Abstract
Near-surface waters ranging from the Pacific subarctic (58°N) to the Southern Ocean (66°S) contain the neurotoxin domoic acid (DA), associated with the diatom Pseudo-nitzschia. Of the 35 stations sampled, including ones from historic iron fertilization experiments (SOFeX, IronEx II), we found Pseudo-nitzschia at 34 stations and DA measurable at 14 of the 26 stations analyzed for DA. Toxin ranged from 0.3 fg·cell−1 to 2 pg·cell−1, comparable with levels found in similar-sized cells from coastal waters. In the western subarctic, descent of intact Pseudo-nitzschia likely delivered significant amounts of toxin (up to 4 μg of DA·m−2·d−1) to underlying mesopelagic waters (150–500 m). By reexamining phytoplankton samples from SOFeX and IronEx II, we found substantial amounts of DA associated with Pseudo-nitzschia. Indeed, at SOFeX in the Antarctic Pacific, DA reached 220 ng·L−1, levels at which animal mortalities have occurred on continental shelves. Iron ocean fertilization also occurs naturally and may have promoted blooms of these ubiquitous algae over previous glacial cycles during deposition of iron-rich aerosols. Thus, the neurotoxin DA occurs both in coastal and oceanic waters, and its concentration, associated with changes in Pseudo-nitzschia abundance, likely varies naturally with climate cycles, as well as with artificial iron fertilization. Given that iron fertilization in iron-depleted regions of the sea has been proposed to enhance phytoplankton growth and, thereby, both reduce atmospheric CO2 and moderate ocean acidification in surface waters, consideration of the potentially serious ecosystem impacts associated with DA is prudent.
Keywords: carbon sequestration, toxicity, harmful algal blooms
The increasing frequency and geographic extent of toxic algal blooms along populated coastlines is generally attributed to human activities (1, 2). Pseudo-nitzschia, a ubiquitous genus of marine and estuarine diatom, is a relatively recently discovered contributor to such events and includes ≈30 species ranging from tropical to polar waters (3–5). Approximately a dozen Pseudo-nitzschia species are known to produce the amino acid neurotoxin domoic acid (DA) (5, 6). Generally, humans discover phytoplankton toxins, including DA, when consuming contaminated shellfish harvested from coastal waters. In contrast, algal toxins have yet to be detected in commercial oceanic products (e.g., ocean fish harvested for human consumption), leading to the view that toxin-producing algae occur only in coastal areas, not in truly oceanic waters. In the case of Pseudo-nitzschia, however, some researchers suggest that all species will be shown to produce DA when tested with sufficiently sensitive methods (7). Pseudo-nitzschia spp. are implicated in coastal harmful algal blooms (HABs) worldwide (5, 6) and are recognized as contaminating a wide range of animals—from invertebrates to marine birds and mammals (8–10).
With the cosmopolitan nature of many Pseudo-nitzschia (4), and its toxin-producing ability well known in continental margin and shelf regions, the concern becomes whether DA is present in truly oceanic and coastal waters. Coastal populations with particularly high DA cell quotas (pg of DA·cell−1) and cell numbers >104·L−1 can be associated with animal mortalities, with 102 to 104 ng of DA·L−1 levels occurring during blooms (11, 12). In contrast, oceanic Pseudo-nitzschia cells are typically smaller and, hence, likely contain less DA; for example, a recent study shows the small cosmopolitan P. turgidula from an oceanic NE Pacific site to contain ≈0.1 fg of DA·cell−1 in unamended growth containers with local water (13).
The special responsiveness of Pseudo-nitzschia to iron additions is well known and recently has been associated with their uncommon ability to store this trace metal (14). This responsiveness is of particular concern because iron fertilization has been proposed in oceanic, high nutrient, low chlorophyll (HNLC) waters to reduce atmospheric CO2 and moderate ocean acidification through enhanced carbon fixation by phytoplankton (15). Reports of DA from HNLC or truly oceanic waters, either from water samples or clonal cultures, are rare: Field grow outs of oceanic P. turgidula, mentioned above, contain DA in situ (13), whereas earlier studies at the site did not find measurable DA in that same species (16).
Here, we address the possible presence of DA in oceanic waters at various sites in the Pacific and re-examine archived samples from mesoscale iron-enrichment experiments for which one of us (K.H.C.) was expedition leader.
Results
Station Data and Pseudo-nitzschia Abundance.
This study reports results from 35 water samples obtained on five ocean expeditions in the Pacific (Fig. 1 and Fig. S1). Data are summarized in Table 1 and Table S1, including station positions, oceanic regions, Pseudo-nitzschia abundance, DA, and nutrients (iron and nitrate) levels.
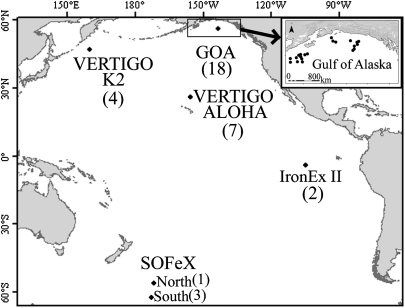
Fig. 1.
Sites of five major ocean research cruises from which samples were obtained for measurements of domoic acid (DA) and/or Pseudo-nitzschia abundance. Samples from GOA-Gulf of Alaska (2007), VERTIGO K2 (2005), and VERTIGO ALOHA (2004) represent natural oceanic waters (i.e., seaward of the continental shelf in ≥200m of water), whereas SOFeX (2002) and IronEx II (1996) samples were obtained from blooms promoted by the artificial addition of Fe, also in deep water (≥1,000m) oceanic regions. The number of stations sampled at each site is given in parentheses. See Table S1 for station locations, geographic descriptions, DA concentrations in cells and water, and Pseudo-nitzschia abundances.
