Abstract
Viral infectivity factor, an accessory protein encoded in the HIV-1 genome, induces G2 cell cycle arrest; however, the biological significance and mechanism(s) remain totally unclear. Here we demonstrate that the TP53 pathway is involved in Vif-mediated G2 cell cycle arrest. Vif enhances the stability and transcriptional activity of TP53 by blocking the MDM2-mediated ubiquitination and nuclear export of TP53. Furthermore, Vif causes G2 cell cycle arrest in a TP53-dependent manner. HXB2 Vif lacks these activities toward TP53 and cannot induce G2 cell cycle arrest. Using mutagenesis, we demonstrate that the critical residues for this function are located in the N-terminal region of Vif. Finally, we construct a mutant NL4-3 virus with an NL4-3/HXB2 chimeric Vif defective for the ability to induce cell cycle arrest and show that the mutant virus replicates less effectively than the wild-type NL4-3 virus in T cells expressing TP53. These data imply that Vif induces G2 cell cycle arrest through functional interaction with the TP53/MDM2 axis and that the G2 cell cycle arrest induced by Vif has a positive effect on HIV-1 replication. This report demonstrates the molecular mechanisms and the biological significance of Vif-mediated G2 cell cycle arrest for HIV-1 infection.
Keywords: AIDS, NL4-3, HXB2, APOBEC3G, infectivity
HIV-1 viral infectivity factor (Vif), one of six HIV-1 accessory proteins, plays a crucial role in the viral life cycle by antagonizing the host restriction factors APOBEC3G (A3G) and APOBEC3F (A3F) (1, 2). Vif forms a ubiquitin ligase (E3) complex with Cullin5 and Elongin B/C (Vif-BC-Cul5) and functions as a substrate recognition subunit of this complex to induce the ubiquitination and subsequent degradation of A3G/F (3, 4). Two recent studies not only implicate Vif as a unique viral protein involved in G2 arrest, but also suggest that the induction of G2 arrest is a unique Vif function (5, 6). However, neither study addresses the mechanism by which Vif induces G2 arrest or the question of why HIV-1 uses two different viral proteins to arrest cells in the G2 phase of the cell cycle.
Multiple overlapping TP53-dependent and TP53-independent pathways regulate the G2/M transition in response to genotoxic stress (7–9). In the TP53-dependent pathway, TP53 inhibits Cdc2 activity through its transcriptional targets, including p21 (10), GADD45 (11), and 14–3-3σ (12). In the TP53-independent pathways, activation of the protein kinases Chk1 and Chk2 by Atm and Atr inhibits Cdc2 by inactivating Cdc25, the phosphatase that normally activates Cdc2. Viral protein R (Vpr) has been shown to affect several proteins involved in the G2/M transition, including Cdc2, Wee1, Cdc25, Atr, and Chk1 (13–16). These Vpr functions have been interpreted as the molecular mechanisms of Vpr-induced G2 arrest; however, the mechanisms of Vif-induced G2 arrest are totally unknown.
We previously reported that MDM2 is a E3 ligase for Vif and induces the ubiquitination and degradation of Vif to regulate HIV-1 replication (17). We showed that Vif binds to the central domain of MDM2, which functions in the MDM2 regulation of TP53 stability and activity. As several proteins have been reported to bind this domain and regulate the effect of MDM2 on TP53, here we have tested whether Vif regulates TP53 activity, resulting in G2 arrest. We show that Vif stabilizes and activates TP53 to induce G2 arrest in infected cells and that the Vif-induced G2 arrest positively supports HIV-1 replication. Our study reveals that Vif positively regulates viral replication in a TP53-dependent manner. This report demonstrates the biological significance of HIV-1 Vif-induced G2 arrest.
Results
Vif Enhances TP53 Stability and Activity by Blocking MDM2.
