Fig. 1H). Thus, similar to that in mammals, deficiency of p38 in gut epithelia could promote gut injury (18, 19).
p38 MAPKs Protect of Drosophila from Septic Injury.
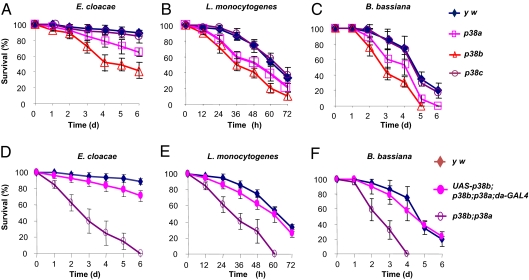
To further evaluate the role of p38a, p38b, and p38c in fly immunity, we examined adult flies that were either pricked with bacteria (Enterobacter cloacae and Listeria monocytogenes) or rolled with fungal spores (Beauveria bassiana). The p38a and p38b mutants, but not the p38c mutant, showed some increase in susceptibility to these microbial infections, with the p38b mutant being the most susceptible (Fig. 2 A–C). The adult p38b;p38a double-mutant flies were more sensitive to infection than that of p38a or p38b single-mutant flies (Fig. 2 D–F). The p38b;p38a double-mutant flies were also challenged by septic injury with E. coli, P. aeruginosa, Staphylococcus aureus, and Aspergillus fumigatus, respectively, and compared with y w control, the mutant flies were hypersensitive to P. aeruginosa, S. aureus, and A. fumigatus, but were not sensitive to E. coli (Fig. S4). A rescue of p38b expression in p38b;p38a mutants by overexpression of UAS-p38b with da-Gal4 increased the survival rate of infected flies to a level close to wild-type (Fig. 2 D–F). Hence, the p38 pathway protects Drosophila from septic injury-induced death from a broad spectrum of pathogenic microbes, and p38b plays a major role in the p38 pathway-mediated host defense.
Fig. 2.
The p38-deficient flies are more sensitive to septic injury. (A–C) The survival rates of infected adult flies of p38a (p38a13), p38b (p38b156A), and p38c (p38c7) mutants, and wild-type flies (y w). (D–F) The survival rates of infected adult flies of p38b;p38a (p38b156A; p38a13) mutants, p38b;p38a mutants with overexpression of UAS-p38b driven by da-Gal4, and wild-type flies (y w). The data were obtained by using groups of 30 female or male adults, aged 2 to 4 d, pricked with E. cloacae or L. monocytogenes or rolled in B. bassiana spores. Six replicates were used for the determination of the survival rates. Error bars represent SEM in each group. p38a compared with y w, P < 0.02; p38b and p38b;p38 compared with y w, P < 0.002 and 0.0001, respectively.
Expression of a Variety of Immune- or Host Defense-related Genes Are Altered in p38b Mutants.
We next carried out a gene-expression profiling of p38b mutants in comparison with wild-type (y w) flies. A genome-wide microarray analysis with 15,000 probes was performed on flies treated with or without E. cloacae or L. monocytogenes as described (20). The expression pattern of the genes was analyzed using Cluster and Treeview (Fig. S5). One-hundred ninety-seven genes were down-regulated and 112 genes were up-regulated in p38b (Fig. S5 and Dataset S1). Ninety-three of the 197 down-regulated genes and 20 of the 112 up-regulated genes were genes that responded to both E. cloacae and L. monocytogenes treatments (Fig. S5). Thirty-four of the down-regulated genes and 27 of the up-regulated genes in p38b mutants belong to Drosophila immune-regulated genes (21). In addition, 39 of the down-regulated genes and 18 of the up-regulated genes were also suggested to play a role in host defense based on annotation (Dataset S2). In p38b mutants, five AMP genes (Def, DptB, Dro, Dros, Mtk) were up-regulated, whereas three AMP genes (AttA, AttC, CecB) were down-regulated. Nine cytochrome P450 genes and six GSTs were down-regulated. However, three cytochrome P450 genes (Cyp4d21, Cyp4e3, Cyp6a13) were also up-regulated. All four Tot genes and two Hsp (Hsp60D, Hsp70Bc) genes were down-regulated (Dataset S2). We used qRT-PCR to validate the microarray results of several genes belonging to the AMP, cytochrome P450, turandont (Tot), and Hsp families in p38b mutant and p38b;p38a double-mutant flies treated with or without septic injuries, and validated the microarray data (Fig. S6). The p38 pathway could both positively and negatively regulate a number of different immune- or host defense-related genes, and thus should affect host defense in both positive and negative ways. The increased susceptibility to infection in p38 mutants could be a result of the sum of the effects caused by the changes in gene expression.