Table 1.
Pseudo-nitzschia abundance, DA cell quotas, and estimated DA concentrations in the waters of six oceanic regions of the Pacific
| Cruise |
||||||
| Measurement | Gulf of Alaska | VERTIGO ALOHA | VERTIGO K2 | IronEx II | SOFeX (N) | SOFeX (S) |
| Location (no. of stations sampled) | NE Subarctic Pacific (18) | Subtropical Pacific (7) | NW Subarctic Pacific (4) | Equatorial Pacific (2) | Sub-Antarctic Pacific (1) | Antarctic Pacific (3) |
| Pseudo-nitzschia abundance, cells·L−1 | 8.6 × 103 ± 1.3 × 104 (n = 14) | 9.8 × 101 ± 2.5 × 102 (n = 14) | 3.4 × 103 ± 3.7 × 103 (n = 11) | 1.3 × 106 ± 1.3 × 106 (n = 2) | 3.7 × 105 (n = 1) | 7.5 × 104 ± 1.3 × 106 (n = 3) |
| Range | b.d.l./4.2 × 104 | b.d.l./9.4 × 102 | 7.6 × 100 to 1.3 × 104 | 3.5 × 105 to 2.3 × 106 | Not applicable | 2.0 × 103 to 2.2 × 105 |
| DA cell quota pg·cell−1 | 0.03 ± 0.07 (n = 6) | m.d. | 0.9 ± 0.7 (n = 4) | 0.04 ± 0.02 (n = 2) | m.d. | 0.85 ± 0.23 (n = 2) |
| Range | 0.0003–0.16 | m.d. | 0.4–1.9 | 0.02–0.05 | m.d. | 0.69–1.0 |
| DA in water range, ng·L−1 | 0.0002–1.4 | m.d. | 1.7–3.6 | 18–45 | m.d. | 2.0–220 |
Average Pseudo-nitzschia abundances, cellular domoic acid (DA) quotas, and water levels of DA are presented here for 35 oceanic stations sampled over 12 y on five different research cruises. Ranges are also presented. The designation “b.d.l.” indicates DA was below the detection limit, which could reflect either the sparcity of the cells in the water or the possibility that many other species were present in the sample, hence making the contribution of DA, even in cells with reasonably high DA levels, a small fraction of the total material in that sample and, thus, below the detection limit. Missing data are indicated by “m.d.” Cell quota averages are based on samples where DA was detected (i.e., measurements that were below our detections limits were not included in the computation of the average) (Table S1, legend). It was assumed all species/cells have equal DA levels. DA in the water was then computed by multiplying the cell abundance by the DA cell quotas (at stations where DA was detected). Values represent means ± 1 SD and sample replicates (n) appear in parentheses. Of 35 stations sampled, Pseudo-nitzschia was present at 34 stations. Samples from 26 stations were analyzed for DA with detectable levels found at 14 stations (see Table S1 for complete data set).
Domoic Acid in Cells and Water.
DA was detected in 14 of 26 samples analyzed for DA by cELISA (Table S1). DA was further verified, structurally, in a sample collected from IronEx II by using LC-MS/MS (Methods). Cell toxin quotas, estimated by dividing cell counts of Pseudo-nitzschia by the particulate DA concentration (i.e., assumes all species/cells have equal DA levels), were 0.3 fg·cell−1 to 0.16 pg·cell−1 at eastern subarctic stations in the Gulf of Alaska, whereas those at VERTIGO K2 were 0.4–1.9 pg of DA·cell−1 (Table 1). In iron-enrichment studies at SOFeX South, DA ranged from 0.7 to 1.0 pg of DA·cell−1; DA in the water reached 220 ng·L−1, the highest level observed in this study (Table 1). IronEx II samples, collected 12 y before our analyses, had Pseudo-nitzschia toxin quotas of 0.02–0.05 pg·cell−1, an order of magnitude lower (possibly related to sample age: see Methods). Pseudo-nitzschia numbers, however, were as high as 106 cells·L−1, and toxin levels reached 45 ng of DA·L−1. The highest cell toxin levels were encountered at VERTIGO K2 and the second highest at SOFeX. The resulting values for DA·cell−1 and DA·L−1 are shown in Tables 1 and 2 and Table S1. There was a highly significant correlation between DA levels and Pseudo-nitzschia abundance at the Pacific sites, indicating waters with high concentrations of this diatom genus typically will have associated high levels of the associated neurotoxin (P < 0.001, n = 25; Fig. S2).
Table 2.
