Abstract
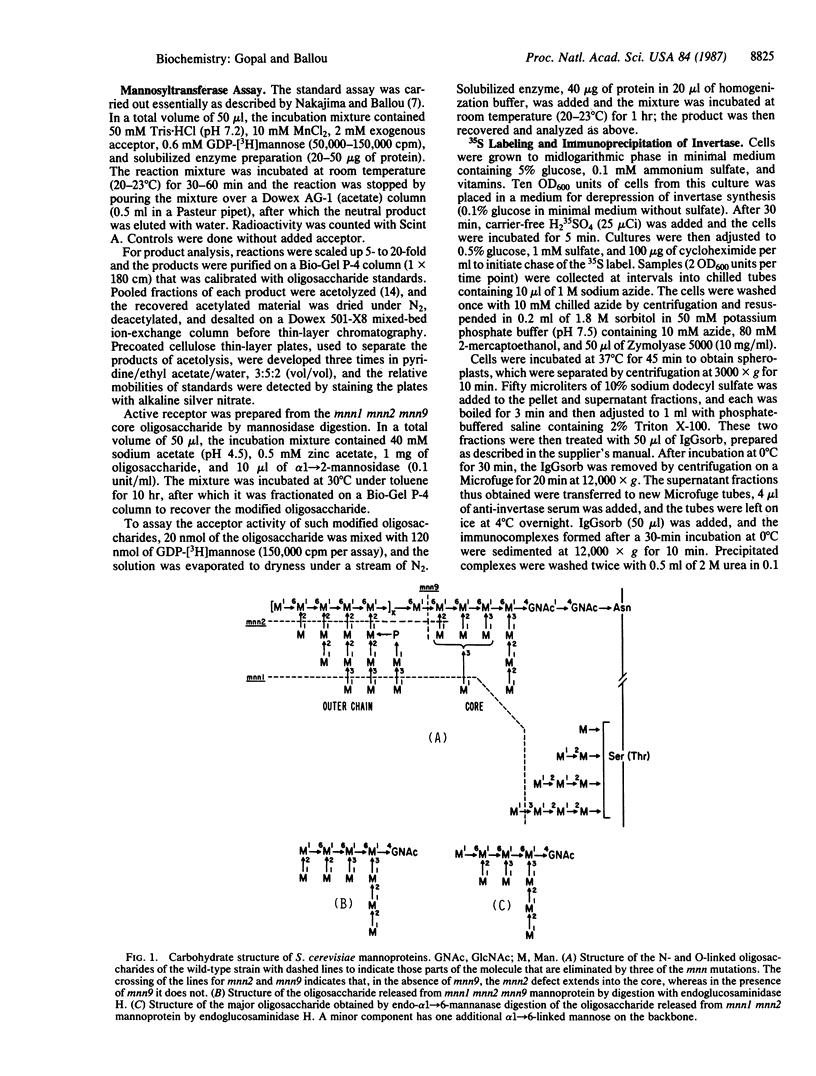
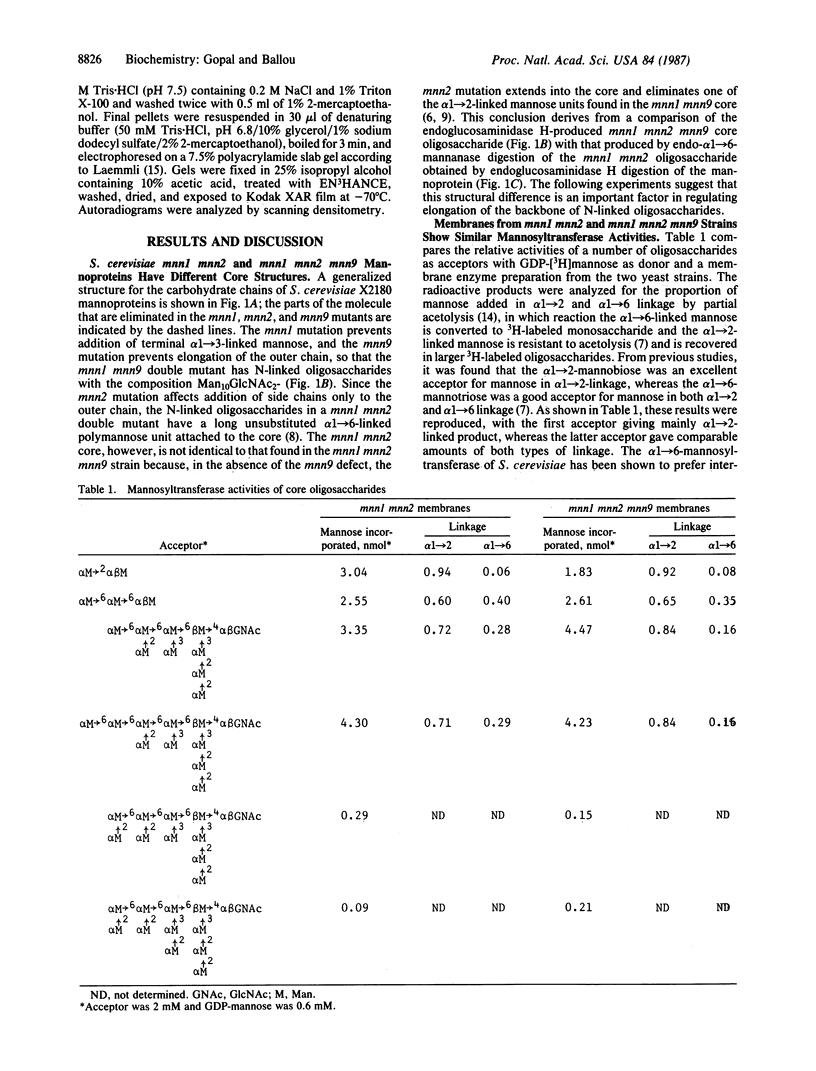
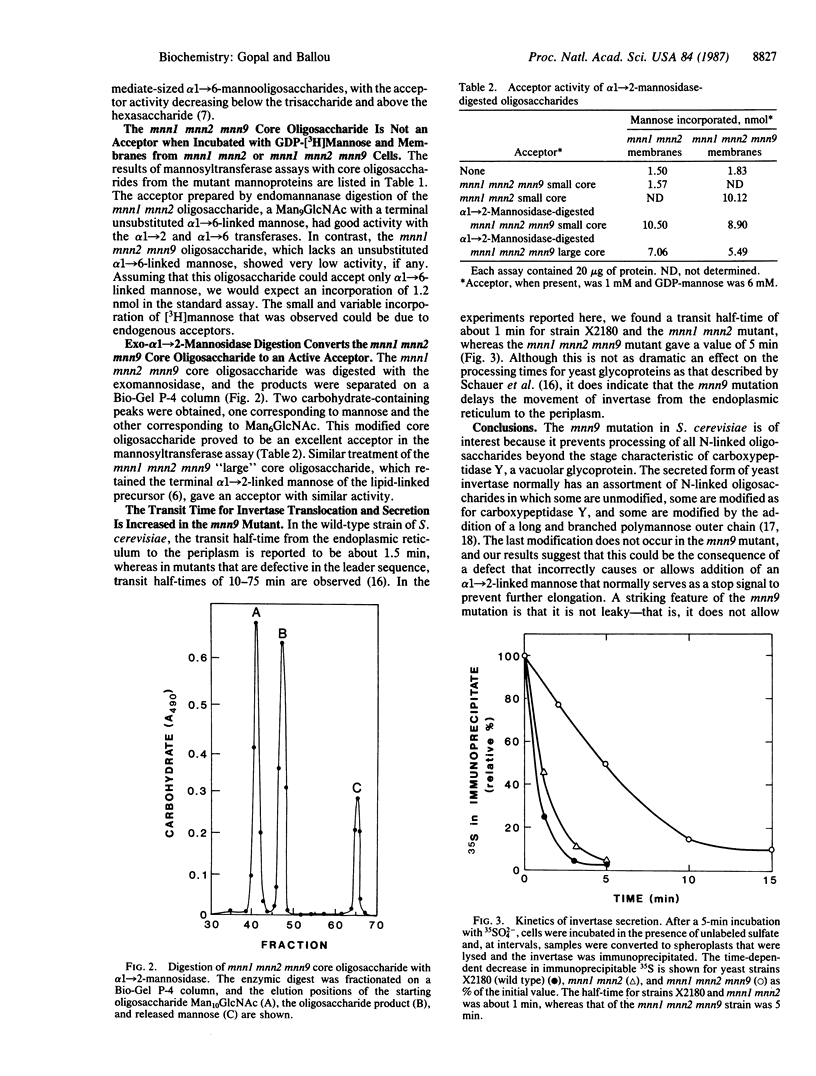
The yeast Saccharomyces cerevisiae X2180 strain with the mnn1 mnn2 mnn9 mutations, all of which affect mannoprotein glycosylation, synthesizes N-linked oligosaccharides having the following structure: (Formula: see text) whereas the mnn1 mnn2 mutant extends the alpha 1----6-linked backbone of some of the core oligosaccharides by adding 20-30 mannose units. Membrane fractions from the mnn1 mnn2 and mnn1 mnn2 mnn9 mutants are equally effective in catalyzing transfer from GDP-[3H]mannose to add mannose in both alpha 1----2 and alpha 1----6 linkages to an oligosaccharide having the following structure: (Formula: see text) but neither membrane preparation can utilize the homologous mnn1 mnn2 mnn9 oligosaccharide as an acceptor. Thus, addition of the alpha 1----2-linked mannose side chain to the terminal alpha 1----6-linked mannose in oligosaccharides of the mnn9 mutant inhibits the elongation reaction and may serve as an important structural control of mannoprotein glycosylation. The mnn9 mutation also increases the transit time for invertase secretion, meaning that this mutation could affect the processing machinery in the Golgi apparatus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. E., Kern K. A., Raschke W. C. Genetic control of yeast mannan structure. Complementation studies and properties of mannan mutants. J Biol Chem. 1973 Jul 10;248(13):4667–4671. [PubMed] [Google Scholar]
