Abstract
Regulated secretion of neurotransmitters and neurohumoural factors from dense core secretory vesicles provides essential neuroeffectors for cell-cell communication in the nervous and endocrine systems. This study provides comprehensive proteomic characterization of the categories of proteins in chromaffin dense core secretory vesicles that participate in cell-cell communication from the adrenal medulla. Proteomic studies were conducted by nano-HPLC Chip MS/MS tandem mass spectrometry. Results demonstrate that these secretory vesicles contain proteins of distinct functional categories consisting of neuropeptides and neurohumoural factors, protease systems, neurotransmitter enzymes and transporters, receptors, enzymes for biochemical processes, reduction/oxidation regulation, ATPases, protein folding, lipid biochemistry, signal transduction, exocytosis, calcium regulation, as well as structural and cell adhesion proteins. The secretory vesicle proteomic data identified 371 distinct proteins in the soluble fraction and 384 distinct membrane proteins, for a total of 686 distinct secretory vesicle proteins. Notably, these proteomic analyses illustrate the presence of several neurological disease-related proteins in these secretory vesicles, including huntingtin interacting protein, cystatin C, ataxin 7, and prion protein. Overall, these findings demonstrate that multiple protein categories participate in dense core secretory vesicles for production, storage, and secretion of bioactive neuroeffectors for cell-cell communication in health and disease.
Keywords: secretory vesicles, soluble, membrane, mass spectrometry, proteomics, proteins, functions, cell-cell communication
Introduction
The nervous system utilizes dense core secretory vesicles for regulated secretion of chemical neurotransmitters and neurohumoural factors that are represented by neuropeptides, catecholamines, and related neuroeffector molecules for cell-cell communication (1–5). These secretory vesicles represent the primary subcellular site for the biosynthesis, storage, and secretion of neurotransmitters and hormones utilized for cell-cell communication in the nervous and endocrine systems for health and disease.
The dense core secretory vesicles of chromaffin cells of the peripheral sympathetic nervous system are a representative model for neurochemical enzymes utilized in brain for the biosynthesis of neuroeffectors composed of neuropeptides and catecholamines (dopamine, norepinephrine, and epinephrine) (5–7). The majority of prior studies have studied individual proteins of these dense core secretory vesicles (8–13). However, a more global understanding of secretory vesicle components is essential to gain knowledge of the repertoire of protein systems that function in this organelle. Elucidation of the proteome characteristics of dense core secretory vesicles can provide valuable insight into the functional protein processes for production and secretion of neuroeffectors, the goal of this study.
The high sensitivity of current mass spectrometry (MS) instrumentation, coupled with efficient HPLC (high-pressure liquid chromatography) separation of peptides, allows proteomic investigations to identify hundreds of proteins from small amounts of samples. Furthermore, enrichment of moderate to low abundant proteins in chromaffin secretory vesicles for this study was achieved by removal of the abundant chromogranin A protein. Peptide identifications from mass spectrometry data were obtained using two independent search algorithms for database searching, combined with searches against a shuffled decoy database for estimation of false discovery rate (FDR) for tryptic peptide identifications. The overall proteomic data resulted in identification of 371 soluble and 384 membrane proteins from dense core secretory vesicles, for a total of 686 distinct secretory vesicle proteins.
Significantly, proteomic data illustrated distinct biochemical functions in dense core secretory vesicles composed of proteins for neuropeptides and neurohumoural factors, protease systems, neurotransmitter enzymes, receptors, biochemical enzymes, regulation of redox status, protein folding, ATPases, lipid and carbohydrate functions, signal transduction and GTP-binding proteins, and proteins for exocytosis. Interestingly, several proteins known to participate in neurological diseases were indicated consisting of the amyloid precursor protein (APP), huntingtin-interacting protein, ataxin 7, and prion protein that represent key elements involved in the mechanisms of Alzheimer’s disease (14–18), Huntington’s disease (19–22), spinocerebellar ataxia (23–25), and prion disease (26–28). These secretory vesicles also contain the CLN8 protein involved in neurodegeneration and mental retardation of EPMR (epilepsy and mental retardation) (29–32), and the P20-CGGBP protein involved in the fragile X syndrome of mental retardation (33). Furthermore, these vesicles also contain regulatory factor X4 involved in bipolar disorder (34), and KIAA0319 that is involved in dyslexia (35).
Overall, proteomic investigation of dense core secretory vesicles revealed functionally distinct categories of protein systems in this organelle, with several involved in neurological disease. These proteomic data illustrate a view of the secretory vesicle ‘system’ for secretion of neuroeffectors mediating neuronal and endocrine cell-cell communication in health and disease.
Materials and Methods
Purification of chromaffin secretory vesicles from bovine adrenal medulla and preparation of soluble and membrane components
Dense core secretory vesicles, represented by chromaffin secretory vesicles (also known as chromaffin granules), were purified from fresh bovine adrenal medulla by differential sucrose density gradient centrifugation, as described previously (37,38), involving extensive wash steps to obtain purified chromaffin granules. We have documented the high purity of this preparation of isolated secretory vesicles by electron microscopy (Fig. 1) and biochemical markers (36–38). Sucrose gradient purification results in a preparation of purified, intact chromaffin secretory vesicles that lack biochemical markers for the subcellular organelles of lysosomes (acid phosphatase marker) (38), cytoplasm (lactate dehydrogenase marker) (37), mitochondria (fumarase and glutamate dehydrogenase markers) (36,37), and endoplasmic reticulum (glucose-6-phosphatase marker) (37). Enzyme markers have been measured in the purified chromaffin secretory vesicle preparation as 1% or less of total homogenate markers, which, thus, indicate the high purity of these isolated secretory vesicles (36–38).
Figure 1. Purity of isolated chromaffin granules (secretory vesicles) evaluated with (Met)enkephalin as a marker for chromaffin granules and with acid phosphatase as a marker for lysosomes.
(a) Preparation of purified chromaffin granules by differential density centrifugation. The flow chart illustrates the purification scheme for chromaffin granules from bovine adrenal medulla homogenate, achieved by differential centrifugation. The homogenate (in 0.32 M sucrose buffer) is centrifuged at 365 × g to remove nuclei (P1, pellet 1) from the supernatant (S1, soluble fraction 1) that represents a crude fraction of chromaffin granules. The Granules of pelleted by centrifugation at 12,000 × g and washed three times in 0.32 M sucrose buffer to obtain enriched chromaffin granules (P5 fraction) that undergoes purification on a 1.6/0.32 M sucrose gradient subjected to ultracentrifugation (120,000 × g) to obtained a pellet of purified chromaffin granules.
(b) Analyses of crude fraction of chromaffin granules on multi-step sucrose gradient. The crude chromaffin granule fraction (P2) was analyzed on a multi-step sucrose gradient of 2.2 M to 1.2 M sucrose as described in the methods. Gradient fractions were assayed for (Met)enkephalin (●) that is present in chromaffin granules, and for the lysosomal enzyme marker acid phosphatase (○). The crude P2 fraction of chromaffin granules contains enkephalin and acid phosphatase.
(c) Analyses of purified chromaffin granules on multi-step sucrose gradient. The purified chromaffin granules were analyzed on the multi-step sucrose gradient of 2.2 M to 1.2 M sucrose as described in the methods. Gradient fractions were assayed for (Met)enkephalin and acid phosphatase. The presence of the purified chromaffin granules is indicated by the peak of (Met)enkephalin. The multi-step gradient showed no peak of acid phosphatase, indicating effective removal of lysosomes. These data document the purity of the chromaffin granule preparation.
(It is noted that cytosolic proteins may possibly associate with the outside of the granule membrane during homogenization, and after freeze-thawing, such cytosolic proteins may become present in the soluble fraction giving the interpretation that they might be luminal proteins of the granules. Nonetheless, cytosolic proteins are likely to have importance because cellular function of the chromaffin granule must involve cytoplasmic proteins for regulated movement to achieve exocytosis.)
In addition, this study further assessed the removal of the lysosomal enzyme marker acid phosphatase from the purified preparation of chromaffin granules compared to unpurified sample of chromaffin granules obtained at an early step in the purification procedure (illustrated in figure 1a). Purified and unpurified granules were analyzed on a multi-step sucrose gradient of 2.2 to 1.2 M sucrose (2.2, 2.1, 2.0, 1.9, 1.8, 1.7, 1.6, 1.5, 1.4, and 1.2 M sucrose steps each consisting of 2.5 ml) by ultracentrifugation at 120,000 × g in a SW28 rotor (25,000 rpm) at 4° C for 100 min. Gradient fractions of 0.5 ml were collected from the bottom of the tube (2.2 M sucrose), and fractions were assayed for (Met)enkephalin by RIA as previously described (5) as a marker for chromaffin granules, and acid phosphatase activity as a marker for lysosomes as described previously (38). Results show that the purified chromaffin granules lack acid phosphatase activity, indicating effective removal of lysosomes of density near that of chromaffin granules (explained in fig. 1 of results). These new data and established purity in the literature (36–38) document the purity of these chromaffin secretory vesicles for this study.
Soluble and membrane components of the purified chromaffin granules were prepared by lysing (by freeze-thawing) purified chromaffin granules in isotonic buffer conditions consisting of 150 mM NaCl in 50 mM Na-acetate, pH 6.0, with a cocktail of protease inhibitors (10 μM pepstatin A, 10 μM leupeptin, 10 μM chymostatin, 10 μM E64c, and 1 mM AEBSF). The lysed granules were centrifuged at 100,000 × g (SW60 rotor) at 4° C for 30 minutes. The resultant supernatant was collected as the soluble fraction. The pellet was collected as the membrane fraction, and washed two times by re-suspending in the lysis buffer and centrifugation (100,000 × g, 30 min). The final pellet was resuspended in the lysis buffer and designated as the membrane fraction.
The soluble and membrane fractions were each subjected to removal of the abundant chromogranin A (CgA) protein, by its binding to calmodulin-Sepharose (GE Healthcare, formerly Amersham Biosciences, Piscataway, NJ) (39). The soluble fraction and membrane fraction (solubilized in 50 mM CHAPS) were each incubated with a slurry of calmodulin-Sepharose at 4° C overnight in equilibration buffer (50 mM Tris-HCl, pH 7.5, 0.1 M NaCl, 2 mM CaCl2, and protease inhibitors consisting of 5 μM E64c, 5 μM leupeptin, 5 μM chymostatin, 5 μM pepstatin A, 5 μM bestatin, 1 μM GEMSA, and 50 mM PMSF). The mixture was centrifuged and the supernatant collected as the soluble fraction without CgA. This step removed approximately 90–95% of CgA, based on assessment by anti-CgA western blots.
Proteins in the membrane fraction were concentrated by chloroform-methanol precipitation. To the membrane fraction (400 μg in 300 μl) was added MeOH (400 μl), chloroform (100 μl), and deionized water (300 μl) with mixing between each step, followed by centrifugation (14,000 × g for 1 min). The top aqueous layer was removed, while retaining the protein precipitate at the top of the chloroform layer; after addition of MeOH (400 μl), mixing, and centrifugation (14,000 × g for 2 min), the pelleted protein was collected for trypsin digestion (40).
Reduction/alkylation and trypsin digestion of samples
Reduction and alkylation were performed using Tris-(2-carboxyethyl)-phosphine hydrochloride (TCEP) (Pierce, Rockford, IL) reduction followed by cysteine alkylation with iodoacetamide (Sigma-Aldrich, St. Louis, MO). Briefly, a sample of the CG soluble fraction (8.6 μg) was lyophilized, redissolved in 12 μl 20% acetonitrile, followed by addition of 4 μl 100 mM TCEP in 20% acetonitrile (28.7 mg/ml TCEP) and reduction at 55°C for 20 minutes. The reduced sample was cooled to room temperature, 4 μl of 100 mM iodoacetamide in 20% acetonitrile was added and incubated for 30 minutes at room temperature (in the dark) for alkylation of free cysteine residues. For proteins of the membrane fraction (400 μg), the proteins precipitated by chloroform-methanol (described above) were subjected to the same procedure for reduction and alkylation using 20 μl of TCEP solution and 5 μl of iodoacetamide solution.
The reduced and alkylated proteins of the chromaffin granule soluble (CGS) and membrane (CGM) fractions were subjected to trypsin digestion. The CGS protein sample (8.67 μg in approximately 1 μl) was diluted by addition of 20 μl 25 mM ammonium bicarbonate (pH 7.0) and 1 μl 100 mM CaCl2, and 10 μl trypsin stock solution was added (200 ng total trypsin, using stock solution consisting of 20 ng/μl sequencing grade trypsin in 25 mM ammonium bicarbonate, pH 7.0, trypsin was from Promega #V5111). Final trypsin digestion conditions for CGS (31.5 μl total volume) were 0.275 μg/ml CGS protein, 6.5 ng/μl trypsin, 25 mM ammonium bicarbonate, pH 7.0, and 3.2 mM CaCl2. For the CGM sample (100 μg protein in ~10 μl), it was prepared by addition of 90 μl trypsin (1800 ng total trypsin, using stock solution consisting of 20 ng/μl trypsin in 25 mM ammonium biocarbonate, pH 7.0), 95 μl ammonium bicarbonate, pH 7.0, and 5 μl 100 mM CaCl2. The trypsin digestion conditions for CGM (200 μl total volume) were 0.5 μg/μl protein, 9.0 ng/μl trypsin, 25 mM ammonium bicarbonate, pH 7.0, and 2.5 mM CaCl2. Trypsin digestion of CGS and CGM samples was conducted by incubation at 37° C for 18 hours.
Nano-liquid chromatography tandem mass spectrometry (nano-HPLC Chip MS/MS)
Soluble and membrane chromaffin granule sample digests were subjected to nano-LC-MS/MS, loaded at 2.9 μg and 1.0 μg total protein for each LC-MS/MS analysis. All LC-MS/MS analyses were performed in triplicate on an Agilent XCT Ultra ion trap mass spectrometer coupled to an Agilent 1100 nano-HPLC system fitted with a HPLC-Chip system. The LC separation was performed on an Agilent C18 analytical HPLC chip (Agilent Zorbax C18 Chip, 150 mm × 75 μm, 40 nl trap) and utilized a gradient of solvent B (acetonitrile with 0.25% formic acid) in solvent A (water with 0.25% formic acid). The gradient progressed from 3% B to 45% B in 40 minutes followed by an increase to 75% B in 10 minutes. The mass spectrometer was set for data dependant scanning in MS/MS mode on the three most abundant ions present in the MS scan. The exclusion time was set to 0.1 minutes, isolation window set to 4 amu, and voltages set to −1850V (capillary), −500V (counterelectrode) and 1.30V (fragmentation). Smart ion target was set to 500,000 to correct for background ions. The maximum injection time was set to 100 ms. All other default settings were used and left unaltered in all experiments.
Analyses of MS/MS data and database search parameters using Spectrum Mill bioinformatics platform (Agilent Technologies, Santa Clara, CA)
The Spectrum Mill database search platform used 1.4 amu for precursor mass tolerance and 0.8 amu fragment mass tolerance with all other default parameters retained. The search was unrestricted and included non-tryptic peptide identifications to prevent forced identifications for non-tryptic peptides. Protein identifications resulted from searches against a bovine protein database (extracted from the NCBI-nr protein sequence database consisting of 38,197 protein entries). We employed a 1.0 amu precursor mass tolerance and 0.3 amu fragment mass tolerance against the IPI bovine database. The search was restricted to tryptic and semi-tryptic peptides. In all searches, carbamidomethylation was included as a variable modification to compensate for the possibility of incomplete alkylation.