Pseudo-nitzschia species and toxin levels for samples from oceanic Pacific locations in this study
| Cruise/station | Location | Domoic acid, pg·cell−1 | Sample age at time of analysis | Dominant Pseudo-nitzschia species |
| GOA /16 | NE Subarctic Pacific | b.d.l. | 1 mo | P. fraudulenta |
| P. pseudodelicatissima | ||||
| GOA /28 | NE Subarctic Pacific | b.d.l. | 1 mo | P. heimii |
| P. cf granii* | ||||
| P. turgidula*,† | ||||
| GOA /53 | NE Subarctic Pacific | 0.003 | 1 mo | P. turgidula*,† |
| P. pseudodelicatissima | ||||
| P. inflatula | ||||
| GOA /55 | NE Subarctic Pacific | 0.0004 | 1 mo | P. turgidula*,† |
| GOA /58 | NE Subarctic Pacific | 0.0003 | 1 mo | P. pseudodelicatissima |
| P. turgidula*,† | ||||
| GOA /62 | NE Subarctic Pacific | b.d.l. | 1 mo | P.cf lineola† |
| VERTIGO K2 /4 | NW Subarctic Pacific | 0.84 | 2 y | P. turgidula*,† |
| VERTIGO K2 /76 | NW Subarctic Pacific | 1.9 | 2 y | P. turgidula*,† |
| IronEx II /240 | Equatorial Pacific | 0.02 | 12 y | P. inflatula |
| P. delicatissima | ||||
| P. heimii | ||||
| P. cuspidata | ||||
| IronEx II /331 | Equatorial Pacific | 0.05 | 12 y | P. lineola† |
| P. roundii | ||||
| P. turgidula*,† | ||||
| P. heimii | ||||
| SOFeX /36 (South) | Antarctic Pacific | 1.0 | 4 y | P. lineola |
| P. granii*,† | ||||
| P. pseudodelicatissima | ||||
| P. cf turgiduloides | ||||
| SOFeX /40 (South) | Antarctic Pacific | 0.69 | 4 y | Rare overall |
| Mostly P. lineola† |
*Species associated with DA production from grow outs of oceanic phytoplankton samples from station PAPA in the NE Subarctic Pacific and/or from in situ water samples at that station (16).
†Toxin observed from in situ water samples from oceanic stations in the present study.
Species identifications using electron microscopy (SEM and TEM) were made on samples from 12 sites based on the availability of net tow material and corresponding DA samples. The “Domoic acid, pg·cell-1” column presents data showing the average cell quota for the Pseudo-nitzschia in that sample. The designation “b.d.l.” indicates DA was below the detection limit, which could reflect either the sparcity of the cells in the water or the possibility that many other species were present in the sample, hence making the contribution of DA, even in cells with reasonably high DA levels, a small fraction of the total material in that sample and, thus, below detection limit. The “sample age at time of analysis” is the time from collecting the sample at sea until the time the sample was analyzed. “Dominant Pseudo-nitzschia species” were identified from aliquots of the net tows using electron microscopy (Table S2). At 5 of the 12 stations, only 1 species was found and, thus, the DA cell quotas could be assigned to those species, whereas in the 7 other samples 2–4 species were evident and, thus, the listed pg·cell−1 reflects an average for the mix of those species.
Pseudo-nitzschia Species.
Pseudo-nitzschia was observed at 34 of 35 stations in this study, with 11 species of Pseudo-nitzschia identified by electron microscopy from 12 different stations (Table 2 and Table S2). Samples usually contained mixed populations of small oceanic and cosmopolitan Pseudo-nitzschia species. P. turgidula occurred in six samples, five of which contained measureable DA, and was the overwhelming dominant in two of the three samples with DA levels ≥0.8 pg·cell−1. In samples in which only a single Pseudo-nitzschia species was observed and toxin was detected, we attribute toxin quotas to that species: for P.cf turgidula, 0.4 fg–1.9 pg DA·cell−1, for P. lineola 0.7 pg·cell−1, with the latter implicated as a possible toxin producer for the first time.
Daily Sinking Rates of Pseudo-nitzschia Cells.
Intact cells (ones with protoplasts) that were sinking into the mesopelagic zone from the upper ocean were also collected by using sediment traps on VERTIGO cruises. Daily fluxes (cells·m−2·d−1) at 150 m represented from 0.2 to 1% of the overlying euphotic zone Pseudo-nitzschia stocks at K2 and from 0.02–0.04% at the more oligotrophic ALOHA station (Table 3). In one deployment (VERTIGO K2D2), sinking cells at 300 and 500 m exceeded those leaving the euphotic zone by >10-fold, a phenomenon expected at the end of a bloom when deeper cells reflect the larger surface populations from earlier bloom phases (e.g., K2D1).
Table 3.
Pseudo-nitzschia standing stocks, daily fluxes of intact cells, and DA flux at VERTIGO K2 to mesopelagic depths
| VERTIGO NBST traps |
||||
| Measurement | ALOHA D1 | ALOHA D2 | K2 D1 | K2 D2 |
| Euphotic zone depth | 125 m | 125 m | 60 m | 60 m |
| Euphotic zone standing stocks (cells·m−2) | 1.1 × 106 | 2.2 × 107 | 4.2 × 108 | 8.6 × 107 |
| Trap depth, daily cell flux, cells·m−2·d−1 | ||||
| 150 m | 0–3.8 | 9.6 × 103 | 4.0 × 106 | 2.1 × 105 |
| 300 m | b.d.l. | b.d.l. | 1.6 × 106 | 2.3 × 106 |
| 500 m | b.d.l. | b.d.l. | 1.8 × 106 | 1.2 × 106 |
| Cell flux at 150 m as % of Pseudo-nitzschia standing stock | 0.02 | 0.04 | 1 | 0.24 |
| Trap depth, daily DA flux, μg·m−2·d−1 | ||||
| 150 m | m.d. | m.d. | 2.2 | 0.4 |
| 300 m | m.d. | m.d. | 0.9 | 4.4 |
| 500 m | m.d. | m.d. | 1.0 | 2.3 |
Pseudo-nitzschia cell fluxes were obtained from counts made on material collected from neutrally buoyant sediment traps (NBST) deployed for 3–5 d at 150, 300, and 500 m on the VERTIGO cruises. DA fluxes were calculated based on DA cell quotas that were obtained from DA measurements on corresponding phytoplankton net tow samples taken during the deployments. Euphotic zone depths (1% light level) were 125 m for ALOHA and 60 m for K2. Standing stocks of Pseudo-nitzschia were derived from counts on water samples taken near the surface (25 m at ALOHA, 25 m at K2) and near the base of the euphotic zones (125 m at ALOHA, 40 m at K2). The designation b.d.l. indicates “below detection limit”. Missing data are indicated by “m.d.” (Materials and Methods and SI Methods).