- Ballou L., Cohen R. E., Ballou C. E. Saccharomyces cerevisiae mutants that make mannoproteins with a truncated carbohydrate outer chain. J Biol Chem. 1980 Jun 25;255(12):5986–5991. [PubMed] [Google Scholar]
- Cohen R. E., Ballou L., Ballou C. E. Saccharomyces cerevisiae mannoprotein mutants. Isolation of the mnn5 mutant and comparison with the mnn3 strain. J Biol Chem. 1980 Aug 25;255(16):7700–7707. [PubMed] [Google Scholar]
- Cohen R. E., Zhang W., Ballou C. E. Effects of mannoprotein mutations on Saccharomyces cerevisiae core oligosaccharide structure. J Biol Chem. 1982 May 25;257(10):5730–5737. [PubMed] [Google Scholar]
- Ichishima E., Arai M., Shigematsu Y., Kumagai H., Sumida-Tanaka R. Purification of an acidic alpha-D-mannosidase from Aspergillus saitoi and specific cleavage of 1,2-alpha-D-mannosidic linkage in yeast mannan. Biochim Biophys Acta. 1981 Mar 13;658(1):45–53. doi: 10.1016/0005-2744(81)90248-5. [DOI] [PubMed] [Google Scholar]
- Karson E. M., Ballou C. E. Biosynthesis of yeast mannan. Properties of a mannosylphosphate transferase in Saccharomyces cerevisiae. J Biol Chem. 1978 Sep 25;253(18):6484–6492. [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem. 1974 Dec 10;249(23):7685–7694. [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Yeast manno-protein biosynthesis: solubilization and selective assay of four mannosyltransferases. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3912–3916. doi: 10.1073/pnas.72.10.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Maitra S. K., Ballou C. E. An endo-alpha1 leads to 6-D-mannanase from a soil bacterium. Purification, properties, and mode of action. J Biol Chem. 1976 Jan 10;251(1):174–181. [PubMed] [Google Scholar]
- Raschke W. C., Kern K. A., Antalis C., Ballou C. E. Genetic control of yeast mannan structure. Isolation and characterization of mannan mutants. J Biol Chem. 1973 Jul 10;248(13):4660–4666. [PubMed] [Google Scholar]
- Schauer I., Emr S., Gross C., Schekman R. Invertase signal and mature sequence substitutions that delay intercompartmental transport of active enzyme. J Cell Biol. 1985 May;100(5):1664–1675. doi: 10.1083/jcb.100.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Atkinson P. H. Structure of yeast external invertase Man8-14GlcNAc processing intermediates by 500-megahertz 1H NMR spectroscopy. J Biol Chem. 1986 Jul 25;261(21):9815–9824. [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Tsai P. K., Frevert J., Ballou C. E. Carbohydrate structure of Saccharomyces cerevisiae mnn9 mannoprotein. J Biol Chem. 1984 Mar 25;259(6):3805–3811. [PubMed] [Google Scholar]