Role of the p38 Pathway in Host Defense Against Microbial Infections Is Partially Mediated by Heat-Shock Factor.
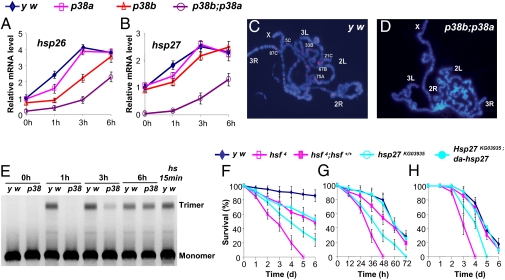
We then focused on heat shock proteins (HSPs) for further studies in addressing the host-defending role of the p38 pathway, because all genes in this group (Hsp60D, Hsp70Bc, Hsp26, and Hsp27) were down-regulated in p38b mutants and p38b;p38a double mutants (Fig. 3 A and B and Fig. S6). Hsf is a key transcription factor that regulates the expression of HSPs (22). Drosophila has a single Hsf, and the activation of Hsf by heat shock is known to be mediated by trimerization and localization of Hsf at specific sites on the polytene chromosome (22). We found a similar localization pattern of Hsf on the polytene chromosome 1 h after E. cloacae infection in wild-type flies (Fig. 3C). In contrast, we did not detect the localization of Hsf on polytene in p38b;p38a double-mutant flies under the same condition (Fig. 3D). Consistently, E. cloacae infection-induced trimerization of Hsf was delayed in p38b;p38a double-mutant flies, indicating that the p38 pathway is involved in regulating Hsf activity (Fig. 3E).
Fig. 3.
Delayed induction of Hsp genes and Hsf activity in p38 mutants. Relative mRNA levels of Hsp26 (A) and Hsp27 (B) in wild-type, p38a, p38b, and p38b;p38a mutants were shown with qRT-PCR normalized with the internal control rp49. Data from three independent experiments are expressed as mean ± SEM; p38b;p38a compared with y w, P < 0.005. The third instar larval polytene chromosomes from wild-type (C) and p38b;p38a double mutants (D) were stained for DNA (DAPI, blue) and Hsf (red) 1 h after septic infection. (E) Hsf trimerization was shown with Western blotting after native gel electrophoresis. (F–H) Hsf (hsf4) and Hsp27 (hsp27KG03935) mutants are more susceptible to septic infection of E. cloacae (F) and L. monocytogenes (G), and natural infection of B. bassiana (H). The susceptibility of Hsf and Hsp27 mutants can be rescued by introduction of a Hsf transgene (hsf4;hsf+/+) and overexpression of Hsp27 (Hsp27KG03935;da > hsp27), respectively. Forty males, aged 2 to 4 d were tested for each group as one experiment. Data from three independent experiments are expressed as mean ± SEM; Hsf and Hsp27 mutants compared with y w, P < 0.0001 and 0.001, respectively.
To assess whether the induction of Hsf and Hsps is a mechanism of host defense, we infected Hsf (Hsf4, a hypomorphic allele) and Hsp27 mutants with E. cloacae, L. monocytogenes, and B. bassiana. Both Hsf and Hsp27 mutants were more susceptible to pathogenic infections (Fig. 3 F–H). Introducing a copy of a wild-type Hsf transgene in a Hsf4 background or restoration of Hsp27 activity (UAS-hsp27 and da-Gal4) in a Hsp27 mutant background largely rescued their phenotypic susceptibility to pathogenic infections (Fig. 3 F–H), indicating that Hsf activation and Hsf-mediated Hsp27 expression are part of the Drosophila host-defense responses.
Deficiency of p38b Results in Sustained JNK Activation.