Discussion
Oceanic Distribution of Pseudo-nitzschia and DA.
Our results show that oceanic waters throughout the Pacific contain Pseudo-nitzschia populations with associated DA, a neurotoxin shown to cause harm to marine communities in productive coastal waters (11). Considering results from 14 field sites in the Pacific, we find DA concentrations in the water to be significantly (P < 0.001) related to Pseudo-nitzschia concentrations, which have reached 105 to 106 cells·L−1 during artificial iron experiments. To date, toxic algae have largely been regarded as constituents of coastal phytoplankton communities, where the toxins have been discovered after highly publicized events of human illness resulting from consumption of contaminated shellfish (17). Recently, DA-producing Pseudo-nitzschia have been shown to be responsible for contaminating pelagic and benthic invertebrates, marine mammals, and even whales in nearshore environments (9, 12, 18, 19). High cell abundances of Pseudo-nitzschia (e.g., densities of 104 to 106 cells·L−1, or even higher) of the large coastal species that produce picogram levels of cellular DA have allowed detection of the toxin in natural waters, especially given the advent of highly sensitive methods such as the ELISA. Oceanic Pseudo-nitzschia, however, are typically less abundant in waters overlying the deep ocean basins, smaller in size, and—with the exception of the recent report by Trick and colleagues (13) of low (femtogram) DA cell quotas and pg of DA·L−1 in truly oceanic waters—have not been known to produce this toxin.
Natural Variability in DA Levels of Pseudo-nitzschia.
Cellular DA quotas in field populations from unfertilized, oceanic waters reported here (Table 1 and Table S1) are at the low (fg of DA·cell−1) to moderate (pg of DA·cell−1) range of those reported from coastal waters. Populations from continental margin and near-coastal regions commonly contain large species (e.g., P. multiseries or P. australis) that can have high in situ toxin quotas, often pg of DA·cell−1 levels (in the case of P. australis, up to 75 pg·cell−1) (12), whereas coastal waters dominated by small species may average <1 pg of DA·cell−1 (12, 20–22). In the present study, most samples contained a variable mix of species, thus likely contributing to some of the toxicity differences among stations. However, three stations were dominated by one species, namely P. turgidula, and cellular DA levels varied by >3 orders of magnitude, i.e., from 0.4 fg·cell−1 in the Northeast (NE) Subarctic (similar to a value recently described from the region; ref. 13) to 1.9 pg·cell−1 in the Northwest (NW) Subarctic Pacific (Tables 1 and 2 and Table S1). Similarly, two stations were dominated by P. lineola—previously not associated with toxin production in oceanic waters—and its cellular DA levels varied from below detection limits (b.d.l.) in the NE Subarctic Pacific to 0.69 pg·cell−1 in the Antarctic Pacific (Tables 1 and 2 and Table S1). These results show that, even within one morphological “species,” cell quotas are highly variable in the field. Indeed, laboratory studies show that multiple environmental variables affect cell toxicity in a species, and toxin quotas in the laboratory may be considerably different (by orders of magnitude) than those in field populations dominated by the same morphological species, presumably due to such factors as the physical and chemical environment, as well as strain differences between field populations and laboratory isolates (21). Numerous chemical variables affect cell toxicity, including concentrations of major limiting nutrients (N, P, Si) (22), trace metals (Fe, Cu) (23–25), and pH (26), to name a few.
Toxin Measurements from Oceanic Iron Fertilization Studies.
Given that archived samples were collected a number of years before the time of analysis, (12 y for IronEx II and 4 y for SOFeX South), toxin estimates presented here may be conservative, if DA degraded during storage (Methods and SI Methods). Our ability to measure toxins, even where toxin quotas were low, was due to our concentration of large numbers of cells in net tows, in contrast to Trick et al. (13), who indicated that previous studies might not have detected DA in oceanic samples because stored, preserved samples were analyzed.
Relevance to Geo-Engineering Solutions for Atmospheric CO2 Reduction and Moderation of Ocean Acidification.