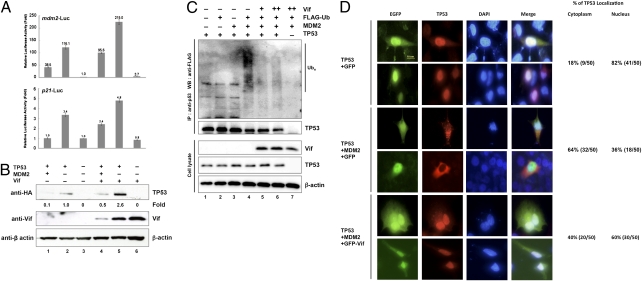
We previously demonstrated that Vif binds to the central domain of MDM2 (17), which is critical for regulating TP53 stability and transcriptional activity. To test whether Vif affects TP53 transcriptional activity, we performed luciferase reporter assays using TP53-null H1299 cells (Fig. 1A). Vif blocks the inhibitory activity of MDM2 on TP53-mediated transcription (lane 4 compared with lane 1 in Fig. 1 A and B) for both p21 and mdm2 promoters. These data confirmed the functional relationship between Vif and TP53, and we therefore next examined the physical interaction between these two proteins. Coimmunoprecipitation assays using the MDM2-binding–defective TP53 mutant TP53(Gln22,Ser23) showed that Vif was able to bind this mutant (Fig. S1). Using a series of Vif deletion mutants, we determined that the N-terminal region of Vif, consisting of aa 4–22, was necessary for TP53 binding (Fig. S2). GST pull-down assays also showed that GST-Vif bound to in vitro–translated TP53 and the mutant TP53(Gln22,Ser23), but GST-VifΔ22 did not (Fig. S3). These data suggest that Vif binds to TP53 directly and independently of MDM2 and that the N-terminal region of Vif is necessary for interaction with TP53. MDM2 specifically targets TP53 for degradation (18, 19), and we therefore examined the effect of Vif on TP53 protein levels (Fig. 1B). TP53 protein levels clearly correlated with luciferase activities as shown in Fig. 1 A and B. Coexpression of MDM2 reduced TP53 levels, and Vif overcame this MDM2 inhibitory effect and restored TP53 protein levels (lane 4 compared with lane 1 in Fig. 1B). Vif also up-regulated TP53 levels even in the absence of ectopically expressed MDM2 as seen in luciferase reporter assays (lane 5 compared with lane 2 in Fig. 1B). We assume that this results from the effect of Vif on the function of endogenous MDM2. Vif levels were also down-regulated when MDM2 was overexpressed (lane 4 compared with lane 5 in Fig. 1B) as we previously reported (17).
Fig. 1.
Vif enhances TP53 stability and transcriptional activity by blocking MDM2-mediated degradation and nuclear export of TP53. (A) Vif overcomes the inhibitory activity of MDM2 on TP53-mediated transcription. H1299 TP53-null lung carcinoma cells were cotransfected with 1-μg reporter plasmids together with expression vectors for TP53, MDM2, and Vif as indicated. Histograms of TP53 transactivation on mdm2 and p21 promoters are shown. Values are presented as an average of three independent experiments. (B) Vif blocks MDM2-mediated degradation of TP53. H1299 cells were transfected with combinations of expression vectors as indicated. Note that the expression vector additions below A also refer to B. Cell lysates were subjected to immunoblotting with the indicated Abs. The amounts of TP53 were quantified by densitometry and shown as the fold ratio relative to that in lane 2. (C) Vif blocks MDM2-mediated ubiquitination of TP53. HEK293T cells were cotransfected with expression vectors for MDM2 and TP53 together with expression vectors for Vif and FLAG-Ubiquitin (FLAG-Ub) as indicated. Cells were treated with MG132 for 6 h, and cell lysates were precipitated with anti-TP53 Ab followed by immunoblotting with the indicated Abs. (D) Vif blocks MDM2-mediated nuclear export of TP53. H1299 cells were transfected with expression vectors for HA-TP53, FLAG-MDM2, EGFP, and EGFP-Vif as indicated. Cells were fixed and probed with anti-HA mAb and then stained with anti-mouse IgG Ab conjugated with Alexa Fluor 594 dye. Cell nuclei were stained with DAPI dilactate. Samples were examined using a fluorescence microscope (Biozero BZ-8100). Quantification of TP53 localization in the cytoplasm or nucleus is shown on the right.
Because MDM2 down-regulates TP53 levels by ubiquitination, we examined the effect of Vif on MDM2-induced TP53 ubiquitination (Fig. 1C). The expression of MDM2 induced the ubiquitination of TP53 (Fig. 1C, lane 4), which was clearly blocked by coexpression of Vif (Fig. 1C, lanes 5 and 6). This suggests that Vif stabilizes TP53 by blocking MDM2-induced ubiquitination.
Because MDM2 exports TP53 from the nucleus (20), we also examined the possibility that Vif blocked MDM2-mediated nuclear export of TP53 using immunofluorescence studies (Fig. 1D). HA-TP53 was primarily located in the nucleus when expressed alone (Top panels), but was translocated into the cytoplasm when coexpressed with MDM2 (Middle panels). HA-TP53 was redistributed to the nucleus when GFP-Vif was expressed with MDM2 (Bottom panels), indicating that Vif blocked MDM2-mediated nuclear export of TP53.
