Abstract
Introduction
Angiotensin II (AngII) activates p38 mitogen-activated protein kinase (MAPK) and elevates arginase activity in endothelial cells. Upregulation of arginase activity has been implicated in endothelial dysfunction by reducing NO bioavailability. However, signaling pathways activated by AngII in the penis are largely unknown.
Aim
We hypothesized that activation of p38 MAPK increases arginase activity and thus impairs penile vascular function in AngII-treated mice.
Methods
Male C57BL/6 mice were implanted with osmotic minipumps containing saline or AngII (42 μg/kg/h) for 14 days and co-treated with p38 MAPK inhibitor, SB 203580 (5 μg/kg/day), beginning 2 days before minipump implantation. Systolic blood pressure (SBP) was measured. Corpus cavernosum (CC) tissue was used for vascular functional studies and protein expression levels of p38 MAPK, arginase and constitutive NOS, and arginase activity.
Main Outcome Measures
Arginase expression and activity; expression of phospho-p38 MAPK, -eNOS and nNOS proteins; endothelium-dependent and nitrergic nerve-mediated relaxations were determined in CC from control and AngII-infused mice.
Results
AngII increased SBP (22%) and increased CC arginase activity and expression (~2-fold), and phosphorylated P38 MAPK levels (30%) over control. Treatment with SB 203580 prevented these effects. Endothelium-dependent NO-mediated relaxation to acetylcholine was significantly reduced by AngII and this effect was prevented by SB 203580 (P<0.01). AngII (2-week) did not alter nitrergic function. However, SB 203580 significantly increased nitrergic relaxation in both control and AngII tissue at lower frequencies. Maximum contractile responses for phenylephrine and electrical field stimulation were increased by AngII (56% and 171%, respectively), and attenuated by SB 203580 treated. AngII treatment also decreased eNOS phosphorylation at Ser-1177 compared to control. Treatment with SB 203580 prevented all these changes.
Conclusion
p38 MAPK inhibition corrects penile arginase activity and protects against erectile dysfunction caused by AngII.
Introduction
Nitric oxide (NO) is the principal mediator of cavernosal smooth muscle relaxation and penile erection [1]. NO is derived from L-arginine by NO synthase (NOS), and both endothelial NOS (eNOS) and neuronal NOS (nNOS) isoforms of the corpus cavernosum (CC) serve as sources to produce relevant levels of NO. Reduced availability of L-arginine to eNOS and nNOS has been implicated in erectile dysfunction in aging-associated endothelial dysfunction [2], atherosclerosis [3] hypercholesterolemia [4], and diabetes [5, 6]. The mechanisms of erectile dysfunction involve oxidative stress and vascular inflammation [7], both of which have been associated with enhanced arginase activity and expression in the vasculature [8–12].
In mammalian cells, L-arginine is used as a substrate by both NOS and arginase. Because arginase and NOS share a common substrate, NO production is likely linked to regulation of arginase activity [13]. Two distinct isoforms of arginase have been identified and both are found in vascular tissue and endothelial and smooth muscle cells [14, 15]. Arginase isoforms compete with NOS for L-arginine and reduce production of NO [12, 16]. Previous studies have shown that arginase exists in human CC, and inhibition of this enzyme results in facilitation of CC relaxation [8, 17].
Angiotensin II (AngII), an active product of the renin-angiotensin system (RAS), is a prominent regulator of erectile function and abnormal levels of AngII induce vasoconstriction, vascular remodeling, and endothelial dysfunction, leading to vascular complications in diabetes and other diseases [18]. AngII, which is found in human CC endothelial and smooth muscle cells [19], appears to play a significant role in the regulation of the erection process [20]. AngII concentrations in penile tissue are higher during detumescence than during tumescence [20]. AngII also mediates activation of NADPH oxidase [21, 22], regulation of cell growth and proliferation through activation of receptor tyrosine kinases, non-receptor tyrosine kinases, and mitogen-activated protein kinases (MAPKs) [23, 24].
p38 MAPK is a member of the superfamily of MAPKs that also includes the extracellular signal-regulated kinase (ERK) and c-jun-NH2-terminal kinase (JNK). Activation of p38 MAPK can be triggered by a variety of cellular stresses including hyperglycemia, oxidative stress and diabetes [25–28]. AngII markedly activates p38 MAPK [29–34] and inhibition of p38 MAPK attenuates organ damage and improves vascular dysfunction in cardiovascular diseases [33–35]. In addition, nitrergic neurovascular dysfunction in CC from diabetic mouse is corrected by chronic treatment with a p38 MAPK inhibitor [28].