Our findings have consequences for proposals to enhance phytoplankton production in HNLC oceanic waters by iron fertilization, as a geo-engineering solution to sequester atmospheric CO2 (27). Over a dozen iron-enrichment studies have been conducted in situ in HNLC environments over more than a decade to test the linkage among iron supply, glacial increases in marine production, and atmospheric CO2 levels. These studies show that Pseudo-nitzschia has frequently been the major diatom responding to iron fertilization (15). Iron plays a prominent role in many enzyme reactions (including nitrate reductase and chlorophyll synthetase), electron transport, and biosynthetic pathways (28). DA, likely related to its chelation affinity for trace metals (29, 30), has been shown to increase iron uptake by Pseudo-nitzschia (25). Furthermore, a recent study (14) shows that Pseudo-nitzschia and a closely related genus are unique among diatoms in their use of ferritin, an iron-storage protein. [Although many other marine diatoms and other algae have iron storage capabilities (28), the mechanisms remain unknown.] In low iron regions of the oceans where inputs of iron are episodic, these characteristics may be adaptive. As such, stored iron becomes available to support further growth when environmental levels become limiting to other local photosynthetic organisms and likely contributes to the success of Pseudo-nitzschia in oceanic environments where iron additions are intermittent. Using several assumptions, Trick et al. (13) calculated the diatom biomass increase resulting from Fe additions in HNLC regions that was needed to provide C export from the surface mixed layer to meet commercial carbon credit goals. They assumed that all C uptake was due to the growth of Pseudo-nitzschia, specifically P. turgidula that contained 15 fg of DA·cell-1. Their results indicated that the responding Pseudonitzschia bloom would result in 1–2 μg DA·L−1 and led them to reject iron fertilization as a responsible carbon sequestration option.
In this present study, we show that high levels of DA have been generated in historic iron enrichment experiments. At IronEx II and SOFeX South, relatively high Pseudo-nitzschia abundances (106 and 105 cells·L−1 respectively), and moderate DA quotas (0.05 and 1 pg of DA·cell−1, respectively) led to high toxin levels in the water: 45 and 220 ng of DA·L−1 respectively. Such DA concentrations approach levels associated with harm to marine predators in coastal waters (11, 12). Ecosystem linkages between DA production and neurotoxin impacts at higher trophic levels, well known in shelf and coastal regions, have not yet been reported in open ocean systems. We show here, however, that Pseudo-nitzschia populations and their associated DA in iron-fertilized HNLC regions have reached levels known to pose an ecosystem threat: An outcome that must be considered when weighing the environmental consequences of climate change (global warming, ocean acidification, sea level rise, increased storm severity, and drought) against others (production of marine toxins and other factors associated with eutrophication).
DA Transport to Mesopelagic Depths.
Our data also suggest that DA can be delivered to depths below the euphotic zone in HNLC waters when Pseudo-nitzschia blooms sink from surface waters. Recent studies have shown that particulate organic matter containing DA, including Pseudo-nitzschia cells with intact protoplasts, occurs in sediment traps as deep as 800 m on the California continental margin (31). In that study, the flux was tightly correlated, and slightly time-lagged, with respect to the overlying toxic blooms and cell descent was rapid, presumably a consequence of surface cell aggregation (32). Our VERTIGO samples from sediment traps at the oceanic North Pacific site, K2, also contained intact Pseudo-nitzschia cells from surface populations of DA-containing Pseudo-nitzschia (Table 3). Although the aliquots available to us from the sediment traps at VERTIGO were too small to test for DA, the presence of intact cells suggests toxic material likely was being delivered, possibly 1–2 μg·m−2·d−1, an amount ≈20% of that found on the California borderland (31). Such delivery rates into the mesopelagic zone could have physiological consequences for underlying communities, even at the present time, under natural conditions.
Enrichment of Surface Ocean Waters with Iron as a Natural Phenomenon.
The deposition of iron-containing dust in the ocean is well known, and, in part, led to the original suggestion by John Martin (33) that aerosol-derived iron delivery to iron-limited HNLC regions of the ocean could result in reduced atmospheric CO2 levels on glacial/interglacial time scales. The addition of iron to waters with otherwise sufficient nutrients to sustain phytoplankton growth results in sequestering atmospheric CO2, when the carbon, now trapped as particulate organic matter in the phytoplankton, settles below the surface mixed layer, no longer exchangeable with the atmosphere. The question is, then, whether periodic iron enrichment by natural aerosols may have resulted in past enhancements of Pseudo-nitzschia growth and, subsequently, in increased levels of DA in the open ocean. Were that the case, toxin-producing phytoplankton may have been a phenomenon not only of modern coastal waters, but also an alternating presence in oceanic waters experiencing natural climate variability. Our results, then, raise the possibility that pelagic communities have already experienced periods of higher neurotoxin concentrations over longer, possibly evolutionary time scales.
In summary, the presence of neurotoxin-producing phytoplankton is a common feature in open ocean environments, and these cells are frequent responders to iron additions. Natural additions of iron occur cyclically on millennial time scales (33), suggesting an associated variation in Pseudo-nitzschia abundance over glacial/interglacial time scales. With the growing acceptance that anthropogenic activities are profoundly affecting today's global ocean, with nearly half of ocean ecosystems strongly and negatively impacted (34), practical, near-term solutions to the climatological impacts of CO2-driven global warming are both timely and necessary. The challenge, however, is to understand, within actionable limits of confidence, the ecosystem consequences of such purposeful manipulations. This study demonstrates that the enhancement of Pseudo-nitzschia, which frequently occurs with oceanic iron fertilization, can result in high and potentially problematic levels of the neurotoxin domoic acid in such environments. This study also suggests that increased attention to such ecosystem alterations is needed before iron fertilization can be recommended as a responsible strategy to reduce atmospheric CO2.
Methods
Sample Sources.