Validation for MS/MS identifications
Protein identifications were evaluated using analysis against proteins known to be present in these samples and by Spectrum Mill database search against a scrambled decoy database (41). Based on these analyses, protein identifications were evaluated by implementing a two-tiered system for peptide identifications. The first tier consisted of proteins with high confidence, peptide scores of ≥10 and Scored Peak Intensity Percent (%SPI) ≥70. When implemented, the False Discovery Rates (FDR) for these protein identifications using tryptic peptides were ~1%, even when single peptides were considered. Protein identification with single tryptic peptides can be confidently achieved as illustrated by previous reports on confident protein identifications based on single peptides (42). The second tier targeted proteins showed confidence levels corresponding to peptide score ≥8 and %SPI >60, combined with the requirement that at least two tryptic peptides of the parent protein were utilized for identification. The levels of these scores were considered to represent identification of proteins based on prior biochemical studies documenting the presence of low abundance proteins in the chromaffin secretory vesicles, which include endopin (43), cathepsin D (44), cathepsin B (45), and prohormone convertase (46). The FDR for this second tier was estimated at 1–2% by decoy database analysis. It is important to note that the FDR determined by decoy database analysis is an estimate and is dependent on the quality of spectra and the randomized database (41). Therefore, we manually evaluated our scoring thresholds in a manner similar to reported by Wang, et al. (41).
Protein organization and clustering
Tryptic peptides shared between several proteins are only counted for the protein that has overall the most matching, unique peptides. Batch Entrez (http://ncbi.nlm.nih.gov/entrez/batchentrez.cgi?db=Protein) was used to generate FASTA formatted protein sequence databases for each GenInfo Identifier (GI) number for proteins identified by the MS experiment. BLASTCLUST was used to perform pairwise comparisons followed by single-linkage clustering of the statistically significant matches (>95% sequence similarity over 90% of the sequence length) (http://www.ncbi.nlm.nih.gov/blast/). The protein list is thus the smallest set of proteins explaining the identified proteins present. Following this analysis, an annotated, non-redundant table of soluble and membrane proteins was compiled (Supplemental Tables A and B).
The functional categories of identified proteins were defined by the gene ontology (GO) resource (http://www.geneontology.org). Further information on the function of proteins was obtained through KEGG and Interact pathway databases, as well as through the MEROPS database to provide additional information relevant to proteases. A series of GO terms in each category was acquired though text searching of specific keywords relating to function and localization. In addition to gene ontologies, both identified and unidentified protein sequences were queried against the the InterPro (http://www.ebi.ac.uk/interpro/) database, SignalP resource (http://www.cbs.dtu.dk/services/SignalP/) and TMHMM resource (http://www.cbs.dtu.dk/services/TMHMM/) in order to assess protein family. All automated searches were enhanced with PubMed searches to assess recent literature where proteins are known to serve multiple functions.
Several peptide sequences were identified that were not functionally annotated in initial database searches. Based on the identified tryptic peptide sequences, predicted mouse and human sequences were aligned back to bovine sequences using the TIGR gene indices (http://tigrblast.tigr.org/tgi/). Proteins demonstrating strong homology to existing bovine sequences were included in the non-redundant assembly of identified chromaffin granule proteins.
Proteomic data (Table 1) combines proteins identified in the sample after the calmodulin affinity step from experiments of this study with proteins identified before the calmodulin affinity step from our previous more limited proteomic study of bovine chromaffin granules [47]. Thus, proteins that may bind to the calmodulin affinity column are included in this complete proteomic data set of proteins identified from this study and our previous, smaller proteomic study of bovine chromaffin granules; the combined proteomic data set is illustrated in Table 1.
Table 1.
Functional Categories of Soluble and Membrane Proteins in Chromaffin Secretory Vesicles.
The functional categories of proteins identified as soluble and membrane components of chromaffin secretory vesicles are illustrated in this table. The main functional categories (large bolded titles) are divided into subcategories. This table combines the extensive group of proteins identified by nano-HPLC Chip MS/MS tandem mass spectrometry in this study with other proteins in these vesicles identified by 1-D gel separation and MS/MS analyses (47).
| Protein Function | Genbank | Soluble | Membrane |
|---|---|---|---|
| Production of Neurotransmitters and Neurohumoural Factors | |||
| Neuropeptides (Proproteins) and Neurohumoural Factors | Genbank | Soluble | Membrane |
| Adrenomedullin* | 27806927 | ||
| Amyloid beta A4 precursor# | 76613693 | ||
| Angiopoietin-4 precursor (ANG-4) | 76633205 | ||
| Cathelicidin 1+ | 27807341 | ||
| Chromogranin A* | 116548 | ||
| Chromogranin B* | 12 | ||
| Chromogranin C* | 27806421 | ||
| Decorin (bone proteoglycan II)* | 54660107 | ||
| Epithelium-derived growth factor (EGF) | 76637576 | ||
| Glycoprotein II+ | 1809215 | ||
| Glycoprotein III (clusterin)* | 27806907 | ||
| Interleukin 27 | 76655433 | ||
| Neuroendocrine secretory protein 55 (NESP-55)* | 2262205 | ||
| Osteocrin | 76671357 | ||
| Osteogenin (BMP3)* | 76620194 | ||
| Platelet basic protein precursor (PBP) | 76619991 | ||
| Proenkephalin* | 83405428 | ||
| Pro-Neuropeptide Y* | 40022234 | ||
| Secretogranin III* | 76663325 | ||
| Transforming growth factor-beta binding protein* | 135671 | ||
| Ubiquitin/ribosomal fusion protein | 28189665 | ||
| VEGF (vascularendothelial growth factor)+ | 27806821 | ||
| VGF nerve growth factor inducible* | 76654056 | ||
| Protease Systems | Genbank | Soluble | Membrane |
| ADAM metallopeptidase with thrombospondin type 1 | 76648708 | ||
| AFG3-like protein 1 | 76677962 | ||
| Alpha-1-antichymotrypsin precursor (ACT) | 76694546 | ||
| Alpha-2-plasmin inhibitor | 76643654 | ||
| Autophagy 4 homolog D (APG4) | 76621759 | ||
| Bleomycin hydrolase | 76643627 | ||
| Carboxypeptidase D* | 76643631 | ||
| Carboxypeptidase E* | 76675819 | ||
| Carboxypeptidase G2 | 76647796 | ||
| Cathepsin B+ | 9955277 | ||
| Cathepsin D* | 76658398 | ||
| Cathepsin L# | 1542853 | ||
| COP9 constitutive photomorphogenic | 74267826 | ||
| COP9 signalosome | 76654132 | ||
| Cystatin C+ | 27806675 | ||
| Cystatin E/M | 61097917 | ||
| Disintegrin and metalloprotease domain 4 | 76684774 | ||
| Endopin 2C# | 62126072 | ||
| HECT, UBA and WWE domain containing 1 | 76659533 | ||
| IAP, Inhibitor of Apoptosis | 76638039 | ||
| Leukotriene A4 hydrolase | 74355010 | ||
| Meltrin | 47564064 | ||
| Metalloprotease 1 | 76672659 | ||
| Neuroendocrine protein 7B2* | 88682959 | ||
| Polyubiquitin | 89994036 | ||
| PP11 serine protease | 76618319 | ||
| Prohormone convertase 1* | 61817689 | ||
| Prohormone convertase 2+ | 13878928 | ||
| Proprotein convertase subtilisin/kexin type 4 | 76622897 | ||
| Reelin precursor | 76677746 | ||
| Serine (or cysteine) proteinase inhibitor | 74268410 | ||
| Tissue inhibitor of metalloproteinase 1 (TIMP-1)+ | 27806161 | ||
| Transmembrane protease, serine 3 | 76608355 | ||
| Ubiquitin* | 76638698 | ||
| Ubiquitin A-52 (residue ribosomal protein fusion product 1) | 76620757 | ||
| Ubiquitin associated protein 2 | 76624820 | ||
| Ubiquitin protein ligase E3B | 76639147 | ||
| Vpr-binding protein | 76649016 | ||
| XIAP associated factor-1 | 78045549 | ||
| YME1-like, metalloprotease | 76632200 | ||
| Neurotransmitter Enzymes/Transporters | Genbank | Soluble | Membrane |
| 4-aminobutyrate aminotransferase precursor | 76678944 | ||
| Bestrophin anion channel | 76629024 | ||
| Calcium channel, voltage-dependent, alpha | 76615073 | ||
| Dopamine beta-monooxygenase* | 1083022 | ||
| Glutamate decarboxylase 1 | 76609605 | ||
| Glutaminase | 86438072 | ||
| Neuronal pentraxin I* | 76669694 | ||
| PNMTase (phenylethanolamine N-methyltransferase)+ | 130374 | ||
| Protein tyrosine phosphatase, receptor | 76614769 | ||
| Rod photoreceptor cng-channel | 1050441 | ||
| Sodium channel protein type IV alpha subunit | 76645224 | ||
| Sodium/potassium/calcium exchanger 1 | 76684545 | ||
| Synaptic vesicle monoamine transporter (VAT2)* | 457486 | ||
| Transient receptor potential cation channel | 76625397 | ||
| Tyrosine 3-monooxygenase (Tyrosine hydroxylase) | 27807401 | ||
| Vesicle amine transport protein 1 (VAT1) | 76671278 | ||
| Vesicular inhibitory amino acid transporter | 76646508 | ||
| Receptors | Genbank | Soluble | Membrane |
| Adiponectin receptor 2 | 76679749 | ||
| Bone morphogenetic protein receptor | 76619536 | ||
| Bradykinin receptor B1 | 76647810 | ||
| Cholinergic receptor, nicotinic, alpha polypeptide 3 | 27807295 | ||
| EC2-V2R pheromone receptor | 76675084 | ||
| EPH receptor A7 | 76679477 | ||
| Estrogen-related receptor gamma | 76636898 | ||
| Fc receptor-like 3 precursor | 76670706 | ||
| Fibroblast growth factor receptor 4 precursor (FGFR-4) | 76622759 | ||
| Gamma-aminobutyric acid receptor | 76637789 | ||
| Glutamate [NMDA] receptor | 76631132 | ||
| Insulin receptor substrate 4 | 76659092 | ||
| Interleukin-1 receptor-like 2 precursor (IL-1Rrp2) | 76628778 | ||
| Muscarinic acetylcholine receptor M5 | 76627274 | ||
| Olfactory receptor 212 | 76683959 | ||
| Olfactory receptor 5A1 | 76636626 | ||
| Olfactory receptor 833 | 76693698 | ||
| Peripheral-type benzodiazepine receptor-associated protein 1 | 76639758 | ||
| Progesterone receptor (PR) | 76678665 | ||
| T-cell receptor alpha chain C region | 76626982 | ||
| Thyroid hormone receptor associated protein 2 | 76639110 | ||
| TNF receptor associated protein | 81673141 | ||
| Vascular endothelial growth factor receptor 3 (VEGFR-3) | 76663199 | ||
| Biochemical Processes | |||
| Enzymes | Genbank | Soluble | Membrane |
| 1-aminocyclopropane-1-carboxylate synthase | 76636325 | ||
| Ash1 (absent, small, or homeotic) | 76612493 | ||
| Arylacetamide deacetylase (AADAC) | 76607894 | ||
| Aspartate aminotransferase 1 | 29135295 | ||
| Bisphosphoglycerate mutase | 61839453 | ||
| C-1-tetrahydrofolate synthase, cytoplasmic | 76628072 | ||
| CG7544-PA | 76643676 | ||
| Cyclophilin B (PPIB) | 74268324 | ||
| Dihydropyrimidinase-related protein 4 (DRP-4) | 61878819 | ||
| Enolase 2 | 88682888 | ||
| Enolase 3 | 88954201 | ||
| Folate receptor 1 precursor | 76635818 | ||
| Glycerol-3-phosphate acyltransferase | 76628542 | ||
| Malate dehydrogenase (MADH2)* | 81674781 | ||
| Metallothionein-like 5 (testis-specific, tesmin) | 76658427 | ||
| Methionine adenosyltransferase II, beta | 81673843 | ||
| NAD synthetase 1 | 73587273 | ||
| Paraoxonase 2 | 61888862 | ||
| Phosphoribosylglycinamide formyltransferase | 61966468 | ||
| Reticulon 4 interacting protein 1 | 76649171 | ||
| Ribonucleoside-diphosphate reductase | 76630263 | ||
| Splicing factor, arginine/serine-rich 15 | 76645770 | ||
| Thioredoxin domain containing protein 5 | 76676581 | ||
| Carbohydrate Functions | Genbank | Soluble | Membrane |
| Alpha-(1,3)-fucosyltransferase (Galactoside 3-L-fucosyltransferase) | 76631232 | ||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase | 61864843 | ||
| Amylo-1,6-glucosidase, 4-alpha-glucanotransferase | 76613348 | ||
| Bactericidal/permeability-increasing protein-like 3 | 76633471 | ||
| Beta 1,4-N-acetylgalactosaminyltransferase-transferase-3 | 76663169 | ||
| Beta-1,3-N-acetylglucosaminyltransferase bGnT-6 | 76645514 | ||
| Beta-1,4-galactosyltransferase 2 | 76614325 | ||
| Chondroitin sulfate glucuronyltransferase | 76616133 | ||
| Galactose-1-phosphate uridylyltransferase isoform 1 | 61555177 | ||
| Galactose-3-O-sulfotransferase 3 isoform 1 | 76658175 | ||
| Glucosidase II* | 76657688 | ||
| Golgi sialoglycoprotein MG-160+ | 76639850 | ||
| Maltase-glucoamylase | 76678173 | ||
| Mannosidase* | 76624022 | ||
| N-acetylglucosaminyltransferase V+ | 76610006 | ||
| Ribophorin II | 76638094 | ||
| UDP-glucose:glycoprotein glucosyltransferase 1 | 76609241 | ||
| Lipid Functions | Genbank | Soluble | Membrane |
| 3,2-trans-enoyl-CoA isomerase, mitochondrial precursor | 76652799 | ||
| Acetyl-Coenzyme A carboxylase beta | 76639133 | ||
| Acyl-Coenzyme A dehydrogenase | 27806205 | ||
| Acyl-Coenzyme A synthetase | 76676487 | ||