Activation of the p38 MAPK signaling pathway has been implicated in elevated arginase activity and expression in macrophages [36, 37]. Our previous study indicates that AngII enhances arginase activity/expression through activation of p38 MAPK in bovine aortic endothelial cells [38]. However, signaling pathways activated by Ang-II in the penis are largely unknown. We hypothesized that AngII increases arginase activity/expression and causes impairment of penile vascular function through activation of p38 MAPK and that inhibition of p38 MAPK prevents arginase upregulation and improves CC relaxation. To further explore the interaction between p38 MAPK and arginase, we determined the effect of the selective isoform p38 α/β MAPK inhibitor SB 203580 in mice treated for two-weeks with AngII. We provide in vivo evidence that two-week treatment with a selective p38 MAPK inhibitor prevents elevation of arginase activity/expression and enhances NO-endothelium dependent relaxation in our AngII model.
Methods
Animals
The protocols were approved by the Education Committee and Experimental Research of the Medical College of Georgia. Ten-week old male C57BL/6J mice were used in this study. Mice were implanted subcutaneously with osmotic mini pumps (Alzet, Durect Corp Cupertino, CA, USA) after they were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (10 mg/mg). Mice were either infused with saline as a control or AngII (42 μg/kg/h) for 2 weeks. The mice were housed in individual cages and provided with standard chow and water ad libitum. Body weight of each mouse was measured before and after the two-week treatment.
Treatment with SB 203580
Two days prior to the implantation of mini pump, and during the two week-infusion of AngII, some mice underwent treatment with the selective p38 MAPK inhibitor, SB 203580 (5 μg/kg/day). Mice were divided into four groups: (i) mice infused with saline only (CTL); (ii) mice infused with saline and treated with SB 203580, ip; (iii) mice infused with AngII only; and (iv) mice infused with AngII and treated with SB 203580. Systolic blood pressure (SBP) was measured by tail cuff plethysmography in conscious mice before starting the treatment and once a week to monitor the progression of hypertension during the two-week treatment period.
Functional Studies in Cavernosal Strips
Mice were anaesthetized and the penes were removed and placed in chilled Krebs solution. Following removal of the vein and urethra, the penile tissue was cleaned from connective and adventitial tissue, and the fibrous septum separating the corpora cavernosa was opened from its proximal extremity toward the penile shaft. A slit was made in the tunica albuginea along the shaft to obtain two strips (approximately 11 × 1 × 1 mm) of corpus cavernosum (CC) from each animal. Each strip was mounted under resting tension of 2.5 mN in a 4-ml myograph chambers filled with Krebs solution (in mM: NaCl, 118; NaHCO3, 25; glucose, 5.6; KCl, 4.7; KH2PO4, 1.2; MgSO4 7H2O, 1.17 and CaCl2 2H2O 2.5) at 37°C (pH 7.4) and continuously aerated with 95% O2 and 5% CO2. Isometric force was recorded using a PowerLab 8/SPTM data acquisition system (Software Chart, version 5, AD Instrument, MA, USA). Tissues were allowed to equilibrate for 1 h before starting the experiments.
After equilibration, the ability of the preparation to develop contraction was assessed by a high KCl solution (80 mM). Cumulative concentration-response curves to acetylcholine (ACh; 10−9 to 10−5 M), an endothelium-dependent vasodilator and sodium nitroprusside (SNP; 10−8 to 10−4 M), a NO donor were obtained in cavernosal strips contracted with phenylephrine (PE; 10−5 M, α1-adrenergic receptor agonist). Cumulative concentration-response curves to the contractile agent PE (10−9 to 10−4 M) were also obtained in the cavernosal tissue.