Euphotic-zone samples of phytoplankton were obtained from five oceanic expeditions (Fig. 1 and Fig. S1). Three of these were natural populations from the North Pacific, and two were populations that developed during mesoscale iron-fertilization experiments in the South Pacific. The North Pacific Gulf of Alaska (GOA) cruise provided samples at 18 sites. The two VERTIGO expeditions (35), also in the North Pacific at ocean observing stations, included one in the northwest Subarctic gyre waters near K2 and the other in subtropical central gyre waters near ALOHA. Both expeditions provided euphotic-zone water as well as sediment trap samples from 150- to 500-m depths. We also reexamined surface water, net-tow samples, and corresponding water samples from K2 and two mesoscale iron-fertilization experiments: SOFeX (2002, Sub-Antarctic and Antarctic Pacific) (36) and IronEx II (1995, Eastern Equatorial Pacific) (37). (See SI Methods and Table S1 for the complete dataset.)
Natural Abundances of Pseudo-nitzschia in the Euphotic Zone.
Water from Niskin bottles provided samples of Pseudo-nitzschia at euphotic zone depths (10–30 m) for cell counts. On the GOA, SOFeX, and IronEx II cruises, counts were made from filter preparations. Counts on the VERTIGO samples were made on 50–100 mL preserved (4% borate buffered formalin) and DAPI-stained aliquots in the shore laboratory by using Utermöhl methods (38) (see SI Methods for details).
Pseudo-nitzschia Standing Stock and Flux Estimates.
On VERTIGO cruises, we measured both euphotic zone stocks and cells with intact protoplasts descending to mesopelagic depths (Table 3). Replicate counts were made from the upper and lower euphotic zone several times during sediment trap deployments. These values were then averaged and integrated over the depth of the euphotic zone [120 m at ALOHA, and 60 m at K2 (39) to estimate Pseudo-nitzschia standing stocks (cells·m−2) during deployment periods (SI Methods)].
During the two VERTIGO cruises, at stations ALOHA and K2, we sampled sinking material from four deployments, using neutrally buoyant sediment traps (39). Each 3- to 5-d deployment consisted of replicate traps at 150, 300, and 500 m. Counts of sinking cells were made from settled subfractions of the trap material (SI Methods). From deployment duration and trap surface collection area, we estimated fluxes of Pseudo-nitzschia (i.e., cells·m2·d−1). By dividing this flux by the integrated Pseudo-nitzschia cell population (standing stock) in the overlying euphotic zone, we estimated the % surface standing stock of Pseudo-nitzschia reaching trap depths daily (SI Methods).
Electron Microscopy: Pseudo-nitzschia Studies.
Net tows provided material for identification of species from selected sites. We examined selected samples for which we also had abundance estimates and detectable DA. Both scanning (SEM) and transmission (TEM) electron microscopy were used on the natural mixes of phytoplankton, using methods slightly modified from the literature (9, 40) (SI Methods and Table S2).
Collecting and Preparation of Phytoplankton for Domoic Acid Analyses.
Based on the detection limit of the indirect cELISA (ELISA), ≈10 pg of DA·mL−1, and the anticipated low cellular DA level, we recognized the need to harvest sufficient cells to detect DA, especially because filters might clog from other, more abundant material in the water. Therefore, we concentrated the larger phytoplankton, including chain-forming Pseudo-nitzschia, by using 20- to 30-μm mesh phytoplankton nets hauled from 10 to 15 m to the surface. Such net tows provided the concentrates used to measure DA on the five expeditions.
Net-tow samples for DA analyses were handled differently, depending on the expedition. On the GOA cruise, a measured volume of the net tow concentrate was gently filtered immediately after collection onto 25 mm GF/F filters and placed in vials in liquid nitrogen for DA analysis at the shore laboratory (completed within 6 mo of collection). The remaining cell concentrates from net tows were fixed with 4% hexamine-buffered formalin and stored in glass containers in the dark at 4 °C for counts (cells·ml−1) and EM species confirmation. In the laboratory, the frozen filters containing particulate, cellular material that included Pseudo-nitzschia cells were placed in vials containing 10 mL of 20% methanol. The vials were vortexed, sonicated for 2 min (30–40 W) by using a Misonix Sonicator 3000 probe, and centrifuged for 10 min at 3000 × g to free water-soluble DA possibly remaining in the cellular matrix. After mixing, the samples were syringe-filtered through 0.2-μm polycarbonate membrane filters, and the supernatant diluted 1:10 (minimum) with Standard/Sample buffer to minimize interferences.
On VERTIGO, SOFeX, and IronEx II cruises, DA was measured in archived net-tow samples fixed with 4% formalin at sea and stored for periods from 2 y (VERTIGO) to 12 y (IronEx II) (Table 2). Storage temperatures varied: VERTIGO samples at 4 °C and SOFeX and IronEx II at room temperature. We anticipated some leakage of water-soluble DA from cells into the seawater during storage. Thus, we measured DA both in filter concentrates and filtrate. Filters were treated as described above (SI Methods).
ELISA Assay for Domoic Acid.
DA was measured routinely by using high-sensitivity Biosense cELISA kits, using protocols provided by the manufacturer (41, 42). cELISA measures both DA and, to some extent, its isomers. We chose cELISA rather than HPLC for routine measurements because of the former's higher sensitivity and ability to measure DA in formalin-fixed samples. HPLC, in contrast, cannot accurately measure DA in samples with formalin (41, 42). Each sample was run in duplicate and at several dilutions. The color intensity was read by using a standard microplate absorbance reader at 450 nm (see SI Methods for complete method).
Structural Confirmation of the Toxin.