On the basis of the above results, we conclude that Vif enhances TP53 stability and transcriptional activity by blocking MDM2-mediated degradation and nuclear export of TP53.
To further elucidate the mechanisms by which Vif blocks MDM2 function, we examined the physical interactions among these three proteins. Immunoprecipitation assays showed that coexpression of Vif partially blocked the binding of TP53 to MDM2 (Fig. S4), suggesting that the binding of vif to TP53 or MDM2 inhibits the interaction between TP53 and MDM2.
Vif Induces G2 Cell Cycle Arrest via the TP53 Pathway.
It has recently been shown that Vif induces G2 arrest in HIV-1–infected cells independently of Vpr (5, 6). To test the possibility that Vif induced G2 arrest by stabilizing and activating TP53, we examined the effect of Vif on cell cycle in various cell lines. In TP53-null H1299 cells, Vif alone did not induce G2 arrest, although G2 arrest was induced upon coexpression of ectopic TP53 with Vif (Fig. 2A, Middle panels). Vpr induced G2 arrest even in the absence of TP53 (Fig. 2A, Bottom panels). In contrast, in 293T cells, which expressed TP53, Vif induced G2 arrest (Fig. 2C, Upper panels), and coexpression of p53 siRNA inhibited Vif-induced G2 arrest (Fig. 2C, Lower panels, and Fig. 2D). In addition, in HCT116 and its derivative cell lines, Vif induced G2 arrest only in wild-type parental cells (Fig. 2E, Top panels), but not in p53−/− or p21−/− cells (Fig. 2E, Middle and Bottom panels, respectively). Immunoblot studies of these cells clearly showed that cellular levels of TP53 and P21 were up-regulated and those of Cdc2 and CyclinB1 were down-regulated in wild-type HCT116 cells when Vif was expressed (Fig. 2F, Left panels). In contrast, in HCT116 p53−/− cells, such changes were not detected (Center panels), whereas in HCT116 p21−/− cells, only TP53 levels were up-regulated (Right panels). These data suggest that Vif induces the up-regulation of TP53 and activation of the TP53-induced cascade including up-regulation of P21 and down-regulation of Cdc2 and CyclinB1, leading to G2 arrest (21), whereas Vpr causes G2 arrest in a TP53-independent manner.
Fig. 2.
HIV-1 Vif causes G2 cell cycle arrest via the TP53 pathway. (A) H1299 TP53-null cells were transfected with expression vectors for EGFP, EGFP-Vif, or EGFP-Vpr with or without a TP53 expression vector. Two days after transfection, cells were fixed and stained with propidium iodide and analyzed by FACS by gating on GFP(+) cells. The y axis denotes cell counts and the x axis represents DNA content. Cell cycle profiles were analyzed by ModFit LT 3.0, and the percentage of cells in G1 (red), G2 (blue), or S (green) and the G2:G1 ratio are given in the upper right of each histogram. (B) Cell lysates from the experiment shown in A were subjected to immunoblotting with the indicated Abs. GFP-Vif was clearly detected with anti-Vif mAb, although it was weakly detected with anti-GFP Ab. (C) Knockdown of TP53 relieves Vif-induced G2 cell cycle arrest. HEK293T cells were transfected with expression vectors for EGFP or EGFP-Vif together with p53 siRNA or control siRNA. Two days after transfection, cell cycle analysis was performed as described above. (D) Cell lysates from the experiment in C were subjected to immunoblotting with the indicated Abs. (E) TP53 and P21 are required for G2 cell cycle arrest induced by Vif. HCT116, HCT116 p53−/−, and HCT116 p21−/− cells were transfected with expression vectors for EGFP or EGFP-Vif and analyzed as described above. (F) TP53 and P21 are required for the reduction in Cdc2 and cyclin B1 levels. Cell lysates from the experiment in E were subjected to immunoblotting with the indicated Abs. (G) Representative cell cycle profiles of NL4-3–infected CEM-SS are shown. CEM-SS cells were infected with NL4-3 wild type or ΔVif virus. Two days after infection, cells were stained with anti-p24 mAb and propidium iodide and analyzed by FACS by gating on p24+ cells. Cells were pretreated with Pifithrin-alpha (90 μM) for 24 h before cell cycle analysis.