| Aminophospholipid transporter (APLT)+ | 27807317 | ||
| Arachidonate 12-lipoxygenase | 76643357 | ||
| Carnitine O-palmitoyltransferase I | 76658339 | ||
| Cerebroside sulfate activator (SAP-1)+ | 115502446 | ||
| Diacylglycerol kinase, beta | 76681581 | ||
| Fatty acid binding protein 11 | 89994084 | ||
| Glucocerebrosidase precursor | 76612148 | ||
| High density lipoprotein-binding protein | 76634040 | ||
| Lipin-2 | 76651852 | ||
| Low-density lipoprotein receptor-related protein | 76609709 | ||
| N-acylsphingosine amidohydrolase+ | 76655702 | ||
| Patatin-like phospholipase domain containing 2 | 76658479 | ||
| Phospholipase A1 member A (Pla1a) | 83405374 | ||
| Transport-secretion protein | 76658477 | ||
| Internal Conditions of Secretory Vesicles | |||
| Reduction-Oxidation | Genbank | Soluble | Membrane |
| Biliverdin reductase A precursor | 76615466 | ||
| Catalase | 78369302 | ||
| Cytochrome b561* | 27807323 | ||
| Cytochrome C oxidase+ | 117102 | ||
| Cytochrome P450* | 76669152 | ||
| Dimethylaniline monooxygenase [N-oxide-forming] 2 | 76637349 | ||
| Endoplasmic reticulum oxidoreductin 1-Lbeta | 76656155 | ||
| Glutathione peroxidase 3* | 585223 | ||
| Myeloperoxidase precursor (MPO) | 76642663 | ||
| NAD(P) transhydrogenase, mitochondrial precursor | 128400 | ||
| Ubiquinol-cytochrome C reductase complex+ | 136691 | ||
| ATPases and Nucleotide Metabolism | Genbank | Soluble | Membrane |
| ADP-ribosylation factor-like 10C | 76648606 | ||
| ANT 1 (adenine nucleotide translocator 1)+ | 32189340 | ||
| ATP H+ transporting VI+ | 4502315 | ||
| ATP Synthase (gamma chain)+ | 2493093 | ||
| ATP synthase alpha chain, mitochondrial precursor | 76652040 | ||
| ATP6IP1 protein | 28461231 | ||
| ATPase type 13A2 | 74267862 | ||
| ATPase, aminophospholipid transporter (APLT), Class I, type 8A* | 27807317 | ||
| ATPase, H+ transporting* | 102 | ||
| ATPase, H+ transporting, lysosomal, V1 subunit C | 28603816 | ||
| ATPase, H+ transporting, subunit A | 27807453 | ||
| ATPase, H+ transporting, V1 subunit B* | 28603772 | ||
| ATP-binding cassette sub-family D | 61863306 | ||
| ATP-binding cassette, sub-family A | 76652817 | ||
| Concentrative Na+-nucleoside cotransporter | 76661614 | ||
| Ectonucleoside triphosphate diphosphohydrolase 1 protein | 76654745 | ||
| HT028 (ATPase)* | 61823467 | ||
| MSTP042+ | 75832069 | ||
| Proton-associated sugar transporter A | 76671385 | ||
| TER ATPase (transitional endoplasmic reticulum)+ | 73586667 | ||
| V-ATPase (vacuolar ATPase accessory subunit B)+ | 549205 | ||
| V-ATPase (vacuolar ATPase accessory subunit D) | 62460538 | ||
| V-ATPase (vacuolar ATPase accessory subunit E1) | 27807375 | ||
| V-ATPase (vacuolar ATPase accessory subunit F1)* | 94574271 | ||
| V-ATPase (vacuolar ATPase accessory subunit SFD alpha isoform) | 2895578 | ||
| V-ATPase (vacuolar ATPase polypeptide IV) | 89602 | ||
| V-ATPase (vacuolar ATPase synthase subunit H)+ | 12643366 | ||
| Protein Folding | Genbank | Soluble | Membrane |
| C1GALT1-specific chaperone 1 | 74356336 | ||
| Chaperonin 10+ | 1167 | ||
| Cysteine string protein (CSP)* | 1232163 | ||
| Glucose-regulated protein precursor (GRP 78) | 76630569 | ||
| Heat Shock Protein 27* | 71037405 | ||
| Heat Shock Protein 40* | 76619510 | ||
| Heat shock protein 60 | 76648520 | ||
| Heat shock protein 70 | 73586960 | ||
| Peptidylprolyl isomerase B+ | 27806469 | ||
| Prion protein | 21666990 | ||
| Wiskott-Aldrich syndrome protein interacting protein (WIP) | 76609571 | ||
| Transporters (solute) | Genbank | Soluble | Membrane |
| Organic anion transporter 3 | 42538740 | ||
| P87 | 259174 | ||
| Sodium-dependent glucose transporter SGLT-I | 14486596 76661712 |
||
| Solute carrier family 2 | |||
| Solute carrier family 22, anion/cation transporter | 76657700 | ||
| Solute carrier family 25, mitochondrial carrier glutamate | 27807185 | ||
| Solute carrier family 26, anion transporter | 76668427 | ||
| Solute carrier family 38, carrier protein | 94966787 | ||
| Solute carrier family 39, zinc transporter | 76651620 | ||
| Solute carrier family 4, sodum borate transporter | 30794360 | ||
| Solute carrier family 6 | 76693476 | ||
| Regulated Secretion Mechanisms | |||
| Signal Transduction and GTP-Binding Proteins | Genbank | Soluble | Membrane |
| 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase | 76648298 | ||
| 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase-like 4 | 76637596 | ||
| Activator of S phase kinase | 76671561 | ||
| Adenylate cyclase-inhibiting G alpha protein | 27805887 | ||
| A-kinase anchor protein 4 | 41386786 | ||
| AHNAK-related protein* | 76657680 | ||
| Ankyrin repeat domain 37 | 76668139 | ||
| AXL receptor tyrosine kinase | 76684583 | ||
| Breast cancer membrane protein 11 | 76614919 | ||
| Calcium/calmodulin-dependent protein kinase IIA | 76623502 | ||
| Calcium/calmodulin-dependent protein kinase IV | 76679349 | ||
| CAMP-dependent protein kinase inhibitor beta | 76625537 | ||
| Catenin | 76656278 | ||
| CDK5 and ABL1 enzyme substrate 2 (Interactor with CDK3 2) | 76632977 | ||
| Centaurin-gamma 1 GTPase | 76618822 | ||
| Cyclic nucleotide-gated channel beta subunit 1e | 3309626 | ||
| DIRAS family, GTP-binding RAS | 73587391 | ||
| Doublecortin kinase 2 | 76668033 | ||
| Dual specificity protein phosphatase 2 | 76628632 | ||
| FK506 binding protein+ | 25066280 | ||
| Frizzled 9 precursor (Frizzled-9) | 76653703 | ||
| FYVE, RhoGEF and PH domain containing 2 | 76650063 | ||
| FYVE, RhoGEF and PH domain containing protein 5 | 76661294 | ||
| Guanine nucleotide binding protein (G protein), alpha* | 30794332 | ||
| Guanine nucleotide binding protein (G protein), beta* | 1085447 | ||
| Guanine nucleotide binding protein (G protein), type B | 399711 | ||
| Guanine nucleotide binding protein (G protein), gamma* | 27807509 | ||
| Guanine nucleotide exchange factor | 77362757 | ||
| Guanylate kinase-associated protein | 76647874 | ||
| Heart alpha-kinase | 76662373 | ||
| Immunity-related GTPase family, Q1 | 76641801 | ||
| Inositol polyphosphate-4-phosphatase, type II | 76660578 | ||
| Intestinal cell kinase | 76650734 | ||
| IP3 receptor associated cGMP kinase | 7341097 | ||
| Leucine-rich repeat kinase 1 | 76646793 | ||
| Lin-7 | 76641963 | ||
| Mitogen-activated protein kinase 1+ | 28461209 | ||
| Mitogen-activated protein kinase 5 | 76660373 | ||
| Mitogen-activated protein kinase 8 | 76656852 | ||
| Mitogen-interacting protein kinase I* | 77736562 | ||
| Myomegalin | 76670843 | ||
| N-ethylmaleimide-sensitive factor | 76645272 | ||
| Phosphatase and tensin homolog | 76655292 | ||
| Phosphatidylinositol 4-kinase beta | 38372429 | ||
| Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase PTEN | 76671072 | ||
| Phosphoinositide-3-kinase adaptor protein 1 | 76608515 | ||
| PKCI-1-related HIT protein+ | 14211923 | ||
| Plexin B2 precursor (MM1) | 76617505 | ||
| Polo-like kinase 2 | 76660620 | ||
| Preferentially expressed antigen in melanoma like 5 | 92096623 | ||
| Protein kinase C, epsilon type (nPKC-epsilon) | 76629287 | ||
| Protein kinase, DNA-activated, catalytic polypeptide | 76634333 | ||
| Protein phosphatase 1, regulatory (inhibitor) | 76645130 | ||
| Protein phosphatase 1J (PP2C domain containing) | 76613182 | ||
| PYRIN-containing APAF1-like protein 7 | 76614247 | ||
| Rab-1 | 76629651 | ||
| Rab-12 | 76651878 | ||
| Rab-14* | 76668547 | ||
| Rab-15 | 76628095 | ||
| Rab-2 | 76686605 | ||
| Rab-21+ | 2500067 | ||
| Rab-27A | 61875226 | ||
| Rab-27B | 24459171 | ||
| Rab-2B | 76627105 | ||
| Rab-33B | 76638452 | ||
| Rab-34 | 73587147 | ||
| Rab-35+ | 76639295 | ||
| Rab-37 | 76645964 | ||
| Rab-39A | 76611110 | ||
| Rab-3A | 27806127 | ||
| Rab-3B | 27806113 | ||
| Rab-3C+ | 86438380 | ||
| Rab-4B | 76641702 | ||
| Rab-5B | 76618738 | ||
| Rab-6B | 76608256 | ||
| Rab-7+ | 74354082 | ||
| Rab-8B | 76627752 | ||
| Receptor-type tyrosine-protein phosphatase N2 | 76676438 | ||
| Regulator of G-protein signalling 2, 24kDa | 76636875 | ||
| Retinitis pigmentosa | 21303187 | ||
| Rho guanine nucleotide exchange factor 12 | 76635393 | ||
| Rho guanine nucleotide exchange factor 4 | 76609133 | ||
| Serine/threonine-protein kinase 38 | 76650116 | ||
| Serine/threonine-protein kinase PLK2 | 76660624 | ||
| SET binding factor 1 | 76617331 | ||
| Signal-induced proliferation-associated 1 | 76628293 | ||
| Son of sevenless homolog 1 | 76629071 | ||
| Src homology 3 domain-containing guanine nucleotide exchange factor | 76676576 | ||
| Sterile alpha and TIR motif containing 1 | 76643315 | ||
| Synaptojanin 2 binding protein | 74354054 | ||
| Testis-specific serine kinase 6 | 76621013 | ||
| Tousled-like kinase 1 | 76609603 | ||
| Transducin protein 4 | 76666047 | ||
| Tuberin (Tuberous sclerosis 2 homolog protein) | 76652568 | ||
| Tyrosine-protein kinase JAK2 | 76661504 | ||
| Tyrosine-protein phosphatase-like N precursor (R-PTP-N)* | 76610663 | ||
| Very large G-protein coupled receptor 1 | 76660074 | ||
| Virus-induced signaling protein | 86438388 | ||
| WD-repeat protein | 76612957 | ||
| Wingless-type MMTV integration site family, member 10B | 76618188 | ||
| WNK lysine deficient protein kinase 2 | 76664151 | ||
| Wnt inhibitory factor 1 precursor (WIF-1) | 76618509 | ||
| Vesicular Trafficking and Exocytosis | Genbank | Soluble | Membrane |
| Clathrin+ | 27806689 | ||
| Coatomer alpha subunit (Alpha-coat protein) | 76615759 | ||
| Dynamin 2 | 76660288 | ||
| Epsin-2 | 76644169 | ||
| Formin binding protein 4 | 76636519 | ||
| Golgi autoantigen* | 76641568 | ||
| Huntingtin interacting protein 1 | 76649404 | ||
| Islet cell autoantigen 512 | 5305476 | ||
| Kinesin | 76648026 | ||
| Piccolo (presynaptic cytomatrix protein) | 76615069 | ||
| Pleckstrin | 76608906 | ||
| SEC31-like 2 | 76654762 | ||
| Sec5 protein | 76661880 | ||
| Sorting nexin 4 | 76640294 | ||
| Synapsin Ia | 108935 | ||
| Synaptophysin* | 33112658 | ||
| Synaptotagmin 1* | 27806387 | ||
| Synaptotagmin II | 76687864 | ||
| Synaptotagmin VI | 76613150 | ||
| Synaptotagmin VII* | 76657575 | ||
| Synaptotagmin-4 | 61809317 | ||
| Syntaxin-1A | 417841 | ||
| THUMP domain containing 1 | 76653091 | ||
| Tomosyn | 76626187 | ||
| Unc-18 protein | 631583 | ||
| VAMP 3 (cellubrevin)* | 61845787 | ||
| Vesicular membrane protein p24 | 76651226 | ||
| Calcium Regulation | Genbank | Soluble | Membrane |
| Annexin A1 | 74 | ||
| Annexin A2* | 27807289 | ||
| Annexin A4* | 1063258 | ||
| Annexin A6* | 76623595 | ||
| Annexin A7 | 76656523 | ||
| Annexin A11+ | 113969 | ||
| Bestrophin isoform 1 | 61842255 | ||
| Calcium binding protein P22 | 76688289 | ||
| Calnuc (Nucleobindin)+ | 189308 | ||
| Mucolipin 3 | 81673761 | ||
| SPARC-like 1 | 74354032 | ||
| Voltage-dependent T-type calcium channel alpha-1 | 76674163 | ||
| Morphological Functions of Secretory Vesicles | |||
| Structural Proteins | Genbank | Soluble | Membrane |
| Bone proteoglycan II | 28189579 | ||
| Brevican | 88682949 | ||
| Cartilage acidic protein 1 | 76654689 | ||
| Centromere protein I (CENP1) | 86821813 | ||
| Centromeric protein E (CENP-E) | 76676943 | ||
| CG15021-PA | 76648468 | ||
| CG2843-PA | 76644813 | ||
| Collagen, type I | 76686475 | ||
| Collagen, type VI | 76667061 | ||
| Collagen, type XVIII | 76608417 | ||
| Collagen, type XXVII | 76625290 | ||
| Collogen cyanogen bromide, type II | 5354051 | ||
| Colonic and hepatic tumor over-expressed protein | 76636503 | ||
| Cytokinesis 8 | 76624391 | ||
| Desmoplakin | 76651410 | ||
| Diaphanous 3 | 76661030 | ||
| Drebrin 1 | 76622581 | ||
| Dynein | 76648966 | ||
| Echinoderm microtubule associated protein like 5 | 76673417 | ||
| Elastin microfibril interfacer 1* | 61845535 | ||
| Fibrinogen | 75812954 | ||
| Fibronectin type III | 76613259 | ||
| Galectin-related inter-fiber protein | 76654374 | ||
| Gap junction protein, (connexin 31.9) | 76644686 | ||
| Hydrocephalus inducing | 76673899 | ||
| KIAA1914 protein | 76655117 | ||
| Microtubule associated serine/threonine kinase 2 | 76614276 | ||
| Microtubule-associated protein 4 | 27806553 | ||
| Myosin, heavy polypeptide 9+ | 27807325 | ||
| Myosin, light polypeptide kinase | 76609105 | ||
| Nebulin | 76609853 | ||
| Nesprin-2 | 76628088 | ||
| Obscurin | 76620689 | ||
| Pericentrin 2 | 76607770 | ||
| Periphilin 1 | 76618432 | ||
| Proline arginine rich coiled coil 1 | 76614586 | ||
| Proteoglycan 3 | 76705880 | ||
| Proteolipid protein 1 (PLP1) | 74354814 | ||
| Radial spokehead-like 3 | 76625577 | ||
| Spectrin domain with coiled-coils 1 | 76644035 | ||
| Sperm associated antigen 4-like | 76633467 | ||
| Symplekin | 76641008 | ||
| Talin 1+ | 76627770 | ||
| Testican 2+ | 76656478 | ||
| Tubulin* | 76628240 | ||
| Cell Adhesion/Cell-Cell Interactions | Genbank | Soluble | Membrane |
| Ankyrin 3 | 76656250 | ||
| Cadherin* | 76640658 | ||
| CD18 antigen | 2407809 | ||
