Abstract
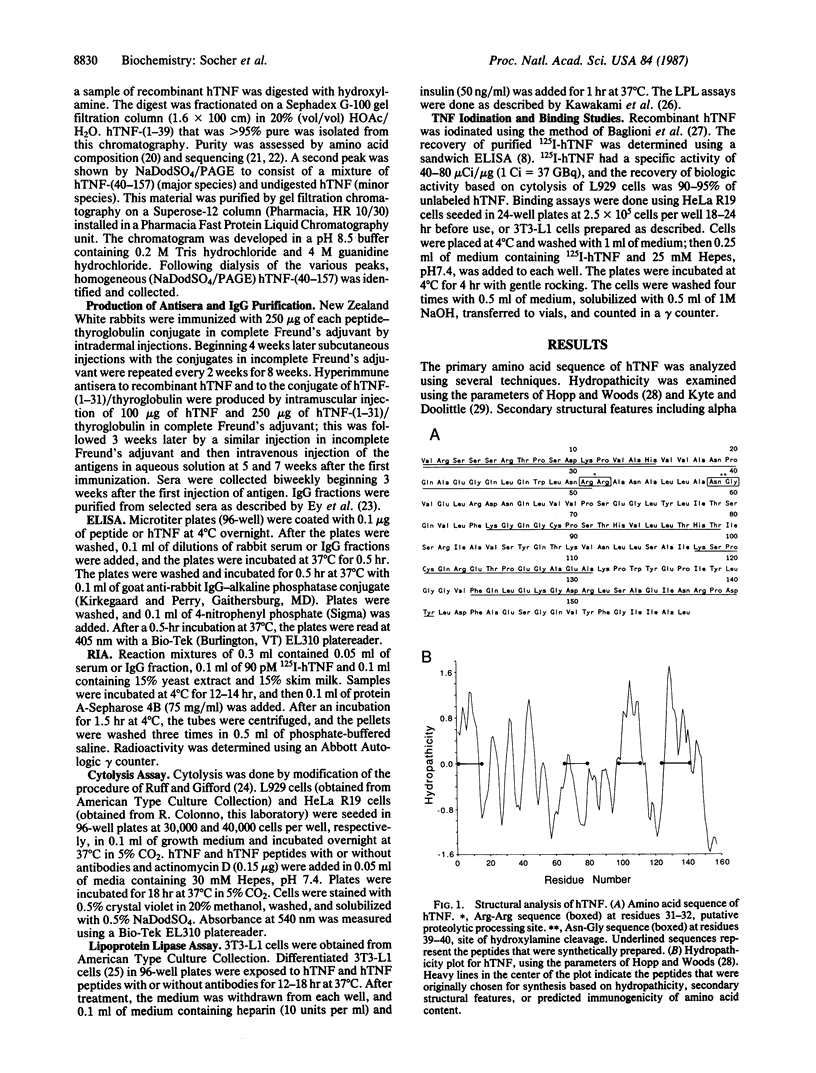
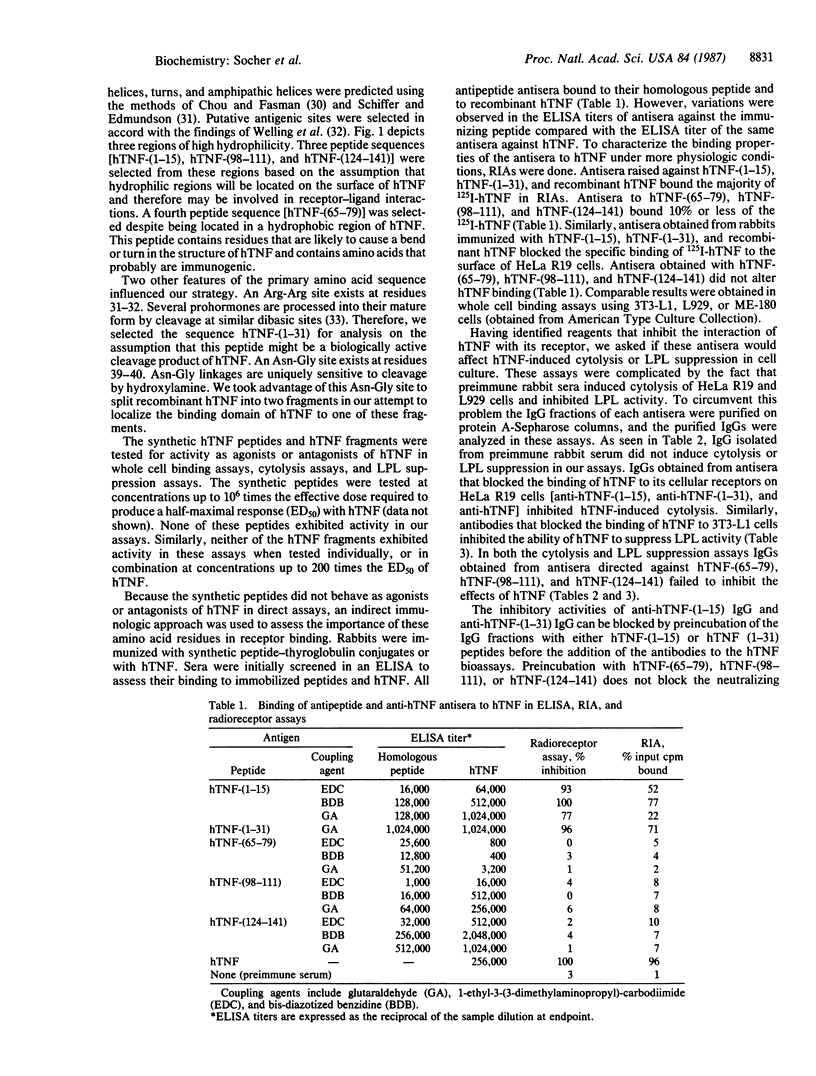
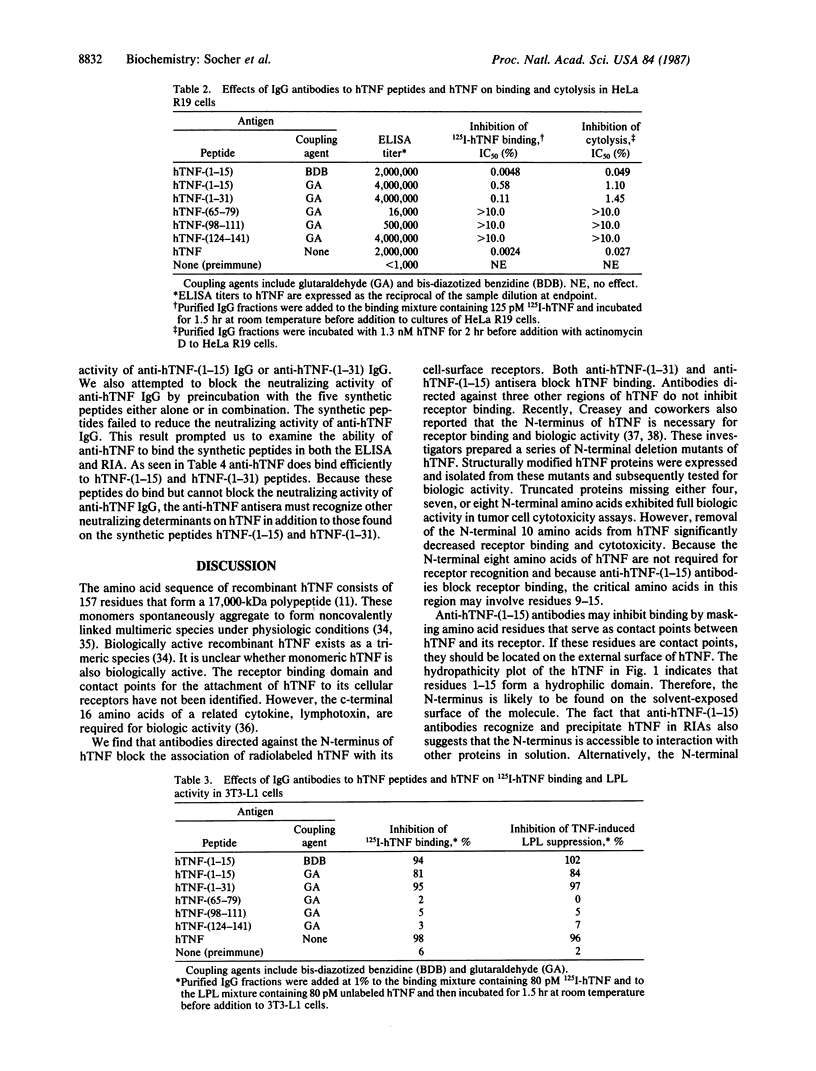
Human tumor necrosis factor (hTNF) mediates a variety of biologic activities, which are dependent on the attachment of hTNF to cell-surface receptors. To identify regions of the hTNF protein involved in binding hTNF to its receptor, we prepared five synthetic peptides [hTNF-(1-15), hTNF-(1-31), hTNF-(65-79), hTNF-(98-111), and hTNF-(124-141)] and two hydroxylamine cleavage fragments [hTNF-(1-39) and hTNF-(40-157)] of hTNF. The hTNF-synthetic peptides and hTNF fragments were tested in hTNF receptor binding assays and in two biologic assays: cytolysis of tumor cells and suppression of lipoprotein lipase in adipocytes. Neither the synthetic peptides nor hTNF fragments were active agonists or antagonists in these assays. The synthetic peptides were also conjugated to thyroglobulin, and peptide-specific antisera were raised. All five peptide-thyroglobulin conjugates induced antibody responses to the immunizing peptide and to hTNF. Each antiserum was tested for antagonist activity in hTNF binding assays. Only antisera raised against hTNF-(1-15) or hTNF-(1-31) and antisera against whole hTNF blocked binding. IgGs purified from these three antisera also block hTNF-induced cytolysis and lipoprotein lipase suppression. We conclude that antibodies that recognize the N-terminus of hTNF block the attachment of hTNF to its cellular receptor and inhibit the biologic effects of hTNF.