We further examined the effect of Vif on cell cycle in T cells in the context of viral infection. Vif-expressing NL4-3 virus caused G2 arrest in CEM-SS cells, a human T lymphoblastoid cell line permissive for HIV-1 infection without expression of Vif, and cell cycle arrest was relieved by treatment with a chemical inhibitor of TP53, pifithirin-α (Fig. 2G). This finding suggests that viral infection with Vif expression also induces G2 arrest in T cells in a TP53-dependent manner.
N-Terminal Region of Vif Is Critical for the Induction of G2 Arrest.
We next examined whether other Vif strains could induce G2 arrest and found that Vif derived from the HXB2 strain was not able to induce G2 arrest (Fig. 3A, Upper panels). We thus examined the effect of HXB2 Vif on TP53 protein levels and transcriptional activity. NL4-3 Vif overcame the inhibitory effect of MDM2 on TP53 (Fig. 3 B and C, lane 4 compared with lane 1), but HXB2 Vif did not (lane 7 compared with lane 1). These data suggest that HXB2 Vif lacks the ability to induce G2 arrest via the TP53 pathway. To determine the residues required for this function, we compared the NL4-3, HXB2, and SG3 Vif amino acid sequences because SG3 Vif has also been reported to cause G2 arrest (6) (Fig. 3D). Because the N-terminal region of Vif is important for binding to both TP53 and MDM2 (Fig. S2 and Fig. S3), we focused on this region and identified potential residues at positions 31, 33, 36, 47, and 50, which were conserved in NL4-3 and SG3, but not in HXB2. We mutated NL4-3 in these residues and tested the effects of these mutants on cell cycle. We found that both mutants of NL4-3 Vif carrying either the triple NL4-3 to HXB2 mutations at positions 31, 33, and 36 or the double mutations at positions 47 and 50 lost the ability to induce G2 arrest, whereas the reverse mutations in HXB2 Vif rendered it capable of inducing G2 arrest (Fig. 3A, Lower panels). We further examined whether these mutants affect TP53 transcriptional activity. The mutants of NL4-3 Vif to HXB2, defective for the ability to induce G2 arrest, lost the ability to overcome the MDM2 activity, whereas the mutant of HXB2 to NL4-3, capable of inducing G2 arrest, regained the ability to overcome the MDM2 activity (Fig. S5). In addition, we performed coimmunoprecipitation assays to test the binding of these mutants to TP53 (Fig. S6). HXB2 Vif and the mutants of NL4-3 to HXB2 retained the ability to bind TP53 to a similar extent to that of NL4-3 Vif. These data indicate that these amino acids are important for Vif function to induce G2 arrest but dispensable for Vif binding to TP53.
Fig. 3.
The N-terminal region is critical for the Vif function of inducing G2 arrest. (A) Cell cycle profiles of H1299 cells transfected with various mutants of NL4-3 or HXB2 Vif. (B) Luciferase reporter assays and (C) immunoblotting were performed as shown in Fig. 1. (D) The sequences of the N-terminal domains of NL4-3, SG3, and HXB2 Vif proteins were aligned. Residues that differ only in HXB2 Vif are indicated by asterisks (*).
G2 Arrest Caused by Vif Positively Supports Viral Replication.
We also examined cell cycle profiles in T cells upon infection with VSV-G–pseudotyped NL4-3/ΔEnvΔVpr-Luc viruses using TP53-deficient Jurkat cells (22), permissive CEM-SS, and nonpermissive CEM cells, which have a functional TP53, and primary CD4+ T cells. We used NL4-3/ΔEnvΔVpr-Luc virus to remove the effect of Vpr on cell cycle and also constructed a chimeric NL4-3/ΔEnvΔVpr-Luc virus replacing the N-terminal aa1-85 with aa1-85 of HXB2 Vif (Fig. 4A). The wild-type NL4-3/ΔEnvΔVpr-luc (Fig. 4B, Left panels) induced G2 arrest in CEM-SS, CEM, and primary CD4+ T cells (second and third panels from Top and Bottom panel, respectively), but not in TP53-deficient Jurkat cells (Top panel), whereas the mutant virus expressing a chimeric Vif did not induce G2 arrest in any of these cells (Right panels). These data suggest that the N-terminal domain of Vif is critical for inducing G2 arrest and that functional TP53 is necessary for Vif-induced G2 arrest following HIV-1 infection in T cells.
Fig. 4.