| CD63 antigen* | 45439308 | ||
| CD81 antigen (target of antiproliferative antibody 1) | 73586978 | ||
| Cell adhesion molecule JCAM | 76608323 | ||
| Contactin 1+ | 1060861 | ||
| Dishevelled-associated activator of morphogenesis 1 | 76665330 | ||
| Dystonin | 76649774 | ||
| Epithelial V-like antigen 1 | 62751654 | ||
| Integrin alpha-8 precursor | 76632354 | ||
| KIAA0319 (dyslexia) | 76674660 | ||
| Laminin beta-1 chain precursor | 76615137 | ||
| Leucine rich repeat and fibronectin type III domain containing | 76658000 | ||
| Nidogen-2 precursor (NID-2) (Osteonidogen) | 76627657 | ||
| Swan isoform 4 (neurexin) | 76634905 | ||
| Thrombospondin-3 precursor | 76612130 | ||
| UCC1+ | 76664807 | ||
| Vinexin (SH3-containing adapter molecule-1) | 76624656 | ||
| Other Protein Categories | |||
| Cell growth and development | Genbank | Soluble | Membrane |
| Gametogenetin | 76641302 | ||
| Mitotic-specific cyclin B1 | 76615016 | ||
| Tectonic | 76639025 | ||
| Immune | Genbank | Soluble | Membrane |
| Anti-testosterone antibody+ | 440 | ||
| Cardiotrophin-1 (CT-1) | 76687585 | ||
| Collectin-43 | 50355694 | ||
| Complement component 6 | 88954145 | ||
| Complement component 7 | 88954301 | ||
| Ig gamma-1 chain C region, membrane-bound form | 76678688 | ||
| Ig heavy chain* | 1575493 | ||
| Ig lambda chain V-I region BL2 precursor | 76690231 | ||
| IgG Fc receptor FcRN | 7339746 | ||
| Immunoglobulin superfamily, member 2 | 76612971 | ||
| Killer cell immunoglobulin-like receptor 3DL1 | 76687295 | ||
| Large proline-rich protein (BAT2) | 76650909 | ||
| Major histocompatibility complex, class I-related | 76660744 | ||
| Myeloid/lymphoid or mixed-lineage leukemia | 76644552 | ||
| Stromal cell derived factor 4 | 73586919 | ||
| TOLL-like receptor 7 | 76151015 | ||
| UL16 binding protein 3 | 76693242 | ||
| Transcription and Translation | Genbank | Soluble | Membrane |
| Ataxin 7 | 76613103 | ||
| BCOR protein (BCL-6 corepressor) | 76670256 | ||
| Bromodomain and PHD finger containing, 1 isoform 6 | 76648396 | ||
| CAMP responsive element binding protein 5 | 76615443 | ||
| Cbp/p300-interacting transactivator | 76668278 | ||
| CCAAT/enhancer binding protein zeta | 76629167 | ||
| Cell division cycle 16 | 76634504 | ||
| Cell division cycle 42 | 76611597 | ||
| Centrosomal protein 2 | 76633389 | ||
| C-Fos | 32481980 | ||
| Chromodomain helicase DNA binding protein 7 | 76634422 | ||
| Chromosome condensation (RCC1) and BTB (POZ) domain | 76667931 | ||
| Cleavage and polyadenylation specific factor 1 | 27807297 | ||
| Clock isoform 4 | 76619910 | ||
| Core-binding factor, beta subunit | 76640732 | ||
| CPEB3 (cytoplasmic polyadenylation element binding protein 3) | 76661464 | ||
| DEAD (Asp-Glu-Ala-Asp) box polypeptide 48 | 76645558 | ||
| DNA cross-link repair 1A protein | 76655155 | ||
| DNA methyltransferase 1 associated protein 1 | 89994078 | ||
| DNA replication complex GINS protein PSF2 | 76639980 | ||
| DNA-binding protein SATB1 | 76608636 | ||
| DRE1 protein | 76636127 | ||
| Eukaryotic translation initiation factor 2 | 76629540 | ||
| Eukaryotic translation initiation factor 3 | 74353982 | ||
| Exosome component 10 | 76637212 | ||
| Fidipidine | 76610089 | ||
| Forkhead box K1 | 76654354 | ||
| Fusilli | 76634878 | ||
| General transcription factor II H | 76650520 | ||
| High mobility group protein 4 | 61878473 | ||
| High-mobility group protein 3 | 76658822 | ||
| Histone acetyltransferase GCN5 | 76644862 | ||
| Histone H2B 291B | 76625998 | ||
| HIV TAT specific factor 1 | 76658704 | ||
| HP1-BP74 | 76611630 | ||
| Hypothetical zinc finger protein KIAA1196 | 76632891 | ||
| Hypoxia-inducible factor-3 alpha | 76642167 | ||
| Ladybird homeobox homolog 1 | 76654810 | ||
| Luc7 | 76652552 | ||
| Methyl-CpG binding domain protein 1 | 86437962 | ||
| Myoneurin | 77567823 | ||
| Neuro-oncological ventral antigen | 76667375 | ||
| Nibrin+ | 76634945 | ||
| Nuclear autoantigen Sp-100 | 76610804 | ||
| Nuclear RNA export factor 3 | 76659002 | ||
| Nucleolar complex associated 3 | 76640610 | ||
| Nucleoporin | 76646535 | ||
| P20-CGGBP | 76608705 | ||
| PGC-1 related co-activator | 76654860 | ||
| PHD finger protein 22 | 76684907 | ||
| Pinin, desmosome associated protein | 27807293 | ||
| Polyhomeotic 1 | 76616376 | ||
| Pre-B-cell leukemia transcription factor interacting protein 1 | 76612171 | ||
| Regulatory factor X4 isoform c | 76619030 | ||
| Replication initiator 1 | 76616173 | ||
| Retinoic acid induced 16 | 76624642 | ||
| Ribosomal protein L23a | 76613395 | ||
| Ribosomal protein L29 | 74268009 | ||
| Ribosomal protein L4 | 62460480 | ||
| Ribosomal protein S27a | 76620759 | ||
| Ribosomal protein S6 kinase polypeptide 3 | 76659718 | ||
| RNA-binding protein 11 | 94966923 | ||
| RNA-binding protein 28 | 76615708 | ||
| RNA-binding protein EWS 8 | 76639325 | ||
| RPS27A protein | 73586974 | ||
| Schlafen 10 | 76642925 | ||
| Serologically defined colon cancer antigen 33 | 76651519 | ||
| Sex comb on midleg-like protein 2 | 76676174 | ||
| SFRS5 splicing factor | 76662260 | ||
| Shugoshin-like 2 | 76610144 | ||
| Sp4 transcription factor | 76673507 | ||
| Spermatogenic leucine zipper 1 | 94966881 | ||
| Target of myb1, Tom-1 protein | 78042494 | ||
| TATA box binding protein like 2 | 76660220 | ||
| Transcription elongation factor B polypeptide 3 binding protein 1 | 76622840 | ||
| Transcription factor PU.1 | 76636497 | ||
| Transcription factor RAM2 | 76614939 | ||
| Transcription factor Sp1 | 76617820 | ||
| Transcriptional intermediary factor 1-gamma (TIF1-gamma) | 76613148 | ||
| Treacle protein | 76683158 | ||
| Woc | 76672108 | ||
| Valosin-containing protein (VCP) | 77735541 | ||
| Zinc finger CCCH-type containing 12A | 76614519 | ||
| Zinc finger protein 111 | 76616182 | ||
| Zinc finger protein 262 | 76614570 | ||
| Zinc finger protein 385 | 76617575 | ||
| Zinc finger protein 398 | 76616180 | ||
| Zinc finger protein 469 | 76640087 | ||
| Zinc finger protein 608 | 76667970 | ||
| Zinc finger protein 623 | 76634062 | ||
| Zinc finger protein 644 | 76613525 | ||
| Zinc finger protein 694 | 76610151 | ||
| Zinc finger protein 8 | 76685031 | ||
| Zinc finger protein 84 | 76642204 | ||
| Zinc finger protein 85 | 76641574 | ||
| Miscellaneous | |||
| Miscellaneous | Genbank | Soluble | Membrane |
| Absent in melanoma 1 protein | 76608722 | ||
| Albumin | 162648 | ||
| Apical-like protein (APXL protein) | 76659839 | ||
| Apoptotic chromatin condensation inducer | 76626863 | ||
| BCL2-like 14 | 82571795 | ||
| Beta2-Microglobulin* | 41386683 | ||
| Breast cancer antiestrogen resistance 3 | 66792756 | ||
| Bromodomain and WD repeat domain containing 2 | 76655253 | ||
| C9orf55 | 76659997 | ||
| Cas-Br-M ecotropic retroviral transforming sequence-like 1 | 76615140 | ||
| CG7593-PA | 76657521 | ||
| Chromosome 17 open reading frame 27 | 76670292 | ||
| Chromosome 9 open reading frame 19 | 61812939 | ||
| Desmocollin 2 | 76651700 | ||
| ELG protein | 76643730 | ||
| ESCO2 (establishment of cohesion) | 76624097 | ||
| F33H2.2 | 76638347 | ||
| Golgi phosphoprotein 3 | 76650110 | ||
| HBx-Hepatitis B virus X interacting+ | 74268261 | ||
| Hemoglobin* | 12655818 | ||
| Kelch protein 3 | 76623241 | ||
| KIAA1862 | 76616193 | ||
| KIAA1900 | 76663072 | ||
| Lysosome membrane protein II (LIMP II) | 76620042 | ||
| Membrane-associated ring finger 3 (C3HC4) | 76660388 | ||
| Membrane glycoprotein 1 precursor (LAMP-1) | 1683365 | ||
| MGC133986 protein | 86437964 | ||
| MICAL-2 | 76676434 | ||
| Mitochondrial carrier homolog 1 | 76650069 | ||
| Mitochondrial import inner membrane translocase subunit Tim17 A | 76680711 | ||
| Odd Oz/ten-m homolog 2 | 76623795 | ||
| Otolin-1 | 76607840 | ||
| Partitioning defective-6 homolog alpha (PAR-6 alpha) | 76633889 | ||
| PC4 and SFRS1 interacting protein 1 | 76624197 | ||
| Phosphorylated CTD interacting factor 1 | 76633669 | ||
| PRBP (plasma retinol-binding protein)+ | 132403 | ||
| Pyrin domain containing 4 | 82571578 | ||
| R119.5 | 76665822 | ||
| RAD52 | 66792838 | ||
| Ran-binding protein 3 (RanBP3) isoform 2 | 76622053 | ||
| Ring finger protein 111 | 76627819 | ||
| Ryanodine receptor 1, skeletal muscle | 76641296 | ||
| SCD6 homolog A | 77736251 | ||
| SID1 transmembrane family member 1 precursor | 76684359 | ||
| Spastin isoform 1 | 76682638 | ||
| Spermatogenesis associated 13 | 76631579 | ||
| Spermatogenesis associated 5 | 76687571 | ||
| Storkhead box 2 | 76655606 | ||
| Tankyrase 1 | 76655843 | ||
| Tetraspanin 7* | 76665086 | ||
| Transcobalamin II+ | 27806385 | ||
| Transmembrane protein 16B | 76673511 | ||
| Transmembrane protein 63C | 76628460 | ||
| Transmembrane protein 79 | 59858019 | ||
| Tripartite motif protein 7 | 76622789 | ||
| Tumor necrosis factor, alpha-induced protein 3 | 76626126 | ||
| WW domain binding protein 11 | 76633774 | ||
| Vacuolar protein sorting factor 4B | 76652249 | ||
| Y37D8A.2 | 76669076 | ||
| Unknown | Genbank | Soluble | Membrane |
| CG11617-PA | 76632406 | ||
| CG6379-PA | 76650147 | ||
| CG13957-PA | 76609707 | ||
| CG17569-PB | 76665263 | ||
| CG32045-PC | 76631513 | ||
| CG3338-PA | 76643561 | ||
| CG4751-PA | 76614067 | ||
| CG5987-PA | 76644502 | ||
| CG7709-PA | 76642510 | ||
| FLJ44048 protein | 76668361 | ||
| H43E16.1 | 76680309 | ||
| Hypothetical gene supported by AK075558; BC021286 | 76637226 | ||
| Hypothetical protein FLJ13868 | 61554841 | ||
| Hypothetical protein LOC539970 | 74267976 | ||
| Hypothetical protein XP_583091 | 76659995 | ||
| Hypothetical protein XP_584302 | 61823940 | ||
| Hypothetical protein XP_585938 | 76658228 | ||
| Hypothetical protein XP_590236 | 76616022 | ||
| Hypothetical protein XP_592499 | 76672076 | ||
| Hypothetical protein XP_594162 | 76637663 | ||
| Hypothetical protein XP_598213 | 76619718 | ||
| Hypothetical protein XP_600405 | 76690587 | ||
| Hypothetical protein XP_600782 | 76678727 | ||
| Hypothetical protein XP_868808 | 76649193 | ||
| Hypothetical protein XP_869427 | 76635764 | ||
| Hypothetical protein XP_873356 | 76769235 | ||
| Hypothetical protein XP_580444 | 76634839 | ||
| Hypothetical protein XP_596183 | 76638981 | ||
| Hypothetical protein XP_608396 | 76678883 | ||
| Hypothetical protein XP_876844 | 76638096 | ||
| Hypothetical protein XP_876914 | 76638098 | ||
| Hypothetical protein XP_881202 | 76639161 | ||
| Hypothetical protein XP_882313 | 76632294 | ||
| Hypothetical protein XP_882414 | 76662668 | ||
| Hypothetical protein XP_882587 | 76630059 | ||
| Hypothetical protein XP_883381 | 76631223 | ||
| Hypothetical protein XP_883390 | 76640533 | ||
| LPR protein | 619 | ||
| Neuroblastoma-amplified protein | 76666100 | ||
| Protein for IMAGE:8054235 | 83406129 | ||
| Protein for MGC:140076 | 92098401 | ||
| RIKEN cDNA 0610040D20 | 61870202 | ||
| RIKEN cDNA 9130210N20 | 76634018 | ||
| Sushi repeat-containing protein | 94966791 | ||
| TAG-278 | 76685297 | ||
| THAP domain containing 4 | 83759162 | ||
| Transmembrane 9 superfamily protein member 4 | 76633198 | ||
| Unknown (protein for MGC:127406) | 73587279 | ||
| Unknown (protein for MGC:140139) | 92096913 | ||
| YEATS domain containing 2 | 76607509 | ||
| ZK1067.4 | 76650437 | ||
| ZK742.2 | 76620462 |
Proteins indicated by ‘+’ were found in the Wegrzyn et al.; 2007 study (47) but not in this study. Proteins indicated by ‘*’ are proteins identified in both the Wegryzn et al.; 2007 study (47) and this study. In addition, protein indicated by ‘#’ were identified by previous focused studies on the amyloid precursor protein (55, 143), cathepsin L (8), and endopin 2C (56). Thus, this table represents the overall proteomic characterization of chromaffin secretory vesicle proteins.
Analyses of selected chromaffin granule proteins by western blots and immunofluorescence confocal microscopy
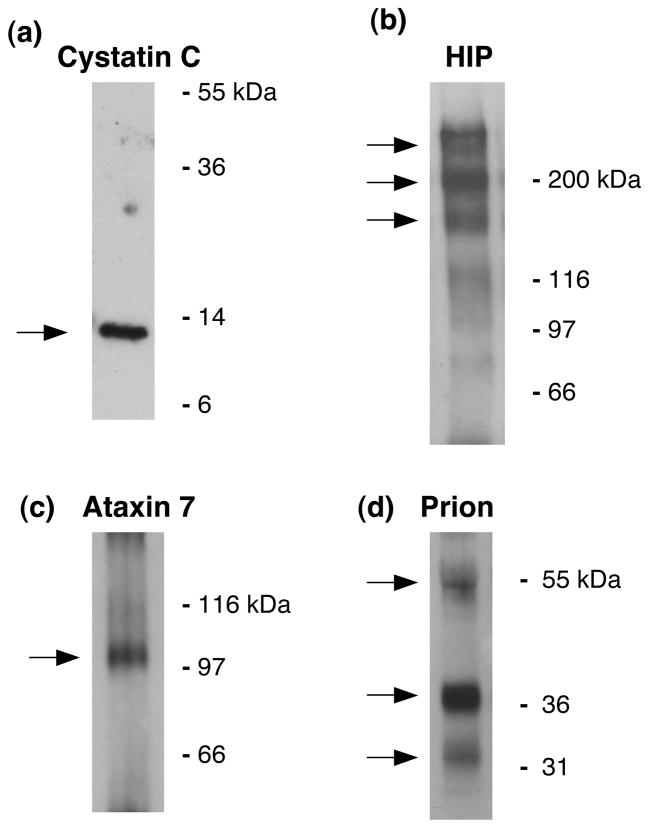
Western blot analyses of chromaffin granules were utilized to assess the presence of several proteins related to neurological diseases. Western blots of cystatin C, huntingtin interacting protein, ataxin 7, and prion protein were conducted using SDS-PAGE gel electrophoresis and western blots methods as we have described previously (48–51). Cystatin C in western blots was detected with anti-cystatin C (at 1:1000 dilution, from US Biological, Swampscott, MA). Huntingtin interacting protein was analyzed in western blots of chromaffin granules with anti-SET2 antisera by immunoprecipitation prior to western blots (at 1:1000 dilution, from Chemicon, Temecula, CA) as described previously (51, 52). Ataxin 7 was detected by anti-ataxin 7 generated by the La Spada laboratory (antisera K (49) utilized at 1:2000, after immunoprecipitation of ataxin 7 performed as we have described previously (51, 52). Detection of prion in western blots utilized monoclonal antibody SAF-84 (1 μl/ml, from Cayman Chemical, Ann Arbor, MI).