Net tow material from IronEx II, the oceanic iron fertilization experiment with high concentrations of Pseudo-nitzschia, was selected for analysis, because higher toxin levels are required for structural confirmation of DA as compared with quantization by cELISA methods. We used liquid chromatographic separation coupled with mass spectrometric detection (LC-MS/MS) to confirm DA presence in a formalin-preserved net-tow sample, mostly using methods described (31). DA was identified and quantified from the signal of the parent mass (312.14 Da) and two daughter masses (266.10 and 168.00 Da). Samples were analyzed in the multiple reaction monitoring mode, using an Agilent 1100 HPLC coupled to a Micromass-Quattro mass spectrometer equipped with an electrospray ion-spray, with methods adapted from Burns and Ferry (43). The mobile phase was a mixture of 0.1% aqueous formic acid in deionized water (A) and 0.1% aqueous formic acid in acetonitrile (B). The initial condition was 95:5 A/B for 4 min, followed by a linear gradient over 11 min ending at 5:95 A/B. The ratio of A and B was reset to the initial condition over the following 7 min to reestablish initial conditions. A 4-min solvent diversion was used to avoid salt contamination of the ion source. Additional modifications included a sample injection volume of 20 μL and the use of caffeine as an internal standard. Caffeine was monitored from the parent mass (195.10 Da) and two daughter masses (138.00 and 110.00 Da). The detection limit for the filtrate was ≈1.2 ng DA. LC-MS/MS DA concentrations in IronEx II averaged 161 ± 30 μg of DA·L−1, comparable with the 130 μg·L−1 measured by cELISA in the same sample.
Supplementary Material
Acknowledgments
Invaluable help was provided by S. Bates, who reviewed the manuscript; N. Lundholm, who confirmed Pseudonitzschia species in Iron-Ex II and SOFeX samples; K. Buessler for his leading role on VERTIGO cruises; and S. Tanner, S. Smith, and T. Coale for invaluable assistance at sea on Vertigo and GOA cruises. We acknowledge helpful comments of four anonymous reviewers of this manuscript. Funding sources included GOA-NSF OCE-0741481; Vertigo-NSF OCE-0327640; SOFeX-National Science Foundation OCE-9911481 (to K.H.C.) and OCE-9530762 (to K.H.C.); the Department of Energy DE-FG03-01ER63093 and Subcontract 6491879 (to K.H.C.); IronEx II-US Office of Naval Research Grant N00014-94-1-0125 (to K.H.C.), and by National Science Foundation Grant OCE-90 -9217518 (to K.H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006968107/-/DCSupplemental.
References
- 1.Parsons ML, Dortch Q, Turner RE. Sedimentological evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnol Oceanogr. 2002;47:551–558. [Google Scholar]
- 2.Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- 3.Hubbard KA, Rocap G, Armbrust EV. Inter-and intraspecific community structure within the diatom genus Pseudo-nitzschia (Bacillariophyceae) J Phycol. 2008;44:637–649. doi: 10.1111/j.1529-8817.2008.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasle GR. Are most of the domoic acid-producing species of the diatom genus Pseudo-nitzschia cosmopolites? Harmful Algae. 2002;1:137–146. [Google Scholar]
- 5.Bates SS. Domoic-acid producing diatoms: Another genus added! J Phycol. 2000;36:978–985. [Google Scholar]
- 6.Trainer VL, Hickey BM, Bates SS. Diatoms. In: Walsh PJ, et al., editors. Oceans and Human Health: Risks and Remedies from the Sea. New York: Elsevier Science Publishers; 2008. pp. 219–238. [Google Scholar]
- 7.Wells ML, Trick CG, Cochlan WP, Hughes MP, Trainer VL. Domoic acid: The synergy of iron, copper, and the toxicity of diatoms. Limnol Oceanogr. 2005;50:1908–1917. [Google Scholar]
- 8.Kvitek RG, et al. Domoic acid contamination within eight representative species from the benthic food web of Monterey Bay, California. Mar Ecol Prog Ser. 2008;367:35–47. [Google Scholar]
- 9.Bargu S, Coale SL, Busman M, Doucette GJ, Silver MW. Krill: A potential vector of domoic acid in marine food webs. Mar Ecol Prog Ser. 2002;237:209–216. [Google Scholar]
- 10.Work TM, et al. In: Toxic Phytoplankton Blooms in the Sea. Smayda TJ, editor. Amsterdam: Elsevier; 1993. pp. 643–649. [Google Scholar]
- 11.Schnetzer A, et al. Blooms of Pseudo-nitzschia and domoic acid in the San Pedro Channel and Los Angeles harbor areas of the Southern California Bight, 2003-2004. Harmful Algae. 2007;5:372–387. [Google Scholar]
- 12.Scholin CA, et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–84. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- 13.Trick CG, et al. Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proc Natl Acad Sci USA. 2010;107:5887–5892. doi: 10.1073/pnas.0910579107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchetti A, et al. Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature. 2009;457:467–470. doi: 10.1038/nature07539. [DOI] [PubMed] [Google Scholar]
- 15.de Baar HJW, et al. Synthesis of iron fertilization experiments: From the iron age in the age of enlightment. J Geophys Res Oceans. 2005;110:1–24. [Google Scholar]