Replication of HIV-1 in T cells. (A) Diagram of the chimeric NL4-3/ΔVpr-Luc virus with the N-terminal region (aa 1–85) of HXB2 Vif and no Vpr expression due to mutation. A luciferase reporter gene replaced the nef gene coding sequence. (B) The wild-type NL4-3/ΔEnvΔVpr-Luc (Left panels) induces G2 arrest in infected CEM-SS cells, CEM cells, and primary CD4+ T cells, but not in TP53-deficient Jurkat cells, whereas the mutant virus expressing a chimeric Vif (Right panels) cannot induce G2 arrest in any of these cells. Two days after infection, cells were stained with anti-p24 mAb and propidium iodide and analyzed using the same method as in Fig. 2G. (C) CEM-SS, CEM, and primary CD4+ T cells (Top, Middle, Bottom, respectively) were challenged with normalized stocks of wild-type, ΔVif, and chimeric Vif NL4-3-Luc viruses, and viral replication was monitored as the supernatant accumulation of p24. CD4+ T cells were isolated from PBMC, and cells were stimulated with 200 U/mL IL-2 and 1 μg/mL PHA for 1 wk. p24 antigen levels were measured by ELISA.
Finally, we examined the effect of Vif-mediated G2 arrest on HIV-1 replication using the NL4-3-Luc virus expressing the NL4-3-Luc/HXB2 chimeric Vif (Fig. 4C). The chimeric virus showed less efficient replication than the parental NL4-3-Luc virus in TP53-active CEM-SS, CEM, and CD4+ T cells. Although a ΔVif virus cannot replicate in nonpermissive CEM and CD4+ T cells, it can replicate in permissive CEM-SS cells albeit less efficiently than wild-type virus. In Jurkat cells, NL4-3-Luc virus could not efficiently replicate, presumably because of the replacement of the nef gene with liciferase. We therefore constructed the NL4-3/HXB2 chimeric Vif virus in the intact NL4-3 as a backbone and monitored the replication kinetics in TP53-deficient Jurkat cells. There are no differences in replication profiles between wild-type and chimeric Vif viruses (Fig. S7). These results indicate that the G2 arrest induced through the Vif-TP53 interaction has a positive effect on HIV-1 replication.
Discussion
In this study, we demonstrate that Vif enhances TP53 stability and transcriptional activity by blocking MDM2-mediated degradation and nuclear export of TP53, which results in G2 arrest in infected cells. More importantly, we further demonstrate that the Vif-induced G2 arrest positively supports HIV-1 replication.
We demonstrate that Vif can bind TP53 as well as MDM2 directly. We previously mapped the interaction domain of MDM2 with Vif to aa 168–320, which are located in its central acidic and zinc-finger domains (17). This central domain is different from the primary TP53-binding site located in the N-terminal region, but was recently reported as a second TP53-binding site important for regulation of TP53 stability (23–26). Interestingly, several proteins, including P300, P14ARF, and pRB, bind to the central domain of MDM2 and regulate the stability and function of TP53 via MDM2 (24, 27). P300 can bind to TP53 and MDM2 independently and facilitate the MDM2-mediated inhibition of TP53 function (28, 29). In contrast, P14ARF binds to MDM2 and enhances the stability and function of TP53 by blocking MDM2-mediated ubiquitination and nucleo-cytoplasmic shuttling of TP53 (30–32). pRB binds to MDM2 and enhances the stability and apoptotic function of TP53 via MDM2, but not its transcriptional activity (33–35). Vif enhances TP53 stability and transcriptional activity by blocking MDM2-mediated degradation and nuclear export of TP53. According to our data, Vif can bind independently to TP53 as well as to MDM2, similar to P300, but its effect on TP53 function is exactly the opposite. On the other hand, Vif is a substrate of an E3 ligase like MDM2 as well as an effecter of MDM2 function, similar to pRB, but its effect on TP53 function is somewhat different. The important finding is that Vif is a protein regulating the TP53/MDM2 axis. This unique interaction also suggests that a possible negative autoregulatory circuit exists among Vif, TP53, and MDM2 (36). Stabilization and activation of TP53 by Vif induces the up-regulation of MDM2 (Fig. 1A, Upper), which in turn down-regulates Vif. This circuit keeps cellular Vif levels relatively low, which might be advantageous for HIV-1—that is, Vif levels not too high to affect viral replication, but high enough to antagonize A3G/F. One possible reason for relatively low Vif levels is that overexpression of Vif affects Gag processing, which impairs viral replication (37).