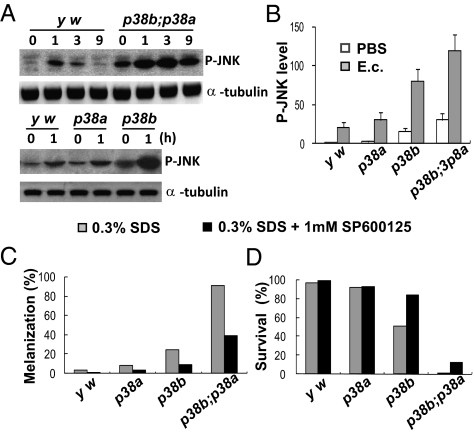
Gene profiling analysis also showed that the expression levels of JNK (bsk) are up-regulated in p38b mutants (Fig. S7A and Datasets S1 and S2). Using a phospho-JNK antibody, we also observed that phospho-JNK in p38a, p38b, and p38b;p38a mutants was higher than that in y w at both a resting stage and after septic injury with E. cloacae and L. monocytogenes (Fig. 4 A and B). Although both p38a and p38b inhibit JNK expression and activation, the effect of p38b was stronger. The inhibition of JNK activation by p38b was at least partially mediated by p38b-mediated expression of a JNK phosphatase puc, because puc was not induced by E. cloacae and L. monocytogenes in p38b mutant flies (Fig. S7B and Datasets S1 and S2). Both the increased expression of JNK and decreased expression of puc might contribute to the sustained JNK activation in p38 mutants.
Fig. 4.
p38 attenuates JNK expression and activity. (A) Wild-type, p38a, p38b, and p38b;p38a mutant flies were infected with E. cloacae for a time period, as indicated. JNK activity determined by immunoblotting with phospho-JNK antibodies. (B) The relative intensity of phospho-JNK (P-JNK) in wild-type, p38a, p38b, and p38b;p38a mutants was measured by Western blotting bands of three independent experiments, with pixel information obtained using Kodak Molecular Imaging Software Version 4.0. (C) The rate of hindgut melanization and (D) rate of survival to adult of the wild-type, p38a, p38b, and p38b;p38a mutants cultured in standard food containing 0.3% SDS with or without the JNK inhibitor SP600125 (n > 200). Data in B represent mean (± SEM) fold changes of P-JNK compared with y w injured with PBS alone; p38b versus y w, P < 0.01; p38b;p38a versus y w, P < 0.001.
To determine whether the sustained JNK activation contributes to the increased susceptibility of p38 mutants to bacterial infection, we fed p38 mutants with a JNK inhibitor and analyzed the rates of melanization in the hindgut and the viabilities of the mutants. Because spontaneous melanization in p38a and p38b single mutants was rare, we used SDS treatment in these experiments. We found that inhibition of JNK increased the survival rates of p38b mutants and decreased the melanization rates in their hindguts (Fig. 4 C and D). These data suggest that sustained activation of JNK contributes to the melanization and lethality of p38b mutants.
Activation of p38 by Microbes Is MAPK Cascade-Dependent but Toll- and Imd-Independent.
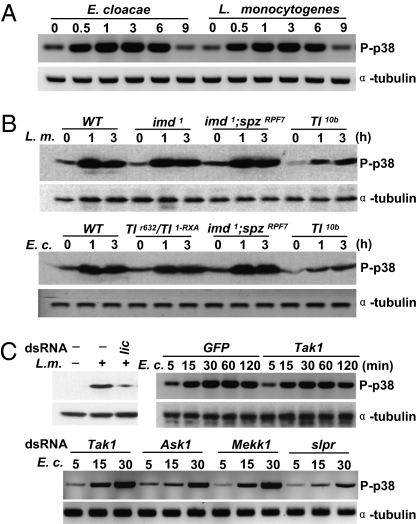
Activation of p38 family members is achieved by phosphorylation on the regulatory dual phosphorylation motif. Western blotting with phospho-p38–specific antibodies showed activation of Drosophila p38 after either Gram-negative or Gram-positive injury (Fig. 5A). Because of the similar size of Drosophila p38a and p38b proteins, we were unable to determine whether phosphorylation occurs on p38a, p38b, or both. Nevertheless, Western blotting with phospho-p38–specific antibodies still could be used to evaluate the activation of fly p38.