- 16.Marchetti A, et al. Identification and assessment of domoic acid production in oceanic Pseudo-nitzschia (Bacillariophyceae) from Iron-limited waters in the northeast Subarctic Pacific. J Phycol. 2008;44:650–661. doi: 10.1111/j.1529-8817.2008.00526.x. [DOI] [PubMed] [Google Scholar]
- 17.Silver MW. Protecting ourselves from shellfish poisoning. Am Sci. 2006;94:316–325. [Google Scholar]
- 18.Fire SE, et al. Domoic acid exposure in pygmy and dwarf sperm whales (Kogia spp.) from southeastern and mid-Atlantic U.S. waters. Harmful Algae. 2009;8:658–664. [Google Scholar]
- 19.Lefebvre KA, Bargu S, Kieckhefer T, Silver MW. From sanddabs to blue whales: The pervasiveness of domoic acid. Toxicon. 2002;40:971–977. doi: 10.1016/s0041-0101(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 20.Baugh KA, Bush KM, Bill BD, LeFebvre KA, Trainer VL. Estimates of specific toxicity in several Pseudonitzschia species from the Washington coast, based on culture and field studies. African J Mar Sci. 2006;28:403–407. [Google Scholar]
- 21.McDonald SM, Sarno D, Zingone A. Identifying Pseudo-nitzschia species in natural samples using genus-specific PCR primers and clone libraries. Harmful Algae. 2007;6:849–860. [Google Scholar]
- 22.Bates SS. In: Physiological Ecology of Harmful Algal Blooms. Anderson DM, Cembella AD, Hallegraeff GM, editors. Heidelberg, Germany: Springer; 1998. pp. 405–426. [Google Scholar]
- 23.Ladizinsky NC. Moss Landing Marine Laboratories; 2003. The influence of dissolved copper on the production of domoic acid by Pseudo-nitzschia species in Monterey Bay, California: Laboratory experiments and field observations. Master's Thesis. [Google Scholar]
- 24.Maldonado M, Hughes M, Rue E, Wells MW. The effect of Fe and Cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol Oceanogr. 2002;47:515–526. [Google Scholar]
- 25.Rue E, Bruland K. Domoic acid binds iron and copper: A possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar Chem. 2001;76:127–134. [Google Scholar]
- 26.Lundholm N, Hansen PH, Kotaki Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar Ecol Prog Ser. 2004;273:1–15. [Google Scholar]
- 27.Chisholm SW, Falkowski PG, Cullen JJ. Oceans. Dis-crediting ocean fertilization. Science. 2001;294:309–310. doi: 10.1126/science.1065349. [DOI] [PubMed] [Google Scholar]
- 28.Geider RJ, La Roche J. The role of iron in phytoplankton photosynthesis and the potential for iron-limitation of primary productivity in the sea. Photosynthesis Res. 1994;39:275–301. doi: 10.1007/BF00014588. [DOI] [PubMed] [Google Scholar]
- 29.Rue EL, Bruland KW. The role of organic complexation on ambient iron chemistry in the equatiorial Pacific ocean and the response of a mesoscale iron addition experiment. Limnol Oceanogr. 1997;42:901–910. [Google Scholar]
- 30.Sunda WG, Huntsman SA. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar Chem. 1995;50:189–206. [Google Scholar]
- 31.Sekula-Wood E, et al. Rapid downward transport of the neurotoxin domoic acid in coastal waters. Nat Geosci. 2009;2:272–275. [Google Scholar]
- 32.Alldredge AL, Gotschalk CC. Direct observations of the mass flocculation of diatom blooms: Characteristics, settling velocities and formation of diatom aggregates. Deep-Sea Res. 1989;36:159–171. [Google Scholar]
- 33.Martin JM. Glacial-interglacial CO2 change: The iron hypothesis. Paleoceanogr. 1990;5:1–13. [Google Scholar]
- 34.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 35.Buessler KO, et al. VERTIGO (VERtical Transport in the Global Ocean): A study of particle sources and flux attenuation in the North Pacific. Deep Sea Res Part II Top Stud Oceanogr. 2008;55:1522–1539. [Google Scholar]
- 36.Coale KH, et al. Southern Ocean iron enrichment experiment: Carbon cycling in high- and low-Si waters. Science. 2004;304:408–414. doi: 10.1126/science.1089778. [DOI] [PubMed] [Google Scholar]
- 37.Coale KH, et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature. 1996;383:495–501. doi: 10.1038/383495a0. [DOI] [PubMed] [Google Scholar]
- 38.Utermöhl H. Toward the improvement of the quantitative phytoplankton method (German) Mitteilungen International Vereiningung für Limnologie. 1958;9:1–39. [Google Scholar]
- 39.Buesseler KO, et al. Revisiting carbon flux through the ocean's twilight zone. Science. 2007;316:567–570. doi: 10.1126/science.1137959. [DOI] [PubMed] [Google Scholar]
- 40.Miller PE, Scholin CA. Identificqtion and enumeration of cultured and wild Pseudo-nitzschia (Bacillariophyceae) using species-specific LSU rRNA-targeted fluorescent probes and filter-based whole cell hybridization. J Phycol. 1998;34:371–382. [Google Scholar]
- 41.Kleivdal H, Kristiansen SI, Nilsen MV, Briggs L. Single-laboratory validation of the biosense direct competitive enzyme-linked immunosorbent assay (ELISA) for determination of domoic acid toxins in shellfish. J AOAC Int. 2007;90:1000–1010. [PubMed] [Google Scholar]
- 42.Kleivdal H, et al. Determination of domoic acid toxins in shellfish by biosense ASP ELISA—a direct competitive enzyme-linked immunosorbent assay: Collaborative study. J AOAC Int. 2007;90:1011–1027. [PubMed] [Google Scholar]
- 43.Burns JM, Ferry JL. Adsorption of domoic acid to marine sediments and clays. J Environ Monit. 2007;9:1373–1377. doi: 10.1039/b713101a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.