More interestingly, several groups recently reported that Vif as well as Vpr induced G2 arrest in HIV-1–infected cells (5, 6). Our data clearly demonstrate that the activation of TP53 is the molecular mechanism involved in Vif-induced G2 arrest. Stabilization and activation of TP53 by Vif induces the up-regulation of P21, leading to the down-regulation of Cdc2 and CyclinB1 levels, resulting in G2 arrest. This is in contrast to Vpr-induced G2 arrest because Vpr induces G2 arrest via a TP53-independent pathway (Fig. 2A). Several laboratories have recently reported the involvement of the DDB-1-Cul4 E3 complex in Vpr-induced G2 arrest (38, 39), although its target substrates and molecular mechanisms remain unclear. Why does HIV-1 have two different proteins regulating G2/M transition? DNA damage activates checkpoint mechanisms and sustains cells at the G2 phase for DNA repair (7, 8). TP53 is involved in the maintenance rather than the initiation of G2 arrest; for example, wild-type HCT116 cells sustain G2 arrest following irradiation, whereas the isogenic TP53-null derivative initially arrests in G2 but then escapes and enters mitosis (10). It seems quite reasonable for HIV-1 to use Vpr and Vif because each regulates TP53-independent and -dependent pathways to initiate and sustain G2 arrest, respectively.
Furthermore, many reports have described a viral connection between TP53 and cell cycle arrest. Herpesviruses, such as herpes simplex virus (40), human herpesvirus 6 (41), and human cytomegalovirus (42–44), cause cell cycle arrest via the TP53 pathway; however, the viral proteins responsible for TP53 stabilization and the precise mechanisms and significance of cell cycle arrest remain unknown. The data in this study might provide a clue to the mechanism and the significance of cell cycle arrest induced by these viruses.
Finally, we demonstrate that the G2 arrest induced by Vif positively supports viral replication. The mutant virus expressing an NL4-3/HBX2 chimeric Vif is defective in its ability to induce G2 arrest although it can antagonize A3G to the same extent as the NL4-3 parental virus (Fig. S8). Replication assays reveal that this chimeric virus replicates more slowly than wild-type virus in CEM-SS, CEM, and CD4+ T cells, which express functional TP53 (Fig. 4C), but not in TP53-deficient Jurkat cells (Fig. S7). The disparity noted between wild-type and NL4-3/HBX2 chimeric viruses is attributable to the ability to induce G2 arrest because these viruses show similar anti-A3G activities (Fig. S8). This finding appears to be consistent with previous observations showing that cells sustained in the G2 phase can produce more virus because of increased LTR and reverse transcriptase activity (45). On the other hand, ΔVif viruses showed a more effective replication profile than an NL4-3/HBX2 chimeric Vif virus in CEM-SS cells, although they cannot replicate in nonpermissive CEM and CD4+ T cells because of the presence of A3G. It is conceivable that a chimeric Vif is expressed at higher levels, which impedes viral replication by affecting Gag processing (37) because it cannot induce an autoregulatory circuit via the TP53 pathway.
Taken together, we here advocate the concept that the interaction between Vif and the TP53/MDM2 axis positively regulates HIV-1 replication. Vif hijacks not only a Cullin5 E3 ligase complex but also the TP53/MDM2 axis to achieve more efficient viral replication. Evolutionarily, HIV-1 seems to have obtained the vif gene to antagonize APOBEC3 proteins. Because Vif protein itself impairs viral replication when overexpressed, we assume that it further interacts with the TP53/MDM2 axis to achieve more efficient viral replication.
Vallunti et al. recently reported that F12-Vif from a nonproducer HIV-1 provirus clone F12 has anti–HIV-1 activity despite degrading A3G as efficiently as NL4-3 Vif does (46). The F12-Vif derivative Chim3-Vif is a chimera Vif generated by replacing the domain (aa 126–170) of wild-type Vif with that of F12-Vif. Lentiviral transduction of Chim3-Vif protected CD4+ T cells from HIV-1 infection by preventing HIV-1 DNA integration and HIV-1–induced G2 cell cycle delay (47, 48). These data also indicate that modification of the cell cycle is important for HIV-1 replication and might be a target for an original therapeutic strategy.
This report shows the molecular mechanisms and the biological significance of G2 arrest induced by Vif. The modification or intervention of this interplay might lead to original therapeutic strategies for HIV-1 infection.
Materials and Methods
Cell Culture and Virus Propagation.