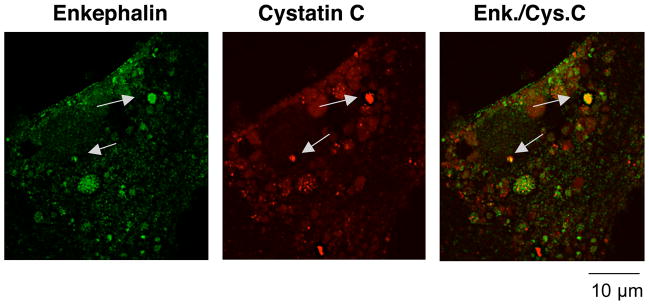
The presence of a neurological disease protein, cystatin C, as an example, in chromaffin cells was assessed by immunofluorescence confocal microscopy to confirm its localization in secretory vesicles. Chromaffin cells in primary culture were prepared from fresh bovine adrenal medulla tissue as previously described (53). Cells were subjected to colocalization studies of enkephalin-containing chromaffin secretory vesicles for the presence of cystatin C (anti-cystatin C rabbit, 1:50 dilution, from US Biological, Swampscott, MA) in enkephalin-containing secretory vesicles (detected by anti-(Met)enkephalin mouse, from Abcam company, Cambridge, MA or from Chemicon-Millipore company, Billerica, MA) by immunofluorescence confocal microscopy, conducted as we have previously described (8, 50). Cystatin C was detected with anti-rabbit IgG-Alexa Fluor 568 (goat) (1:50 dilution, red fluorescence, Molecular Probes, Eugene, Oregon) with comparison to localization of (Met)enkephalin (ME) in secretory vesicles detected with anti-mouse IgG Alexa Fluor 488 (goat) (1:50 dilution, green fluorescence). Immunofluorescent images were obtained with the Delta Vision Spectris Image Deconvolution Systems on an Olympus IX70 confocal microscope using the software Softwrox Explorer from Applied Precision.
Results and Discussion
Identification of an extensive number of chromaffin granules (CG, also known as secretory vesicles) proteins by nano-HPLC Chip MS/MS tandem mass spectrometry
Differential centrifugation was utilized to obtain purified chromaffin granules (CG) from bovine adrenal medulla homogenate, illustrated in figure 1a. This purification scheme is well-established in the field (36–38). Organelles are removed from the CG fraction by a series of centrifugation steps that remove nuclei (P1 fraction), microsomes (S2 fraction), and mitochondria and lysosomes (S3, S4, and S5 fractions, not shown in Fig. 1a). The enriched fraction of CG (P5 fraction) is purified by a 0.32/1.6 M sucrose gradient, resulting in a pellet of purified CG. Comparison on a multi-step sucrose gradient of 2.2 M to 1.2 M sucrose shows that both the crude and purified CG samples contain peaks of (Met)enkephalin at about 1.7–2.0 M sucrose. While the crude CG contains a peak of acid phosphatase (Fig. 1b), a marker for lysosomes, such a peak for lysosomes is absent in the purified CG (Fig. 1c). The purity of these CG have been confirmed by electron microscopy (47) that shows the homogeneity of the preparation. These data and those from other studies (36–38) establish the purity of chromaffin granule obtained by density gradient.
The soluble and membrane fractions of these purified secretory vesicles were separated to provide functional predictions of identified proteins in the soluble secreted pool, or as membrane-related proteins that participate in maintaining integrity of the organelle. Soluble and membrane fractions were obtained by lysis and centrifugation of chromaffin granules in isotonic salt conditions in buffer of pH 6.0 that represents the internal vesicle environment (54).
Furthermore, to enhance nano-LC-MS/MS analyses of moderate to lower abundance proteins, the highly abundant chromogranin A (CgA) protein was removed by affinity chromatography (as described in methods), which removes the major 66–70kDa CgA protein band (Fig. 2). Each of the soluble and membrane fractions were subjected to trypsin digestion and nano-HPLC Chip MS/MS tandem mass spectrometry with the XCT Ultra ion trap mass spectrometer (Agilent) for sensitive analyses of peptides (estimated down to the attomole range). Evaluation of MS/MS spectra by the Spectrum Mill search program yielded identification of proteins in soluble and membrane fractions with ~1% FDR (determined by shuffled decoy database analysis). Subsequently the entire dataset was also searched using an alternate search algorithm, OMSSA, to confirm and validate peptide identifications.
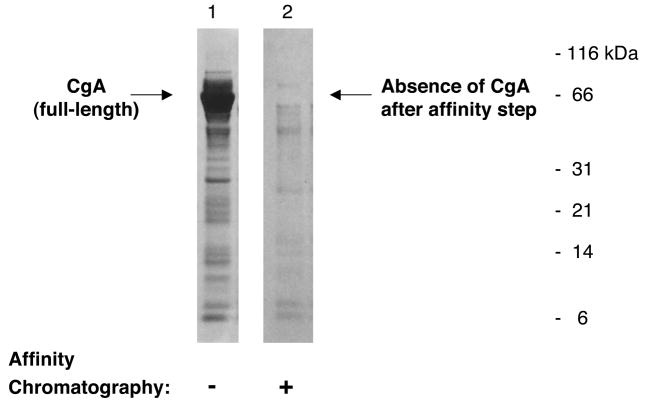
Figure 2. Removal of abundant chromogranin A protein from soluble and membrane fractions of purified chromaffin granules.
Chromaffin granule soluble and membrane fractions were each subjected to calmodulin affinity chromatography to remove the most abundant protein consisting of full-length CgA. The soluble fraction of these chromaffin granules is illustrated (lane 1), showing full-length CgA of ~66–70 kDa that has been identified by mass spectrometry in previous studies (10, 137). After affinity chromatography on calmodulin-Sepharose conducted two times, removal of the full-length CgA is illustrated (lane 2). Equal relative volumes (5 μl) of soluble chromaffin granule sample was applied to lanes 1 and 2 (corresponding to ~2 μg and ~6.5 μg protein, respectively). The CgA depletion step recovered ~30–35% of the original proteins of the soluble chromaffin granule sample. CgA also exists as cleaved proteolytic fragments in the chromaffin granules which presumably are largely removed by the calmodulin-Sepharose affinity step. After the affinity step, the overall pattern of protein bands (lane 2) resembles that of the soluble granule sample before the affinity step, with the exception of removal of CgA protein(s).
Proteins found in the soluble and membrane fractions of the secretory vesicles by the nano-HPLC Chip MS/MS approach are illustrated in Supplemental Tables A and B, respectively. Nano-HPLC Chip MS/MS identified more than 600 proteins in both the soluble and membrane fractions. These results demonstrate the high efficiency of the nano-HPLC Chip MS/MS tandem mass spectrometry system to identify hundreds of proteins from several micrograms of sample per analysis.
Total proteome of chromaffin secretory vesicles
To obtain an overall proteomic view of the purified adrenal medullary secretory vesicles (chromaffin granules) the extensive nano-HPLC Chip MS/MS identification of more than 600 proteins obtained in this study was combined with our prior data of proteins from these secretory vesicles subjected to gel electrophoresis separation prior to MS/MS analyses, (47) and several purified proteins (8, 55–57). The present study identified proteins in chromaffin secretory vesicles after affinity removal of the most abundant chromogranin A (CgA) protein, to allow identification of other proteins of more modest abundance. The prior study of chromaffin secretory vesicles with CgA identified ~100 proteins via one-dimensional gel electrophoresis (47). Thus, the overall proteomic analysis of the chromaffin secretory vesicles of this extensive proteomic study for identification of more than 600 proteins, combined with our prior smaller proteomic study (47), has identified 371 distinct soluble proteins and 384 distinct membrane proteins (Fig. 3, Venn diagram), with 69 proteins present in both soluble and membrane fractions. These data illustrate the presence of a total of 686 distinct proteins identified in the soluble and membrane fractions of chromaffin secretory vesicles.
Figure 3. Venn diagram of common and different proteins in soluble and membrane fractions of chromaffin granules.
This Venn diagram illustrates the the majority of the chromaffin secretory vesicle proteins identified in this study using nano-HPLC Chip MS/MS, combined with several proteins identified in in earlier proteomic studies using gel electrophoresis for protein enrichment [43]. The soluble fraction contained 371 distinct proteins and the membrane fraction contained 384 distinct proteins. Proteins common to both soluble and membrane fractions are illustrated as the intersecting area of the Venn diagram, indicating 69 proteins that were present in both soluble and membrane compartments of these secretory vesicles. The soluble and membrane fractions contained a total of 686 unique proteins in chromaffin secretory vesicles.
Distinct functional categories of proteins in soluble and membrane components of chromaffin secretory vesicles
The categorization of the chromaffin secretory vesicle proteins indicates the presence of distinct functional categories of biochemical systems (Fig. 4, and Table 1). Protein categories were identified for neurotransmitter and neurohumoural mechanisms, as well as diverse biochemical processes that include maintenance of the internal environment of these vesicles. A large portion of proteins participate in regulated secretion via signal transduction and exocytosis. These functions are combined with vesicular trafficking that involves structural proteins. Protein components within these categories are described in detail below.
Figure 4. Comparison of soluble and membrane proteins by pie charts.
The relative portion of proteins in each functional category are compared for the soluble (panel A) and membrane (panel B) fractions of chromaffin secretory vesicles. Each functional category of the pie chart is shown as a distinct color.
Production of neurotransmitters and neurohumoural factors: neuropeptides and neurohumoural factors, protease systems, neurotransmitter enzymes/transporters, and receptors
Proteins involved in secretory vesicle-mediated cell-cell communication were identified as proneuropeptides and processing proteases, neurohumoural agents, enzymes and transporters for classical small molecule neurotransmitters, and receptors. Numerous proneuropeptide and prohormone precursors were identified such as proenkephalin, proNPY, chromogranins, and others that undergo proteolytic processing (5, 8–10, 46, 50, 58–63) to generate active neuropeptides that function as neurotransmitters and hormones. These vesicles also contain several neurohumoural factors such as VGF nerve growth factor and vascular endothelial growth factor (64–66).
Numerous proteases of the serine, aspartyl, cysteine, and metalloprotease classes were identified. Several subtilisin-like prohormone convertases (PC1/3, PC2, PACE 4) were identified, which participate in proneuropeptide processing (5, 67–69). The cysteine protease cathepsin L has been identified by MS/MS tandem mass spectrometry utilizing enrichment prior to MS/MS (5, 8); cathepsin L participates in secretory vesicles for processing proneuropeptides for the production of enkephalin (5, 8), NPY (62), and POMC-derived peptide hormones consisting of β-endorphin, ACTH, and α-MSH (46). The cysteine protease cathepsin B was identified, which was recently discovered to participate in the production of neurotoxic beta-amyloid related to Alzheimer’s disease (45, 70). The aspartyl protease cathepsin D was also present [38]. Numerous metalloproteases were found including the ADAM metallopeptidase, and carboxypeptidases (D, E, and G2) (71–73). Components of the ubiquitin system for protein degradation were identified (74–76). In addition, endogenous protease inhibitors were indicated that included TIMP-1 (77), inhibitor of PC1/3 (78), and cystatins (79). These results indicate the presence of numerous protease and protease inhibitors in these secretory vesicles.
Proteins that participate in the biosynthesis and metabolism of small molecule neurotransmitters, as well as receptors, were identified. Catecholamine synthesizing enzymes were present that include tyrosine hydroxylase, dopamine beta-monooxygenase, and PNMT (phenylethanolamine N-methyltransferase) (80, 81). In addition, transporters for vesicular localization of catecholamines were identified (82–84). Interestingly, several receptor proteins were identified which may be present in secretory vesicles for transport to the plasma membrane [85].
Biochemical processes: enzymes, carbohydrate and lipid functions, protein folding, transporters
The secretory vesicles contained enzymes for a variety of biochemical reactions. Enzymes for numerous biochemical reactions were identified including aspartate aminotransferase for amino acid modification, cofactor related tetrahydrofolate synthase, and enolase (86–88). In addition, a number of carbohydrate and lipid metabolizing enzymes were identified (89–90). These included carbohydrate transferases, mannosidase, and glucosidase. Lipid-related enzymes included arachidonate lipoxygenase, phospholipase, and acyl-CoA synthetase.
Internal environment of secretory vesicles: reduction oxidation, ATPases and nucleotide metabolism
Homeostatic mechanisms for maintaining the unique internal conditions of the secretory vesicle require reduction-oxidation regulation, ATP/nucleotide related proteins for pH regulation, and protein factors for protein folding. Regulation of reducing and oxidative conditions is evident with the presence of cytochromes, perioxidase, catalase, and related proteins. Numerous membrane-associated ATPase isoforms were present which participate in proton transport that maintains the acidic internal pH (pH 5–6) of these secretory vesicles (54, 91–95). Conditions for appropriate protein folding or protein configuration are represented by chaperone proteins that include heat shock proteins, chaperonin, and isomerase (96–98). These regulators of the internal secretory vesicle environment are utilized for effective production of neurotransmitters, hormones, and neurohumoural factors in this organelle.
Regulated secretion: signal transduction and GTP-binding proteins, vesicular trafficking and exocytosis, calcium regulation
Significant representation of secretory vesicle proteins consisted of functions for regulated secretion involving signal transduction and GTP-binding proteins, proteins for exocytosis and vesicular trafficking, and calcium regulation. An extensive collection of Rab GTP-binding proteins (99–103) was present, which are critical for intracellular trafficking and transport of secretory vesicles and in vesicle exocytosis. Furthermore, numerous proteins involved in signal transduction pathways by protein kinases (104–107) and phosphatases (107–109) were identified. The presence of these proteins suggest regulation of the phosphorylation status of target proteins within secretory vesicles. The process of exocytosis of secretory vesicles for release of vesicle contents to the extracellular environment utilizes synaptotagmin isoforms and synaptophysin related proteins (110–112). Also, the presence of kinesin suggests its utilization by dense core secretory vesicles for trafficking to the plasma membrane via interactions with cellular structural proteins (113, 114). Notably, regulated secretion is calcium-dependent, which utilizes proteins that regulate calcium metabolism in secretory vesicles. These proteins include several isoforms of annexins which function in calcium-dependent phospholipid binding during exocytosis (105).
Morphological functions of secretory vesicles: structural proteins, cell adhesion and cell-cell interactions
Numerous structural proteins were identified including collagen, myosin, spectrin, proteoglycan, and tubulin which may function in morphological features of secretory vesicles (116, 117). Such cytoplasmic proteins are utilized for intracellular movement of the secretory vesicles to the plasma membrane for regulated secretion. For example, microtubule and myosin structural proteins may be involved in intracellular granule movement. Such cytoplasmic proteins may be linked to the chromaffin granule membrane through protein interactions, but would lack signal peptide sequences. In addition, several proteins involved in cell adhesion or cell-cell interactions were found, which included cadherin, integrin, laminin, and related components.
Potentially novel functional proteins of secretory vesicles
It was of interest that several identified proteins included those with functions in the immune system, cell growth and development, and transcription and translation. Several proteins with immune system functions were found including immunoglobulins and immunoglobulin receptor. Several studies have demonstrated nervous system stimulation of immunoglobulin secretion (118), as well as immunoglobulin receptor trafficking through regulated secretory vesicles (119). Recent studies have also indicated transport of chemokines in large dense core vesicles as mechanisms for secretion of cytokines (120, 121). Thus, dense core secretory vesicles may be involved in both the regulated secretion of immunological factors as well as neurohumoural factors, hormones, and neurotransmitters.
Proteins involved in translation of RNAs were identified such as ribosomal proteins and RNA-binding proteins. Recent studies have demonstrated localization and translation of mRNAs in axons (122–124), which occur in the vicinity of secretory vesicles that are transported to axons and nerve terminals. It is possible that components for mRNA translation may reside in subregions of the neuroendocrine cell where secretory vesicles undergo transport and trafficking for regulated secretion. An alternative possibility is that contaminating RNA granules may be present in the chromaffin granule preparation, but that is unlikely since RNA granule markers (FMRP, Pur alpha and beta, RACK1, S6, Staufen2, or Syncrip) (125) were not identified in this chromaffin granule proteomic study. Indeed, the purity of the isolated chromaffin granules has been established in prior studies with additional enzyme marker data in this study to demonstrate that chromaffin granules of high purity were utilized for this proteomic study.
In addition, several proteins representing transcription factors were identified. Thus far, little is known about the roles of such factors in secretory vesicles. Furthermore, several miscellaneous and unknown proteins were indicated from MS/MS data analyses.