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., McCandless S., Tavernier J., Fiers W. Binding of human tumor necrosis factor to high affinity receptors on HeLa and lymphoblastoid cells sensitive to growth inhibition. J Biol Chem. 1985 Nov 5;260(25):13395–13397. [PubMed] [Google Scholar]
- Berent S. L., Torczynski R. M., Bollon A. P. Sendai virus induces high levels of tumor necrosis factor mRNA in human peripheral blood leukocytes. Nucleic Acids Res. 1986 Nov 25;14(22):8997–9015. doi: 10.1093/nar/14.22.8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Balian G. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 1977;47:132–145. doi: 10.1016/0076-6879(77)47016-2. [DOI] [PubMed] [Google Scholar]
- Carlino J. A., Lin L. S., Creasey A. A. Use of a sensitive receptor binding assay to discriminate between full-length and truncated human recombinant tumor necrosis factor proteins. J Biol Chem. 1987 Jan 25;262(3):958–961. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Creasey A. A., Doyle L. V., Reynolds M. T., Jung T., Lin L. S., Vitt C. R. Biological effects of recombinant human tumor necrosis factor and its novel muteins on tumor and normal cell lines. Cancer Res. 1987 Jan 1;47(1):145–149. [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Thibault G., Januszewicz P., Cantin M., Genest J. Direct radioimmunoassay of atrial natriuretic factor. Biochem Biophys Res Commun. 