HEK293T cells and the TP53-null lung carcinoma cell line H1299 were maintained in DMEM (Invitrogen) containing 10% FBS and 1% penicillin–streptomycin and glutamine (PSG) (Invitrogen). The human colorectal carcinoma cell line HCT116 and the isogenic derivative lines HCT116 p53−/− and HCT116 p21−/− were kindly provided by B. Vogelstein and cultured in McCoy's 5A medium (Invitrogen) supplemented with 10% FBS and 1% PSG. The leukemic T-cell lines (Jurkat, CEM-SS, and CEM) were maintained in RPMI medium 1640 (Nacalai tesque) containing 10% FBS and 1% PSG. Primary CD4+ T cells were purified by CD4 MultiSort MicroBeads (Miltenyi Biotec) and stimulated with 200 U/mL IL-2 and 1 μg/mL phytohemaglutimin (PHA) at 37 °C for 1 wk. All cells were maintained at 37 °C in a humidified atmosphere of 5% CO2. Virus stocks were prepared by transfection of HEK293T cells with HIV-1 NL4-3 derivatives using the calcium phosphate coprecipitation method.
Additional experimental methods are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. K. Strebel (Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Disease, National Institutes of Health, Bethesda) for the pNL-A1 plasmid and its derivative mutants, Dr. C. deNoronha (Center for Immunology and Microbial Disease, Albany Medical College, Albany, NY) for pEGFP-Vpr, which encodes a red-shifted variant of wild-type GFP-fused Vpr, Dr. B. Vogelstein (Medical Institutions, Baltimore) for HCT116 and the isogenic derivative lines HCT116 p53−/− and HCT116 p21−/−, Dr. X. Lu (Nuffield Department of Clinical Medicine, University of Oxford, Oxford, UK) for the mdm2-Luc plasmid, Dr. D. Beach (Wolfson Institute for Biomedical Research, University College London, London) for p21-Luc plasmid, and Dr. M. Malim (Department of Infectious Diseases, King's College London School of Medicine, London) for the anti-Vif monoclonal antibody (#319) through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. This study was partly supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology and from the Ministry of Health, Labour and Welfare in Japan. This study was also partly supported by grants-in-aid from the Sankyo Foundation of Life Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008076107/-/DCSupplemental.
References
- 1.Stopak K, de Noronha C, Yonemoto W, Greene WC. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol Cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 2.Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 3.Yu X, et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Takaori-Kondo A, Miyauchi Y, Iwai K, Uchiyama T. Ubiquitination of APOBEC3G by an HIV-1 Vif-Cullin5-Elongin B-Elongin C complex is essential for Vif function. J Biol Chem. 2005;280:18573–18578. doi: 10.1074/jbc.C500082200. [DOI] [PubMed] [Google Scholar]
- 5.Sakai K, Dimas J, Lenardo MJ. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc Natl Acad Sci USA. 2006;103:3369–3374. doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, et al. The Vif accessory protein alters the cell cycle of human immunodeficiency virus type 1 infected cells. Virology. 2007;359:243–252. doi: 10.1016/j.virol.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 8.Giono LE, Manfredi JJ. The p53 tumor suppressor participates in multiple cell cycle checkpoints. J Cell Physiol. 2006;209:13–20. doi: 10.1002/jcp.20689. [DOI] [PubMed] [Google Scholar]
- 9.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 10.Bunz F, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 11.Wang XW, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA. 1999;96:3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermeking H, et al. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 13.He J, et al. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Re F, Braaten D, Franke EK, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakai-Murakami C, et al. HIV-1 Vpr induces ATM-dependent cellular signal with enhanced homologous recombination. Oncogene. 2007;26:477–486. doi: 10.1038/sj.onc.1209831. [DOI] [PubMed] [Google Scholar]
- 16.Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem. 2003;278:25879–25886. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- 17.Izumi T, et al. MDM2 is a novel E3 ligase for HIV-1 Vif. Retrovirology. 2009;6:1. doi: 10.1186/1742-4690-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 19.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 20.Tao W, Levine AJ. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci USA. 1999;96:3077–3080. doi: 10.1073/pnas.96.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flatt PM, Tang LJ, Scatena CD, Szak ST, Pietenpol JA. p53 regulation of G(2) checkpoint is retinoblastoma protein dependent. Mol Cell Biol. 2000;20:4210–4223. doi: 10.1128/mcb.20.12.4210-4223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J, Haas M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol Cell Biol. 1990;10:5502–5509. doi: 10.1128/mcb.10.10.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argentini M, Barboule N, Wasylyk B. The contribution of the acidic domain of MDM2 to p53 and MDM2 stability. Oncogene. 2001;20:1267–1275. doi: 10.1038/sj.onc.1204241. [DOI] [PubMed] [Google Scholar]