Chromaffin granule proteins related to neurological diseases
Several key proteins involved in neurological disease mechanisms were present in these secretory vesicles. These vesicles contain several neurodegenerative disease related proteins consisting of the amyloid precursor protein (APP), huntingtin interacting protein, ataxin 7, CLN8 protein, and prion protein (Table 2). The amyloid precursor protein (APP) undergoes proteolytic processing to generate toxic beta-amyloid peptide, a neurotoxic factor involved in the development of Alzheimer’s disease (14–18, 39, 45, 70). Beta-amyloid peptide and proteases for its production have been demonstrated in chromaffin secretory vesicles (39, 45, 70). The protease inhibitor cystatin C is involved in epilepsy (126, 127). The huntingtin interacting protein (19–22) is known to bind to the mutant huntingtin (htt) protein with polyglutamine expansion of Huntington’s disease (128). The ataxin 7 protein is the product of the SCA7 gene that possesses polyGln expansions, representing a CAG triplet-repeat neurodegenerative disease (23–25). The CLN8 protein is involved in genetic EPMR syndrome for epilepsy and mental retardation as a mutant CLN8 autosomal recessive disorder (29–32). The mutant CLN8 gene represents one of several neuronal ceroid lipofuscinoses (NCLs) neurodegenerative disorders characterized by the accumulation of autofluorescent lipopigment in various tissues. The prion protein is a key component of prion neurodegenerative diseases based on misfolding of the prion protein (26–28). It is notable that multiple factors known to participate in severe neurodegenerative diseases are present in chromaffin secretory vesicles.
Table 2.
Proteins Identified in Chromaffin Secretory Vesicles with Neurological and Neurodegenerative Disease Functions.
| Protein | Neurological Disease |
|---|---|
| Amyloid precursor protein | Alzheimer’s Disease |
| Ataxin 7 | Spinocerebellar Ataxia type 7 |
| CLN8 protein | Neuronal Lipofuscinosis, EPMR, epilepsy and mental retardation |
| Cystatin C | Epilepsy |
| Huntingtin interacting protein 1 | Huntington’s Disease |
| KIAA0319 | Dyslexia |
| Nesprin-2 | Muscular Dystrophy |
| P20-CGGBP | Fragile X Syndrome, mental retardation |
| Prion protein | Prion Disease |
| Regulatory factor X4 isoform c | Bipolar Disorder |
Proteins were identified from chromaffin secretory vesicles by LC-MS/MS and subjected to bioinformatic analyses as described in the methods. Several proteins were found which are known to participate in several neurological and neurodegenerative disease conditions.
Furthermore, proteins related to several neurological diseases were identified (Table 2) as the P20-CGGBP protein of the fragile X syndrome for mental retardation (33), the regulatory factor X4 involved in bipolar disorder (34), the KIAA0319 protein involved in dyslexia (35), and nesprin-2 related to muscular dystrophy (129–131). Thus, the dense core secretory vesicle organelle contains several key proteins that participate in neurological diseases.
The presence of selected neurological disease proteins in chromaffin secretory vesicles were assessed by western blots of purified vesicles. Western blots (Fig. 5) show the presence of cystatin C of ~14 kDa (132, 133), huntingtin (htt) interacting protein as several bands of 150–250 kDa that interact with fragments of htt present in brain (48), ataxin 7 of ~98–100 kDa (49, 134), and prion protein of a main and of ~30–35 kDa (135, 136) combined with a 55–60 kDa band (a possible multimer form). Cellular immunofluorescence microscopy indicated the localization of cystatin C (Fig. 6), as example, in chromaffin secretory vesicles that contain the enkephalin peptide neurotransmitter (8). These data show that the LC-MS/MS results can appropriately indicate the presence of such proteins in chromaffin secretory vesicles.
Figure 5. Presence of cystatin C, huntingtin interacting protein, ataxin 7, and prion protein in chromaffin secretory vesicles demonstrated by western blots.
Purified chromaffin secretory vesicles were subjected to western blots for analyses of cystatin C (panel a), huntingtin interactin protein (HIP) (panel b), ataxin 7 (panel c), and prion protein (panel d). Cystatin C of ~12–14 kDa in these vesicles (panel a) is similar in MW (molecular weight) to that reported in other studies [132, 133]. Huntingtin-interacting proteins (3 bands) in the area of ~ 150–250 kDa were observed (panel b). Ataxin 7 of about 98–100 kDa (panel c) is similar to that found in prior studies [49]. Prion protein of several apparent molecular weights of ~30 kDa, 36–40 kDa, and 50–60 kDa were observed (lane d).
Figure 6. Cellular localization of cystatin C with enkephalin-containing secretory vesicles of chromaffin cells in primary culture.
The localization of cystatin C to secretory vesicles that contain the enkephalin neuropeptide was observed by immunofluorescence confocal microscopy of chromaffin cells primary culture. Colocalization of enkephalin (green fluorescence) and cystatin C (red fluorescence) was demonstrated by the yellow fluorescence of merged images. Examples of secretory vesicle colocalization of cystatin C and enkephalin are indicated by the arrows.
Number of proteins identified in distinct functional categories of chromaffin secretory vesicles
Comparisons of the numbers of proteins in each category showed the varying distributions of components within each category in chromaffin secretory vesicles (Table 3). The functional categories of these proteins represent the diverse biochemical systems utilized for secretory vesicle exocytosis of active molecules for cell-cell communication.
Table 3.
Total Number of Proteins Identified in the Soluble and Membrane Secretory Vesicle Compartments in Designated Functional Categories.
| Protein Category | Soluble | Membrane |
|---|---|---|
| Neuropeptides/Neurohumoural | 18 | 19 |
| Protease Systems | 26 | 21 |
| Neurotransmitter Enzymes/Transporters | 9 | 11 |
| Receptors | 14 | 11 |
| Enzymes | 13 | 11 |
| Carbohydrate Functions | 11 | 9 |
| Lipid Functions | 10 | 9 |
| Reduction-Oxidation | 7 | 7 |
| ATPases/Nucleotide Metabolism | 8 | 23 |
| Protein Folding | 2 | 10 |
| Transporters (solute) | 4 | 8 |
| Signal Transduction/GTP-binding | 65 | 76 |
| Vesicular Trafficking and Exocytosis | 17 | 15 |
| Calcium Regulation | 4 | 10 |
| Structural Proteins | 24 | 26 |
| Cell Adhesion/Cell-Cell Interactions | 12 | 10 |
| Cell growth and development | 2 | 1 |
| Immune | 11 | 2 |
| Transcription/Translation | 49 | 47 |
| Miscellaneous | 28 | 37 |
| Unknown | 37 | 15 |
Numbers of proteins found in each functional category for the soluble and membrane compartments of chromaffin secretory vesicles are indicated.
Conclusion: Proteomic data reveals that secretory vesicles utilize multiple categories of proteins utilized for production and release of neuroeffectors for cell-cell communication
Secretory vesicle biosynthesis, storage, and secretion of neuroeffector molecules
This study illustrates the most comprehensive proteomic data of chromaffin secretory vesicles that provide neuroeffectors mediating cell-cell communication from the adrenal medulla. Proteins of these secretory vesicles function in the biogenesis and maturation of secretory vesicles, synthesis and storage of bioactive molecules consisting of neuropeptides and catecholamines, and secretion of bioactive molecules which involves secretory vesicle transport and docking to the plasma membrane for regulated secretion (Fig. 7). These proteins provide the biochemical basis for regulated secretion of active neurotransmitter and neurohumoural agents for cell-cell communication. These data illustrate that the dense core secretory vesicles function with distinct functional protein categories. Production of peptide neurotransmitters and hormones, as well as catecholamine neuroeffectors, within the chromaffin secretory vesicles utilize proteins that control the intravesicular environment with respect to redox conditions, acidity (acid pH at 5.5–6.0), protein folding, as well as carabohydrate and lipid conditions. Transporters are needed for bringing certain neuroeffectors into the chromaffin granule. Regulated secretion of chromaffin granules requires extensive use of signal transduction and GTP-binding proteins, combined with proteins needed for secretory vesicle trafficking and exocytosis (including structural proteins for this purpose). Furthermore, because regulated secretion is calcium-dependent, calcium binding proteins are present to mediate regulated secretion. The proteomic data indicate that secretory vesicles utilize proteins of multiple functions for production, storage, and regulated secretion of neuroeffectors for cell-cell communication.
Figure 7. Multiple protein categories for biosynthesis, storage, and regulated secretion of neuroeffectors for cell-cell communication in health and disease.
The chromaffin granule proteome consists of distinct functional categories of proteins utilized for secretory vesicle production, storage, and regulated secretion of neuroeffector molecules. The architecture of proteins of the soluble (blue area) and membrane (gray area) function in the initial biogenesis and subsequent maturation of secretory vesicles, which produce and store bioactive chemical molecules for secretion. The secreted neurotransmitters and hormones mediate cell-cell communication among physiological target organs.
Complementary biochemical studies assist in substantiating proteomic data
An important criterion for proteomic studies is the subject of confidence and validation of protein identifications obtained from tandem mass spectrometry data. The unique feature of this proteomic study was consideration of protein biochemistry data from these secretory vesicles to guide and enhance appropriate confidence levels in bioinformatic analyses of MS/MS data for protein identification, including single peptide identifications (137). Protein biochemistry studies of chromaffin secretory vesicles provide information of previously identified proteins in this organelle at moderate and low levels; this information can assist in defining reliable scoring thresholds. For example, the serpin endopin 2C is of moderate abundance since it was isolated from these secretory vesicles with a 500-fold enrichment (43), and was observed in this study at intermediate peptide scoring thresholds for identification. Cathepsin B and cathepsin L are low abundance proteins, demonstrated by their enrichment requiring 2 × 105-fold (45) and 2 × 106 – fold purification (8), respectively; after enrichment of these proteins, they were identified with high confidence scoring MS/MS data (8, 45). Confidence levels of these MS/MS data are consistent with previous bioinformatic analyses of MS/MS data (137, 138). These types of biochemical studies assisted in guiding appropriate confidence levels for identification of proteins from tandem mass spectrometry data, including those identified by single peptide (tryptic) identification. Application of biochemical data for relative protein abundances enhances analyses of proteomic data.
Proteomic data of secretory vesicle proteins from adrenal medulla in this study and from other tissues in related studies
Important secretory mechanisms for the chromaffin secretory vesicles are indicated by the expansive nature of these proteomic studies that have identified 686 distinct proteins in chromaffin secretory vesicles. Other studies of regulated secretory vesicles have been reported for the dense core secretory vesicles (144–149) and synaptic vesicles (150–154) for investigation of their proteomic features. While it is of interest to compare the identified proteins among these studies, their different methodologies for purification of organelles and protein extraction conditions, combined with different mass spectrometry instrumentation and different bioinformatic database searches should be considered in details of such comparisons. Nonetheless, features of some of the prior proteomic studies of various secretory vesicle systems are summarized here. A proteomic study of pancreatic zymogen granule membranes yielded identification of 101 proteins by 2-D gel electrophoresis, in-gel trypsin digestion, followed by LC-MALDI (139). Another proteomic study of insulin secretory granules has identified 130 different proteins by SDS-PAGE, excision of gel slices, trypsin digestion, followed by nano-LC-ESI-MS/MS (140). Proteomic analyses of synaptic vesicles, which undergo regulated secretion, identified 410 proteins (141). Analyses of multiple organelles isolated from abundant rat liver yielded identification of proteins of the rough and smooth microsomes, and Golgi fractions of the rat secretory pathway; this study identified 1400 proteins that was possible with multiple fractions from an abundant tissue source (142). However, these rat liver organelle components represent the constitutive secretory pathway present in liver cells, which lack the regulated secretory pathway. It will be of interest for future joint efforts to compare different secretory vesicle systems in different cell types.
Summary
This proteomic study of chromaffin dense core secretory vesicles has provided new knowledge of the protein architecture of the regulated secretory vesicle system. The application of sensitive high throughput nano-HPLC Chip MS/MS to proteomic studies, combined with biochemical information, of the dense core secretory vesicle has revealed the presence of distinct functional protein categories for secretory vesicle production and secretion of bioactive molecules – neurotransmitters and hormones – that control physiological functions through cell-cell communication. Significantly, several proteins involved in neurodegenerative and neurological diseases were identified in these secretory vesicles, suggesting their involvement with the secretory vesicle protein systems in the regulation of cell-cell communication. Overall, this proteomic study has revealed an extensive group of functional protein systems in regulated dense core secretory vesicles for cell-cell communication in health and disease.
Supplementary Material
Acknowledgments
The authors appreciate support of this research by grants to V. Hook consisting of R01DA04271, R01NS24553, and R01MH077305 from the NIH, as well as an award from the Alzheimer’s Association to V. Hook, to D.T. O’Connor consisting of P01HL58120 from NIH, and to A. La Spada consisting of R01EY014061 from the NIH. S. Bark was supported by a NIH Mentored Scientist Award (K01DA023065). The authors also appreciate scientific advice by Dr. Shin-Rong Hwang, and technical assistance by Mr. Thomas Toneff, at the Skaggs School of Pharmacy and Pharmaceutical Sciences, Univ. of Calif., San Diego, La Jolla, CA.