1984 Jul 31;122(2):593–601. doi: 10.1016/s0006-291x(84)80074-1. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Marmenout A., Fransen L., Tavernier J., Van der Heyden J., Tizard R., Kawashima E., Shaw A., Johnson M. J., Semon D., Müller R. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. High affinity binding of 125I-labeled human tumor necrosis factor (LuKII) to specific cell surface receptors. J Exp Med. 1985 Sep 1;162(3):1099–1104. doi: 10.1084/jem.162.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Rabbit tumor necrosis factor: mechanism of action. Infect Immun. 1981 Jan;31(1):380–385. doi: 10.1128/iai.31.1.380-385.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Kirstein M., Fiers W., Baglioni C. Species specificity of human and murine tumor necrosis factor. A comparative study of tumor necrosis factor receptors. J Biol Chem. 1986 Nov 15;261(32):14871–14874. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Welling G. W., Weijer W. J., van der Zee R., Welling-Wester S. Prediction of sequential antigenic regions in proteins. FEBS Lett. 1985 Sep 2;188(2):215–218. doi: 10.1016/0014-5793(85)80374-4. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Pain R. H., Craig S. Tumour necrosis factor is a compact trimer. FEBS Lett. 1987 Jan 26;211(2):179–184. doi: 10.1016/0014-5793(87)81432-1. [DOI] [PubMed] [Google Scholar]