Fig. 5.
A canonical MAP3K-MKK cascade is required for p38 activation by septic infection, but Toll and Imd are dispensable for this pathway. (A) Phospho-p38 in wild-type flies was detected at different time points after septic injury with E. cloacae and L. monocytogenes. (B) Phosphorylation of p38 is unchanged in Imd, Tl, and Imd;spz double mutants (loss-of-function) as well as in Tl10b mutants (a gain-of-function mutation of Tl) following infection with both Gram-positive L. monocytogenes and Gram-negative E. cloacae. (C) Toll homologs or licorne (lic, a MKK3/6 homolog), TAK1, ASK1, Mekk1, and Slpr were knocked down with dsRNAs and the L. monocytogenes- or E. cloacae-induced phophorylation of p38 in S2 cells are shown. Knockdown of Tak1, ASK1, and Mekk1 had no significant effect on bacteria-induced p38 phosphorylation, and lic and Slpr are required for p38 activation. Phosphorylation was assayed by Western blotting using phospho-specific antibodies of p38. α-Tubulin is shown as a loading control. All Western blots have been repeated at least twice and all showed the same tendency.
Toll and Imd are the major pathways in flies that respond to different microbial pathogens. To determine the relationship between the p38 pathway and the Toll pathway, we infected Toll mutant flies with L. monocytogenes and found that p38 phosphorylation was not affected by a Toll loss-of-function mutation and a gain-of-function mutation Toll10b (Fig. 5B). We infected Imd and Imd;spz double mutants with E. cloacae and found a similar level of p38 activation when compared with wild-type flies (Fig. 5B). Knockdown of licorne(lic), the sole ortholog to mammalian MKK3/6, inhibited L. monocytogenes-induced p38 phosphorylation (Fig. 5C and Fig. S7C). Knockdown of hemipterous (hep), an ortholog of mammalian MKK4, did not affect p38 activation by infection. We further determined which MAP3K is involved in the activation of p38 by infection. Knockdown of slipper (slpr), but not Tak1, Ask1, and Mekk1 reduced p38 activation (Fig. 5C). Therefore, our data reveal a p38 MAPK cascade that participates in the host defense in Drosophila, and indicate that the activation of the p38 pathway is mediated by an unidentified signaling mechanism.
Discussion
D. melanogaster has been widely used as a model to study innate immunity; however, the role of the p38 pathway in a fly's host response to microbes remains largely unknown. This study analyzed all p38 orthologs in flies and found that p38a and p38b, but not p38c, participate in the fly's defense against microbes. Although different p38 isoforms can functionally compensate one another to a certain degree, p38b appears to be more important than p38a in host defense against pathogenic infections. Our data suggest that activation of Hsf and suppression of JNK are part of the mechanism used by the p38 pathway in host defense. Although the p38 kinase cascade is similar to that in mammals, the upstream signaling mediators (sensor and adaptors) that lead to the activation of the p38 pathway in flies seems to be different from that of mammals and are currently unknown.
In mammals, the Toll and Imd (RIP) proteins are linked in a signaling cascade, but in flies they are found in independent pathways. Both p38 and NF-κB are often activated simultaneously by signaling from the same receptor in mammals, but here we found that these two pathways are likely activated by different upstream receptors or sensors in flies. Mammalian p38 primarily participates in host defense by regulating cytokine expression, but fly p38 seems to have a minimal role in the specific antimicrobial response, such as AMP expression. We show here that activation of Hsf, a key transcription factor in stress responses, was impaired in p38 mutants, providing unique evidence that the p38 pathway directly or indirectly regulates this transcription factor. Hsf activates the transcription of a large number of genes that regulate protein homeostasis, including the molecular chaperones Hsps. Our data suggest that the p38 pathway regulates Hsf before or during its trimerization. Because of the conservation of Hsf, it is likely that p38 also regulates Hsf in mammals. Because of the role of Hsf in general stress, the anti-infection function of the p38 pathway in flies is at least in part caused by its general stress responses. However, the regulation of Hsf by the p38 pathway in Drosophila appears to be selective for bacterial pathogens, because heat shock-induced activation of Hsf is normal in p38b;p38a mutants.