Abbreviations
- ACTH
adrenocorticotropin hormone
- ADAM
a disintegrin and metalloprotease
- AEBSF
4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride
- APP
amyloid precursor protein
- CG
chromaffin granule
- CGS
chromaffin granule soluble fraction
- CGM
chromaffin granule membrane fraction
- CHAPS
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
- EPMR
epilepsy and mental retardation
- FDR
false discovery rate
- GEMSA
guanidinoethylmercaptosuccinic acid
- GTP
guanosine triphosphate
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- α-MSH
α-melanocyte stimulating factor
- nano-HPLC Chip MS/MS
nano-high pressure liquid chromatography Chip tandem mass spectrometry
- NCL
neuronal ceroid lipofuscinoses
- NPY
neuropeptide Y
- PC
prohormone convertase
- PMSF
phenylmethanesulphonylfluoride
- POMC
proopiomelanocortin
- PNMT
phenylethanolamine N-methyltransferase
- TCEP
Tris-(2-carboxyethyl)-phosphine
- TEM
transmission electron microscopy
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gainer H, Russell JT, Loh YP. The enzymology and intracellular organization of peptide precursor processing: the secretory vesicle hypothesis. Neuroendocrinology. 1985;40:171–84. doi: 10.1159/000124070. [DOI] [PubMed] [Google Scholar]
- 2.Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology. 2006;21:124–33. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- 3.Grabner CP, Price SD, Lysakowski A, Cahill AL, Fox AP. Regulation of large dense-core vesicle volume and neurotransmitter content mediated by adaptor protein 3. Proc Natl Acad Sci USA. 2006;103:10035–40. doi: 10.1073/pnas.0509844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scalettar BA. How neurosecretory vesicles release their cargo. Neuroscientist. 2006;12:164–76. doi: 10.1177/1073858405284258. [DOI] [PubMed] [Google Scholar]
- 5.Hook V, Funkelstein L, Lu D, Bark S, et al. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael SW, Winkler H. The adrenal chromaffin cell. Sci Am. 1985;253:40–49. doi: 10.1038/scientificamerican0885-40. [DOI] [PubMed] [Google Scholar]
- 7.Njus D, Kelley PM, Harnadek GJ. The chromaffin vesicle: a model secretory organelle. Physiologist. 1985;28:235–241. [PubMed] [Google Scholar]
- 8.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, et al. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang SR, O’Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J Neurochem. 2003;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JC, Taylor CV, Gaucher SP, Toneff T, et al. Primary sequence characterization of catestatin intermediates and peptides defines proteolytic cleavage sites utilized for converting chromogranin A into active catestatin secreted from neuroendocrine chromaffin cells. Biochemistry. 2003;42:6938–6946. doi: 10.1021/bi0300433. [DOI] [PubMed] [Google Scholar]
- 11.Mosley CA, Taupenot L, Biswas N, Taulane JP, et al. Biogenesis of the secretory granule: chromogranin A coiled-coil structure results in unusual physical properties and suggests a mechanism for granule core condensation. Biochemistry. 2002;46:10999–1012. doi: 10.1021/bi700704r. [DOI] [PubMed] [Google Scholar]
- 12.Tabares L, Alés E, Lindau M. Alvarez de Toledo G. Exocytosis of catecholamine CA-containing and CA-free granules in chromaffin cells. J Biol Chem. 2001;276:39974–9. doi: 10.1074/jbc.M106498200. [DOI] [PubMed] [Google Scholar]
- 13.Sawada K, Echigo N, Juge N, Miyaji T, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–6. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 15.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, et al. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 17.Sisodia SS, Price DL. Role of the beta-amyloid protein in Alzheimer’s disease. FASEB J. 1995;9:366–70. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- 18.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115:1121–9. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passani LA, Bedford MT, Faber PW, McGinnis KM, Sharp AH, Gusella JF, Vonsattel JP. MacDonald M.E; Huntingtin’s WW domain partners in Huntington’s disease post-mortem brain fulfill genetic criteria for direct involvement in Huntington’s diseaes pathogenesis. Human Molecular Genetics. 2000;9:2175–82. doi: 10.1093/hmg/9.14.2175. [DOI] [PubMed] [Google Scholar]
- 20.Rega S, Stiewe T, Chang DI, Pollmeier B, Esche H, Bardenheuer W, Marquitan G, Putzer BM. Identification of the fullk-length huntingtin-interacting protein p213HBP/HYPB as a DNA-binding factor. Molecular and Cellular Neurosci. 2001;18:68–79. doi: 10.1006/mcne.2001.1004. [DOI] [PubMed] [Google Scholar]
- 21.Wanker EE, Rovira C, Scherzinger E, Hasenbank R, et al. HIP-I: a huntingtin interacting protein isolated by the yeast two-hybrid system. Hum Mol Genet. 1987;6:487–95. doi: 10.1093/hmg/6.3.487. [DOI] [PubMed] [Google Scholar]
- 22.Truant R, Atwal R, Burtnik A. Hypothesis: Huntingtin may function in membrane association and vesicular trafficking. Biochem Cell Biol. 2006;84:912–7. doi: 10.1139/o06-181. [DOI] [PubMed] [Google Scholar]
- 23.David G, Abbas N, Stevanin G, Dürr A, et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet. 1997;17:65–70. doi: 10.1038/ng0997-65. [DOI] [PubMed] [Google Scholar]
- 24.Holmberg M, Duyckaerts C, Dürr A, Cancel G, et al. Spinocerebellar ataxia type 7 (SCA7): a neurodegenerative disorder with neuronal intranuclear inclusions. Hum Mol Genet. 1998;7:913–918. doi: 10.1093/hmg/7.5.913. [DOI] [PubMed] [Google Scholar]
- 25.Michalik A, Martin JJ. Van Broeckhoven C. Spinocerebellar ataxia type 7 associated with pigmentary retinal dystrophy. Eu J Hum Genet. 2004;12:2–15. doi: 10.1038/sj.ejhg.5201108. [DOI] [PubMed] [Google Scholar]
- 26.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prusiner SB. Shattuck lecture--neurodegenerative diseases and prions. N Engl J Med. 2001;344:1516–26. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 28.Kovacs GG, Budka H. Prion diseases: from protein to cell pathology. Am J Pathol. 2008;172:555–65. doi: 10.2353/ajpath.2008.070442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranta S, Lehesjoki AE. Northern epilepsy, a new member of the NCL family. Neurol Sci. 2000;21:S43–7. doi: 10.1007/s100720070039. [DOI] [PubMed] [Google Scholar]
- 30.Ranta S, Savukoski M, Santavuori P, Haltia M. Studies of homogenous populations: CLN5 and CLN8. Adv Genet. 2001;45:123–40. doi: 10.1016/s0065-2660(01)45007-3. [DOI] [PubMed] [Google Scholar]
- 31.Hermansson M, Käkelä R, Berghäll M, Lehesjoki AE, et al. Mass spectrometric analysis reveals changes in phospholipid, neutral sphingolipid and sulfatide molecular species in progressive epilepsy with mental retardation, EPMR, brain: a case study. J Neurochem. 2005;95:609–17. doi: 10.1111/j.1471-4159.2005.03376.x. [DOI] [PubMed] [Google Scholar]
- 32.Striano P, Specchio N, Biancheri R, Cannelli N, et al. Clinical and electrophysiological features of epilepsy in Italian patients with CLN8 mutations. Epilepsy Behav. 2007;10:187–91. doi: 10.1016/j.yebeh.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Deissler H, Wilm M, Genç B, Schmitz B, et al. Rapid protein sequencing by tandem mass spectrometry and cDNA cloning of p20-CGGBP. A novel protein that binds to the unstable triplet repeat 5′-d(CGG)n-3′ in the human FMR1 gene. J Biol Chem. 1997;272:16761–8. doi: 10.1074/jbc.272.27.16761. [DOI] [PubMed] [Google Scholar]
- 34.Glaser B, Kirov G, Bray NJ, Green E, et al. Identification of a potential bipolar risk haplotype in the gene encoding the winged-helix transcription factor RFX4. Mol Psychiatry. 2005;10:920–7. doi: 10.1038/sj.mp.4001689. [DOI] [PubMed] [Google Scholar]
- 35.Velayos-Baeza A, Toma C, Paracchini S, Monaco AP. The dyslexia-associated gene KIAA0319 encodes highly N- and O-glycosylated plasma membrane and secreted isoforms. Hum Mol Genet. 2008;17:859–71. doi: 10.1093/hmg/ddm358. [DOI] [PubMed] [Google Scholar]
- 36.Smith AD, Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967;103:480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gratzl M, Krieger-Brauer H, Ekerdt R. Latent acetylcholinesterase in secretory vesicles isolated from adrenal medulla. Biochim et Biophys Acta. 1981;649:355–366. doi: 10.1016/0005-2736(81)90425-9. [DOI] [PubMed] [Google Scholar]
- 38.Hook VH, Eiden LE. Two peptidases that convert 125I-Lys-Arg-(Met)enkephalin and 125I-(Met)enkephalin-Arg6, respectively, to 125I-(Met)enkephalin in bovine adrenal medullary chromaffin granules. FEBS Lett. 1984;172:212–218. doi: 10.1016/0014-5793(84)81128-x. [DOI] [PubMed] [Google Scholar]
- 39.Yoo SH. Identification of the Ca(2+)-dependent calmodulin-binding region of chromogranin A. Biochemistry. 1992;31:6134–6140. doi: 10.1021/bi00141a025. [DOI] [PubMed] [Google Scholar]
- 40.Zybailov BL, Florens L, Washburn MP. Quantitative shotgun proteomics using a protease with broad specificty and normalized spectral abundance factors. Mol Biosyst. 2007;3:354–360. doi: 10.1039/b701483j. [DOI] [PubMed] [Google Scholar]
- 41.Wang G, Wu WW, Zhang Z, Masilamani RF, Shen RF. Decoy methods for assessing false positives and false discovery rates in shotgun proteomics. Anal Chem. 2009;81:146–159. doi: 10.1021/ac801664q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higdon P, Kolker E. A predictive model for identifying proteins by a single peptide match. Bioinformatics. 2007;23:277–280. doi: 10.1093/bioinformatics/btl595. [DOI] [PubMed] [Google Scholar]
- 43.Hook VYH, Tezapsidis N, Hwang S-R, Sei C, et al. α1-antichymotrypsin-like proteins I and II purified from bovine adrenal medulla are enriched in chromaffin granules and inhibit the proenkephalin processing enzyme “prohormone thiol protease”. J Neurochem. 1999;73:59–69. doi: 10.1046/j.1471-4159.1999.0730059.x. [DOI] [PubMed] [Google Scholar]
- 44.Krieger TJ, Hook VYH. Purification and characterization of a cathepsin D protease from bovine chromaffin granules. Biochemistry. 1992;31:4223–4231. doi: 10.1021/bi00132a011. [DOI] [PubMed] [Google Scholar]
- 45.Hook V, Toneff T, Bogyo M, Greenbaum D, et al. Inhibition of cathepsin B reduces beta-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate beta-secretase of Alzheimer’s disease. Biol Chem. 2005;386:931–940. doi: 10.1515/BC.2005.108. [DOI] [PubMed] [Google Scholar]
- 46.Azaryan AV, Krieger TJ, Hook VYH. Purification and characteristics of the candidate prohormone processing proteases PC2 and PC 1/3 from bovine adrenal medulla chromaffin granules. J Biol Chem. 1995;270:8201–8208. doi: 10.1074/jbc.270.14.8201. [DOI] [PubMed] [Google Scholar]
- 47.Wegrzyn JL, Lee J, Neveu JM, Lane WS, Hook V. Proteomics of neuroendocrine secretory vesicles reveal distinct functional systems for biosynthesis and exocytosis of peptide hormones and neurotransmitters. J Proteome Research. 2007;6:1652–1665. doi: 10.1021/pr060503p. [DOI] [PubMed] [Google Scholar]
- 48.Mende-Mueller LM, Toneff T, Hwang SR, Chesselet M-F, Hook VYH. Tissue-specific proteolysis of huntingtin (htt) in human brain: evidence of enhanced levels of N- and C-terminal htt fragments in Huntington’s disease striatum. J Neurosci. 2001;21:1830–1837. doi: 10.1523/JNEUROSCI.21-06-01830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young JE, Gouw L, Propp S, Sopher BL, et al. Proteolytic cleavage of ataxin-7 by caspase-7 modulates cellular toxicity and transcriptional dysregulation. J Biol Chem. 2007;282:30150–30160. doi: 10.1074/jbc.M705265200. [DOI] [PubMed] [Google Scholar]
- 50.Funkelstein L, Toneff T, Mosier C, Hwang SR, et al. Major role of cathepsin L for producing the peptide hormones ACTH, β–endorphin, and α-MSH, illustrated by protease gene knockout and expression. J Biol Chem. 2008;283:5652–59. doi: 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang SR, Garza C, Mosier C, Toneff T, et al. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J Biol Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- 52.Hook VYH, Toneff T, Aaron W, Yasothronsrikul S, et al. β-endorphin peptide in regulated secretory vesicles of chromaffin cells; evidence for multiple cystein proteolytic activities in distinct pathways for β-secretase activity in chromaffin vesicles. J Neurochem. 2002;81:237–256. doi: 10.1046/j.1471-4159.2002.00794.x. [DOI] [PubMed] [Google Scholar]
- 53.O’Connor DT, Mahata S, Mahata M, Jiang Q, et al. Primary culture of bovine chromaffin cells. Nat Protoc. 2007;2:1248–1253. doi: 10.1038/nprot.2007.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollard HB, Shindo H, Creutz CE, Pazoles CJ, Cohen JS. Internal pH and state of ATP in adrenergic chromaffin granules determined by 31P nuclear magnetic resonance spectroscopy. Biol Chem. 1979;254:1170–7. [PubMed] [Google Scholar]
- 55.Tezapsidis N, Li H-G, Ripellino JA, Efthimiopoulos S, Vassilacopoulou D, Sambamurti K, Toneff T, Yasothornsrikul S, Hook VYH, Robakis NK. Release of nontransmembrane full-length Alzheimer’s amyloid precursor protein from the lumenar surface of chromaffin granule membranes. Biochemistry. 1998;37:1274–1282. doi: 10.1021/bi9714159. [DOI] [PubMed] [Google Scholar]
- 56.Hwang S-R, Stoka V, Turk V, Hook VYH. The novel bovine serpin endopin 2C demonstrates selective inhibition of the cysteine protease cathepsin L compared to the serine protease elastase, in cross-class inhibition. Biochemistry. 2005;44:7757–7767. doi: 10.1021/bi050053z. [DOI] [PubMed] [Google Scholar]
- 57.Hook V. Neuroproteases in peptide neurotransmission and neurodegenerative diseases: applications to drug discovery research. Bio Drugs. 2006;20:105–119. doi: 10.2165/00063030-200620020-00005. [DOI] [PubMed] [Google Scholar]
- 58.Goumon Y, Lugardon K, Gadroy P, Strub JM, et al. Processing of proenkephalin-A in bovine chromaffin cells. Identification of natural derived fragments by N-terminal sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Biol Chem. 2000;75:38355–62. doi: 10.1074/jbc.M007557200. [DOI] [PubMed] [Google Scholar]
- 59.Hwang SR, Hook V. Zinc regulation of aminopeptidase B involved in neuropepide production. FEBS Lett. 2008;582:2527–2531. doi: 10.1016/j.febslet.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taupenot L, Harper KL. O’Connor D.T. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 61.Taylor CV, Taupenot L, Mahata SK, Mahata M, et al. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A. Determination of proteolytic cleavage sites in hormone storage granules. J Biol Chem. 2000;275:22905–22915. doi: 10.1074/jbc.M001232200. [DOI] [PubMed] [Google Scholar]
- 62.Funkelstein L, Toneff T, Hwang S-R, Reinheckel T, Peters C, Hook V. Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J Neurochem. 2008;106:384–389. doi: 10.1111/j.1471-4159.2008.05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hook V, Toneff T, Baylon S, Sei C. Differential activation of enkephalin, galanin, somatostatin, NPY, and VIP neuropeptide production by stimulators of protein kinases A and C in neuroendocrine chromaffin cells. Neuropeptides. 2008;42:503–511. doi: 10.1016/j.npep.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Claffey KP, Wilkison WO, Spiegelman BM. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992;267:16317–16322. [PubMed] [Google Scholar]
- 65.Rindi G, Licini L, Necchi V, Bottarelli L, et al. Peptide products of the neurotrophin-inducible gene vgf are produced in human neuroendocrine cells from early development and increase in hyperplasia and neoplasia. J Clin Endocrinol Metab. 2007;92:2811–2815. doi: 10.1210/jc.2007-0035. [DOI] [PubMed] [Google Scholar]
- 66.Canu N, Possenti R, Ricco AS, Rocchi M, Levi A. Cloning, structural organization analysis, and chromosomal assignment of the human gene for the neurosecretory protein VGF. Genomics. 1997;45:443–446. doi: 10.1006/geno.1997.4945. [DOI] [PubMed] [Google Scholar]
- 67.Seidah NG, Mayer G, Zaid A, Rousselet E, et al. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol. 2008;40:1111–25. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 68.Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–9. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 69.Fugère M, Day R. Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends Pharmacol Sci. 2005;26:294–301. doi: 10.1016/j.tips.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hook V, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce Abeta in transgenic Alzheimer’s disease mice expressing the wild type, but not the Swedish mutant, beta-secretase APP site. J Biol Chem. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 71.Kesteloot F, Desmoulière A, Leclercq I, Thiry M, et al. ADAM metallopeptidase with thrombospondin type 1 motif 2 inactivation reduces the extent and stability of carbon tetrachloride-induced hepatic fibrosis in mice. Hepatology. 2007;46:1620–31. doi: 10.1002/hep.21868. [DOI] [PubMed] [Google Scholar]