- 24.Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- 25.Kawai H, Wiederschain D, Yuan ZM. Critical contribution of the MDM2 acidic domain to p53 ubiquitination. Mol Cell Biol. 2003;23:4939–4947. doi: 10.1128/MCB.23.14.4939-4947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meulmeester E, et al. Critical role for a central part of Mdm2 in the ubiquitylation of p53. Mol Cell Biol. 2003;23:4929–4938. doi: 10.1128/MCB.23.14.4929-4938.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganguli G, Wasylyk B. p53-independent functions of MDM2. Mol Cancer Res. 2003;1:1027–1035. [PubMed] [Google Scholar]
- 28.Grossman SR, et al. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol Cell. 1998;2:405–415. doi: 10.1016/s1097-2765(00)80140-9. [DOI] [PubMed] [Google Scholar]
- 29.Kawai H, Nie L, Wiederschain D, Yuan ZM. Dual role of p300 in the regulation of p53 stability. J Biol Chem. 2001;276:45928–45932. doi: 10.1074/jbc.M107770200. [DOI] [PubMed] [Google Scholar]
- 30.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamijo T, et al. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci USA. 1998;95:8292–8297. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao W, Levine AJ. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh JK, et al. RB regulates the stability and the apoptotic function of p53 via MDM2. Mol Cell. 1999;3:181–193. doi: 10.1016/s1097-2765(00)80309-3. [DOI] [PubMed] [Google Scholar]
- 34.Xiao ZX, et al. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995;375:694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- 35.Yap DB, Hsieh JK, Chan FS, Lu X. mdm2: A bridge over the two tumour suppressors, p53 and Rb. Oncogene. 1999;18:7681–7689. doi: 10.1038/sj.onc.1202954. [DOI] [PubMed] [Google Scholar]
- 36.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- 37.Akari H, et al. High level expression of human immunodeficiency virus type-1 Vif inhibits viral infectivity by modulating proteolytic processing of the Gag precursor at the p2/nucleocapsid processing site. J Biol Chem. 2004;279:12355–12362. doi: 10.1074/jbc.M312426200. [DOI] [PubMed] [Google Scholar]
- 38.Schröfelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci USA. 2007;104:4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem. 2007;282:27046–27057. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- 40.Hobbs WE, II, DeLuca NA. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J Virol. 1999;73:8245–8255. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Øster B, Bundgaard B, Höllsberg P. Human herpesvirus 6B induces cell cycle arrest concomitant with p53 phosphorylation and accumulation in T cells. J Virol. 2005;79:1961–1965. doi: 10.1128/JVI.79.3.1961-1965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillo JP, et al. Human cytomegalovirus IE1-72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response. J Virol. 2005;79:11467–11475. doi: 10.1128/JVI.79.17.11467-11475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song YJ, Stinski MF. Inhibition of cell division by the human cytomegalovirus IE86 protein: Role of the p53 pathway or cyclin-dependent kinase 1/cyclin B1. J Virol. 2005;79:2597–2603. doi: 10.1128/JVI.79.4.2597-2603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, Knutson E, Wang S, Martinez LA, Albrecht T. Stabilization of p53 in human cytomegalovirus-initiated cells is associated with sequestration of HDM2 and decreased p53 ubiquitination. J Biol Chem. 2007;282:29284–29295. doi: 10.1074/jbc.M705349200. [DOI] [PubMed] [Google Scholar]
- 45.Goh WC, et al. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: A mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 46.Vallanti G, Lupo R, Federico M, Mavilio F, Bovolenta C. T lymphocytes transduced with a lentiviral vector expressing F12-Vif are protected from HIV-1 infection in an APOBEC3G-independent manner. Mol Ther. 2005;12:697–706. doi: 10.1016/j.ymthe.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Porcellini S, et al. The F12-Vif derivative Chim3 inhibits HIV-1 replication in CD4+ T lymphocytes and CD34+-derived macrophages by blocking HIV-1 DNA integration. Blood. 2009;113:3443–3452. doi: 10.1182/blood-2008-06-158790. [DOI] [PubMed] [Google Scholar]
- 48.Porcellini S, et al. Chim3 confers survival advantage to CD4+ T cells upon HIV-1 infection by preventing HIV-1 DNA integration and HIV-1-induced G2 cell-cycle delay. Blood. 2010;115:4021–4029. doi: 10.1182/blood-2009-09-243030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.