Recently p38a was shown to be involved in dual oxidase (Duox) induction in Drosophila, and Duox-dependent reactive oxygen species is known to serve on the front line microbicidal function in fly gut (23). Although p38a was shown to play a minor role in the host defense against microorganisms in our study, its role in Duox induction may contribute to the phenotype observed in p38b;p38a mutants. Because Duox plays a role in peritrophic matrix cross-linking in mosquitoes (24), it is possible that the control of Duox expression by the p38 pathway is an underlying mechanism for the defect in peritrophic membrane observed in p38b;p38a flies. Furthermore, p38b was found to play a role in phagocytic encapsulation, which increase survival rate of intracellular bacterial infected Drosophila (25). Although intracellular infection is not a model we used in the present study, the defect in phagocytic encapsulation may contribute to the hypersusceptibility to microbial infection in p38b mutant flies. We showed that p38c had no role in protecting Drosophila from infection, but it may still have some functions during bacterial infection because it was reported that induction of decarboxylase gene by both Gram-positive and -negative septic injury is p38c-dependent (26).
The production and deposition of melanin pigments on invading pathogens and parasites represents a unique, innate immune response in insects (27). This immune response in flies also occurs at the sites of wounding, which may explain the enhancement of infection-induced melanization by DSS or SDS in our experiments. Although the production of melanin is a defense mechanism in insects, we show in this article that heavy melanization of the hindgut leads to fly death, which seems to mimic the consequences of overactive inflammatory signaling in mammals. The work described in this article suggests that melanization of the hindgut in Drosophila can be used as a model system to study host responses to microbial infection. The hindgut melanization may provide a model to study inflammatory bowel disease in flies, as the gut is one of the most ancient organs in animals and the hindgut of flies is homologous to the bowels in mammals (17).
The increased susceptibility to infection in p38 mutants must result from multiple changes caused by p38 deficiency, as microarray analysis identified changes in the expression of a large number of genes. This study shows that the activation of Hsf and suppression of JNK are part of the mechanisms used by the p38 pathway in fly host defense. Like NF-κB, JNK, and Jak-STAT, the evolutionarily conserved p38 pathway also participates in host defense in flies.
Materials and Methods
Fly Husbandry and Fly Stocks.
Flies were raised at 25 °C on standard yeast-cornmeal-agar medium (JazzMix, Fisher Scientific AS153) except as otherwise mentioned. For sterile conditions, plastic vials, cotton balls, and media were autoclaved for 30 min. Media were divided into vials and plugged in a biosafety cabinet. The eggs were collected and surface-sterilized in White's solution for 20 min, as previously described (28). Antibiotic food was prepared as described by adding 1 mL of a 100× stock of antibiotics in 50% ethanol per 100 mL of liquefied food to a final concentration of 500 μg/mL ampicillin, 50 μg/mL tetracycline, and 200 μg/mL rifamycin,. Control antibiotic-free food was prepared by adding the same concentration of ethanol alone. The y1 w67c23; ry506 and related precise excision lines were used as wild-type controls in this study, because P-element insertion lines p38bKG01337, p38cKG05834, dMK2EY11791, and Hsp27KG03935 share the same genetic background. UAS-p38b, UAS-Hsp27, da-Gal4, Hsf4, Hsf+, imd1, Tlr632, Tl1-RXA, Tl10b, and imd1; spzRPF7, have been described elsewhere (2, 22, 29). A full description of all stocks used in this study is included in the SI Materials and Methods.
Immune-Response Assays.
Procedures for septic injury with Gram-negative or -positive bacteria and fungal infection were as described previously (2). See SI Materials and Methods for details.
Complementary DNA Microarray Analysis.
Complementary DNA microarrays containing 15,000 known or predicted genes of the D. melanogaster genome were processed (20).
RT-PCR.
See SI Materials and Methods for details on RT-PCR.
RNA Interference Experiments.
RNA interference was performed as described in SI Materials and Methods.
SDS/PAGE and Immunoblotting.
Standard methods were used. See SI Materials and Methods for details.
Light Microscopy, Imaging, and Transmission Electron Microscopy.
Standard methods were used. See SI Materials and Methods for details.
Statistics.