- 72.Xin X, Varlamov O, Day R, Dong W, et al. Cloning and sequence analysis of cDNA encoding rat carboxypeptidase D. DNA Cell Biol. 1997;16:897–909. doi: 10.1089/dna.1997.16.897. [DOI] [PubMed] [Google Scholar]
- 73.Jung YK, Kunczt CJ, Pearson RK, Dixon JE, Fricker LD. Structural characterization of the rat carboxypeptidase-E gene. Mol Endocrinol. 1991;5:1257–1268. doi: 10.1210/mend-5-9-1257. [DOI] [PubMed] [Google Scholar]
- 74.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 75.Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–87. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 76.Ciechanover A. The ubiquitin proteolytic system: from an idea to the patient bed. Proc Am Thorac Soc. 2006;3:21–31. doi: 10.1513/pats.200510-106JH. [DOI] [PubMed] [Google Scholar]
- 77.Nagase H, Brew K. Designing TIMP (tissue inhibitor of metalloproteinases) variants that are selective metalloproteinase inhibitors. Biochem Soc Symp. 2003;70:201–12. doi: 10.1042/bss0700201. [DOI] [PubMed] [Google Scholar]
- 78.Qian Y, Devi LA, Mzhavia N, Munzer S, et al. The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1. J Biol Chem. 2000;275:23596–601. doi: 10.1074/jbc.M001583200. [DOI] [PubMed] [Google Scholar]
- 79.Turk V, Stoka V, Turk D. Cystatins: biochemical and structural properties, and medical relevance. Front Biosci. 2008;13:5406–20. doi: 10.2741/3089. [DOI] [PubMed] [Google Scholar]
- 80.Kuhar MJ, Couceyro PR, Lambert PD. Catecholamines. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry. 6. Lippincott Williams and Wilkins Publisher; Philadelphia: 1999. pp. 243–262. [Google Scholar]
- 81.Flatmark T, Almas B, Ziegler MG. Catecholamine metabolism: an update on key biosynthetic enzymes and vesicular monoamine transporters. Ann N Y Acad Sci. 2002;971:69–75. doi: 10.1111/j.1749-6632.2002.tb04436.x. [DOI] [PubMed] [Google Scholar]
- 82.Surratt CK, Persico AM, Yang XD, Edgar SR, et al. A human synaptic vesicle monoamine transporter cDNA predicts posttranslational modifications, reveals chromosome 10 gene localization and identifies TaqI RFLPs. FEBS Lett. 2003;318:325–330. doi: 10.1016/0014-5793(93)80539-7. [DOI] [PubMed] [Google Scholar]
- 83.Erickson JD, Eiden LE. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993;61:2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 84.Waites CL, Mehta A, Tan PK, Thomas G, et al. An acidic motif retains vesicular monoamine transporter 2 on large dense core vesicles. J Cell Biol. 2001;152:1159–68. doi: 10.1083/jcb.152.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Julius D, Basbaum AI. A neuropeptide courier for delta-opioid receptors? Cell. 2005;122:496–8. doi: 10.1016/j.cell.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 86.Bousquet-Lemercier B, Pol S, Pave-Preux M, Hanoune J, Barouki R. Properties of human liver cytosolic aspartate aminotransferase mRNAs generated by alternative polyadenylation site selection. Biochemistry. 1990;29:5293–5299. doi: 10.1021/bi00474a011. [DOI] [PubMed] [Google Scholar]
- 87.Erbe RW. Genetic aspects of folate metabolism. Adv Hum Genet. 1979;9:293–354. 367–9. doi: 10.1007/978-1-4615-8276-2_5. [DOI] [PubMed] [Google Scholar]
- 88.Voet D, Voet JG. Biochemistry. 2. John Wiley & Sons; New York: 1995. pp. 444–463. [Google Scholar]
- 89.Voet D, Voet JG. Biochemistry. 2. John Wiley & Sons; New York: 1995. pp. 599–617. [Google Scholar]
- 90.Voet D, Voet JG. Biochemistry. 2. John Wiley & Sons; New York: 1995. pp. 662–720. [Google Scholar]
- 91.Loh YP, Tam WW, Russell JT. Measurement of delta pH and membrane potential in secretory vesicles isolated from bovine pituitary intermediate lobe. J Biol Chem. 1984;259:8238–45. [PubMed] [Google Scholar]
- 92.Taupenot L, Harper KL, O’Connor DT. Role of H+-ATPase-mediated acidification in sorting and release of the regulated secretory protein chromogranin A: Evidence for a vesiculogenic function. J Biol Chem. 2005;280:3885–3897. doi: 10.1074/jbc.M408197200. [DOI] [PubMed] [Google Scholar]
- 93.Paulusma CC, Oude Elferink RPJ. The type 4 subfamily of P-type ATPases, putative aminophospholipid translocases with a role in human disease. Biochim et Biophys Acta. 2005;1741:11–24. doi: 10.1016/j.bbadis.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Nelson N. Structure and function of V-ATPases in endocytic and secretory organelles. J Exp Biol. 1992;172:149–153. doi: 10.1242/jeb.172.1.149. [DOI] [PubMed] [Google Scholar]
- 95.Leon A, McKearin D. Identification of TER94, an AAA ATPase protein, as a Bam-dependent component of the Drosophila fusome. Mol Biol Cell. 1999;10:3825–3834. doi: 10.1091/mbc.10.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohtsuka K, Hata M. Molecular chaperone function of mammalian Hsp70 and Hsp40—a review. Int J Hyperthermia. 2000;16:231–245. doi: 10.1080/026567300285259. [DOI] [PubMed] [Google Scholar]
- 97.Martin J. Protein folding assisted by the GroEL/GroES chaperonin system. Biochemistry. 1998;63:374–381. [PubMed] [Google Scholar]
- 98.Takaki Y, Muta T, Iwanaga SA. Peptidyl-prolyl cis/trans-isomerase (cyclophilin G) in regulated secretory granules. J Biol Chem. 1997;272:28615–28621. doi: 10.1074/jbc.272.45.28615. [DOI] [PubMed] [Google Scholar]
- 99.Chung SH, Takai Y, Holz RW. Evidence that the Rab3a-binding protein, rabphilin3a, enhances regulated secretion. J Biol Chem. 1995;270:16714–16718. doi: 10.1074/jbc.270.28.16714. [DOI] [PubMed] [Google Scholar]
- 100.Pfeffer S, Aivazian D. Targeting RAB GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 101.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;250:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol 2: reviews. 2001:3007.1–3007.7. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 104.Smith CM, Shindyalov IN, Veretnik S, Gribskov M, et al. The protein kinase resource. Trends Biochem Sci. 1997;22:444–6. doi: 10.1016/s0968-0004(97)01131-6. [DOI] [PubMed] [Google Scholar]
- 105.Taylor SS, Knighton DR, Zheng J, Sowadski JM, et al. A template for the protein kinase family. Trends Biochem Sci. 2003;18:84–9. doi: 10.1016/0968-0004(93)80001-r. [DOI] [PubMed] [Google Scholar]
- 106.Nagy G, Reim K, Matti U, Brose N, et al. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–29. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 107.Sefton BM. Overview of protein phosphorylation. Curr Protoc Cell Biol. 2001;Chapter 14(Unit 14.1) doi: 10.1002/0471143030.cb1401s00. [DOI] [PubMed] [Google Scholar]
- 108.Shenolikar S. Analysis of protein phosphatases: toolbox for unraveling cell signaling networks. Methods Mol Biol. 2007;365:1–8. doi: 10.1385/1-59745-267-X:1. [DOI] [PubMed] [Google Scholar]
- 109.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 110.Hu K, Rickman C, Carroll J, Davletov BA. Common mechanism for the regulation of vesicular SNAREs on phospholipid membranes. Biochem J. 2004;377:781–785. doi: 10.1042/BJ20031164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshihara M, Montana ES. The synaptotagmins: calcium sensors for vesicular trafficking. Neuroscientist. 2004;10:566–574. doi: 10.1177/1073858404268770. [DOI] [PubMed] [Google Scholar]
- 112.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 113.Goldstein LS. Kinesin molecular motors: transport pathways, receptors, and human disease. Proc Natl Acad Sci USA. 2001;98:6999–7003. doi: 10.1073/pnas.111145298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;2:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 115.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 116.Lodish H, Berk A, Zipursky SL, Matsudaira P, et al. Molecular Biology of the Cell. 4. W.H., Freeman and Co; New York: 2000. pp. 796–845. [Google Scholar]
- 117.Alberts B, Johnson A, Lewis J, Raff M, et al. The Cell. 4. Garland Science; New York: 2002. pp. 907–980. [Google Scholar]
- 118.Carpenter GH, Proctor GB, Anderson LC, Zhang XS, Garrett JR. Immunoglobulin A secretion into saliva during dual sympathetic and parasympathetic nerve stimulation of rat submandibular glands. Exp Physiol. 2000;85:281–6. [PubMed] [Google Scholar]
- 119.Evans E, Zhang W, Jerdeva G, Chen CY, et al. Direct interaction between Rab3D and the polymeric immunoglobulin receptor and trafficking through regulated secretory vesicles in lacrimal gland acinar cells. Am J Physiol Cell Physiol. 2008;294:C662–74. doi: 10.1152/ajpcell.00623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Jong EK, Vinet J, Stanulovic VS, Meijer M, et al. Expression, transport, and axonal sorting of neuronal CCL21 in large dense-core vesicles. FASEB J. 2008;2:4136–4145. doi: 10.1096/fj.07-101907. [DOI] [PubMed] [Google Scholar]
- 121.Moqbel R, Coughlin JJ. Differential secretion of cytokines. Sci STKE. 2006;338:26. doi: 10.1126/stke.3382006pe26. [DOI] [PubMed] [Google Scholar]
- 122.Wang W, van Niekerk E, Willis DE, Twiss JL. RNA transport and localized protein synthesis in neurological disorders and neural repair. Dev Neurobiol. 2007;67:1166–82. doi: 10.1002/dneu.20511. [DOI] [PubMed] [Google Scholar]
- 123.Sotelo-Silveira JR, Calliari A, Kun A, Koenig E. Sotelo J.R. RNA trafficking in axons. Traffic. 2006;7:508–15. doi: 10.1111/j.1600-0854.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 124.Job C, Eberwine J. Localization and translation of mRNA in dendrites and axons. Nat Rev Neurosci. 2001;2:889–98. doi: 10.1038/35104069. [DOI] [PubMed] [Google Scholar]
- 125.Elvira G, Wasiak S, Blandord V, Tong XK, Seranno A, Fan X, del Rayo Sanchez-Carbente M, Servant F, Bell AW, Boismenu BD, Lacaille JC, McPherson PS, DesGroseiller L, Sossin WS. Characterization of an RNA granule from developing brain. Molecular & Celluar Proteomics. 2006;5.4:635–651. doi: 10.1074/mcp.M500255-MCP200. [DOI] [PubMed] [Google Scholar]
- 126.Lukasiuk K, Pirttilä TJ, Pitkänen A. Upregulation of cystatin C expression in the rat hippocampus during epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Epilepsia. 2002;43:137–45. doi: 10.1046/j.1528-1157.43.s.5.20.x. [DOI] [PubMed] [Google Scholar]
- 127.Aronica E, Van Vliet EA, Hendriksen E, Troost D, et al. Cystatin C, a cysteine protease inhibitor, is persistently up-regulated in neurons and glia in a rat model for mesial temporal lobe epilepsy. Eur J Neurosci. 2001;14:1485–91. doi: 10.1046/j.0953-816x.2001.01779.x. [DOI] [PubMed] [Google Scholar]
- 128.MacDonald ME, Ambrose CM, Duyao MP, Ricchaqrds H, Myers RH, et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;26:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Q, Bethmann C, Worth NF, Davies JD, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–33. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 130.Wheeler MA, Davies JD, Zhang Q, Emerson LJ, et al. Distinct functional domains in nesprin-1alpha and nesprin-2beta bind directly to emerin and both interactions are disrupted in X-linked Emery-Dreifuss muscular dystrophy. Exp Cell Res. 2007;313:2845–57. doi: 10.1016/j.yexcr.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 131.Warren DT, Zhang Q, Weissberg PL, Shanahan CM. Nesprins: intracellular scaffolds that maintain cell architecture and coordinate cell function? Expert Rev Mol Med. 2005;7:1–15. doi: 10.1017/S1462399405009294. [DOI] [PubMed] [Google Scholar]
- 132.Abrahamson M, Mason RW, Hansson H, Buttle DJ, et al. Human cystatin C. Biochem J. 1991;273:621–626. doi: 10.1042/bj2730621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alvarez-Fernandez M, Barrett AJ, Gerhartz B, Dando PM, et al. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem. 1999;274:19195–19203. doi: 10.1074/jbc.274.27.19195. [DOI] [PubMed] [Google Scholar]
- 134.Custer SK, Garden GA, Gill N, Rueb U, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9:1302–11. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- 135.Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–27. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- 136.Marcotte EM, Eisenberg D. Chicken prion tandem repeats form a stable, protease-resistant domain. Biochemistry. 1999;38:667–76. doi: 10.1021/bi981487f. [DOI] [PubMed] [Google Scholar]
- 137.Lee JC, Hook V. Proteolytic fragments of chromogranins A and B represent major components of chromaffin granules, illustrated by 2-D proteomics with NH2-terminal Edman peptide sequencing and MALDI-TOF MS. Biochemistry. 2009;48:5254–5262. doi: 10.1021/bi9002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kapp EA, Schutz F, Connolly LM, Chakel JA, et al. An evaluation, comparison, and accurate benchmarking of several publicly available MS/MS search algorithms: Sensitivity and specificity analysis. Proteomics. 2005;5:3475–3490. doi: 10.1002/pmic.200500126. [DOI] [PubMed] [Google Scholar]
- 139.Chen X, Walker AK, Strahler JR, Simon ES, et al. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics. 2006;5:306–12. doi: 10.1074/mcp.M500172-MCP200. [DOI] [PubMed] [Google Scholar]
- 140.Brunner Y, Couté Y, Lezzi M, Foti M, et al. Proteomics analysis of insulin secretory granules. Mol Cell Proteomics. 2007;6:1007–17. doi: 10.1074/mcp.M600443-MCP200. [DOI] [PubMed] [Google Scholar]
- 141.Takamori S, Holt M, Stenius K, Lemke EA, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–46. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 142.Gilchrist A, Au CE, Hiding J, Bell AW, et al. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–81. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 143.Vassilacopoulou D, Ripellino JA, Tezapsidis N, Hook VYH, Robakis NK. Full-length and truncated Alzheimer amyloid precursors in chromaffin granules: solubilization of membrane amyloid precursor is mediated by an enzymatic mechanism. J Neurochem. 1995;64:2140–2146. doi: 10.1046/j.1471-4159.1995.64052140.x. [DOI] [PubMed] [Google Scholar]
- 144.Gauthier DJ, Sobota JA, Ferraro F, Mains RE. Lazure C. Flow cytometry-assisted purification and proteomic analysis of the corticotropes dense-core secretory granules. Proteomics. 2008;8:3848–3861. doi: 10.1002/pmic.200700969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee HS, Jeong J. Lee K-J. Characterization of vesicles secreted from insulinoma NIT-1 cells. J Proteome Res. 2009:2851–2862. doi: 10.1021/pr900009y. [DOI] [PubMed] [Google Scholar]
- 146.Brunner Y, Schvartz D, Couté Y. Sanchez J-C. Proteomics of regulated secretory organelles. Mass Spectrom Rev. 2009;28:844–867. doi: 10.1002/mas.20211. [DOI] [PubMed] [Google Scholar]
- 147.Chen X. Andrews P.C. Quantitative proteomics analysis of pancreatic zymogen granule membrane proteins. Methods Mol Biol. 2009;528:327–338. doi: 10.1007/978-1-60327-310-7_23. [DOI] [PubMed] [Google Scholar]
- 148.Hickey AJR, Bradley JWI, Skea GL, Middleditch MJ, et al. Proteins associated with immunopurified granules from a model pancreatic islet b-cell system: proteomic snapshot of an endocrine secretory granule. J Proteome Res. 2009;8:178–186. doi: 10.1021/pr800675k. [DOI] [PubMed] [Google Scholar]
- 149.Chen X, Ulintz PJ, Simon ES, Williams JA. Andrews P.C. Global topology analysis of pancreatic zymogen granule membrane proteins. Mol Cell Proteomics. 2008;7:2323–36. doi: 10.1074/mcp.M700575-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coughenour HD, Spaulding RS. Thompson C. The synaptic vesicle proteome: A comparitive study in membrane protein identification. Proteomics. 2004;4:3141–3155. doi: 10.1002/pmic.200300817. [DOI] [PubMed] [Google Scholar]
- 151.Morciano M, Burré J, Corvey C, Karas M, et al. Immunoisolation of two synaptic vesicle pools from synaptosomes: a proteomics analysis. J Neurochem. 2005;95:1732–1745. doi: 10.1111/j.1471-4159.2005.03506.x. [DOI] [PubMed] [Google Scholar]
- 152.Takamori S, Holt M, Stenius K, Lemke EA, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 153.Burré J, Beckhaus T, Schägger H, Corvey C, et al. Analysis of the synaptic vesicle proteome using three gel-based protein separation techniques. Proteomics. 2006;6:6250–6262. doi: 10.1002/pmic.200600357. [DOI] [PubMed] [Google Scholar]
- 154.Morciano M, Beckhaus T, Karas M, Zimmermann H, Volknandt W. The proteome of the presynaptic active zone: from docked synaptc vesicles to adhesion molecules and maxi-channels. J Neurochem. 2009;108:662–675. doi: 10.1111/j.1471-4159.2008.05824.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.