Digital image processing used IDL software (Research Systems) and custom-devised computer algorithms. Statistical data are expressed as mean ± SEM. Student's t test and paired t test were applied when appropriate. A P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs. B. Lemaitre, J. A. Hoffmann, D. Ferrandon [French National Center for Scientific Research (CNRS), Paris, France], S. Rutschmann (Imperial College, London, UK), T. Adachi-Yamada (Nagoya University, Nagoya, Japan), U. Haecker (Lund University, Lund, Sweden), C. Wu (National Cancer Institute, Bethesda, MD), P. Greenberg (University of Iowa, Iowa City, IA), Y. T. Ip (University of Massachusetts, Worcester, MA), R. L. Cagan (Washington University School of Medicine, St. Louis, MO), R. Bodmer (The Sanford-Burnham Medical Research Institute, San Diego, CA), H. Bellen (Baylor College of Medicine, Houston, TX), R. Nusse (Stanford University Medical Center, Stanford, CA), F. Ausubel (Harvard Medical School, Boston, MA), S. Wasserman (University of California, San Diego, CA), and S. Benzer (California Institute of Technology, Pasadena, CA) for valuable Drosophilia and bacterial strains and/or technical suggestions and the Bloomington Stock Center (Indiana University, Bloomington, IN) for providing materials. We thank Yu Cai (National University of Singapore, Singapore) for comments and suggestions on the manuscript. This work was supported in part by Sino-Swiss International Collaboration Grant 2009DFA32760, 973 Program 2009CB522200, 111 Project (B06016), and Grant 30830092 from the National Science Foundation of China.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009223107/-/DCSupplemental.
References
- 1.Imler JL, Hoffmann JA. Toll receptors in Drosophila: A family of molecules regulating development and immunity. Curr Top Microbiol Immunol. 2002;270:63–79. doi: 10.1007/978-3-642-59430-4_4. [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B, et al. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedengren M, et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 5.Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 6.Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–450. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 7.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:711–722. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 9.Park JM, et al. Targeting of TAK1 by the NF-kappa B protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 2004;18:584–594. doi: 10.1101/gad.1168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dostert C, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 11.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 12.Ono K, Han J. The p38 signal transduction pathway: Activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- 14.Han ZS, et al. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol Cell Biol. 1998;18:3527–3539. doi: 10.1128/mcb.18.6.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig CR, Fink JL, Yagi Y, Ip YT, Cagan RL. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 2004;5:1058–1063. doi: 10.1038/sj.embor.7400282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson HM, et al. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami R, Shiotsuki Y. Ultrastructure of the hindgut of Drosophila larvae, with special reference to the domains identified by specific gene expression patterns. J Morphol. 2001;248:144–150. doi: 10.1002/jmor.1025. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka M, et al. Distinct effects of p38alpha deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology. 2010;138:1255–1265. doi: 10.1053/j.gastro.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YJ, et al. Epithelial p38alpha controls immune cell recruitment in the colonic mucosa. PLoS Pathog. 2010;6:e1000934. doi: 10.1371/journal.ppat.1000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou D, et al. Experimental selection for Drosophila survival in extremely low O(2) environment. PLoS ONE. 2007;2:e490. doi: 10.1371/journal.pone.0000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westwood JT, Clos J, Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991;353:822–827. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- 23.Ha EM, et al. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol. 2009;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinzawa N, et al. p38 MAPK-dependent phagocytic encapsulation confers infection tolerance in Drosophila. Cell Host Microbe. 2009;6:244–252. doi: 10.1016/j.chom.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Davis MM, Primrose DA, Hodgetts RB. A member of the p38 mitogen-activated protein kinase family is responsible for transcriptional induction of Dopa decarboxylase in the epidermis of Drosophila melanogaster during the innate immune response. Mol Cell Biol. 2008;28:4883–4895. doi: 10.1128/MCB.02074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang H. Regulation and function of the melanization reaction in Drosophila. Fly (Austin) 2009;3:105–111. doi: 10.4161/fly.3.1.7747. [DOI] [PubMed] [Google Scholar]
- 28.Bakula M. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J Invertebr Pathol. 1969;14:365–374. doi: 10.1016/0022-2011(69)90163-3. [DOI] [PubMed] [Google Scholar]
- 29.Adachi-Yamada T, et al. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.