In another set of experiments, electrical field stimulation (EFS) was applied in cavernosal strips placed between two platinum ring electrodes connected to a grass S88 stimulator (Astro-Med Industrial Park, RI, USA), and EFS was conducted at 20 V, 1-ms pulse width and trains of stimuli lasting 10s at varying frequencies (1–32 Hz). In order to study the nitrergic relaxations, cavernosal tissues were pretreated with bretylium tosylate (3 × 10−5 M) and atropine (10−6 M) to deplete the catecholamine stores and to block muscarinic receptors, respectively. Involvement of NO on EFS-induced cavernosal relaxations was confirmed by using L-NAME (10−4; NOS inhibitor). To evaluate adrenergic nerve-mediated responses, the strips were incubated with L-NAME (10−4 M) plus atropine (10−6 M), before EFS was performed.
Arginase Activity Assay
Mice cavernosal tissues were frozen in liquid nitrogen, pulverized, combined 1:4 (wt:vol) with ice-cold lysis buffer (50 mmol/L Tris-HCl, 0.1 mmol/L EDTA and EGTA, pH 7.5) containing protease inhibitors, phosphates cocktail 1 and 2 and homogenized on ice. The homogenate was sonicated and centrifuged at 14,000 g for 20 minutes at 4°C and the supernatant was removed for enzyme assay. 25 μL of the supernatant was collected in triplicate and then added to 25 μL of Tris-HCl 121 (50 mmol, pH 7.5) containing 10 mmol MnCl2 and the mixture was activated by heating for 10 minutes at 55–60°C. Arginase activity was assayed by measuring urea produced from L-arginine as previously described [16].
Western Blot Analysis
Tissues were homogenized in lysis buffer containing protease inhibitors and centrifuged at 14,000 × g for 20 minutes at 4°C, supernatants collected and protein concentrations determined. Total protein (20 μg) was resolved on a 10% SDS-polyacrylamide pre-cast gel and transferred to polyvinylidene difluoride membrane. The membrane was blocked in advance blocking agent (Amersham) and then incubated with primary antibody (anti-arginase I, BD Transduction Laboratories, 1:1000; anti-arginase II, Santa Cruz Biotechnology, INC, 1:250; anti-eNOS, anti eNOS phosphorylated at Ser1177 and Thr495, Cell Signaling Technology, 1:1000; anti nNOS, Cell Signaling, 1:4000; anti p38 MAPK and anti p38 MAPK phosphorylated at Thr180 and Thr182, Cell Signaling Technology, 1:1000) in Tris-buffered saline/Tween 20 buffer overnight at 4°C. After washing, the membranes were incubated with sheep anti-mouse (Amersham, 1:4000) or donkey anti-rabbit (GE Healthcare, 1:4000) horseradish peroxidase-labeled secondary antibody, respectively, and visualized using an enhanced chemiluminescence kit (Amersham, Piscataway, NJ, USA). The protein expression levels were normalized by α-actin.
Drugs and Chemicals
Acetylcholine, angiotensin II, atropine, bretylium tosylate, cocktail 1, cocktail 2, Nω-nitro-L-arginine methyl ester (L-NAME), sodium nitroprusside, phenylephrine and protease inhibitor were purchased from Sigma Aldrich (St Louis, MO, USA). 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole (SB 203580) was purchased from Calbiochem (EMD Biosciences, Inc, La Jolla, CA, USA). All reagents used were of analytical grade. Stock solutions were prepared in deionized water or ethanol and stored in aliquots at −20°C; dilutions were prepared immediately before use.
Statistical Analysis
Experimental values of relaxation or contraction were calculated relative to the maximal changes from the contraction produced by PE and KCl, respectively, taken as 100% in each tissue. Data are shown as the mean ± SEM of the mean of n experiments. Two-way analysis of variance (ANOVA) was used to evaluate the results followed by bonferroni post hoc test. P<0.05 was considered significant. A program package was used for the statistical analysis of all data (GraphPad Instat, version 5.00; GraphPad Software Inc., San Diego, CA).
Results
AngII-treated mice showed similar weight (26.3 ± 0.41 g) compared with the CTL group (26.2 ± 0.34 g, N=10 in each group). However, weights in SB 203580-treated CTL (23.8 ± 0.79 g) and Ang II-infused mice (24.5 ± 0.75 g) were statistically lower than their respective controls (P <0.05). SBP at the end of treatment was significant increased in AngII-treated mice compared to CTL mice (Table 1). Two-week treatment with the p38 inhibitor, SB 203580, did not alter SBP in CTL mice, but significantly attenuated the elevated SBP in AngII- treated mice by 60 %.
Table 1.
AngII-treated mice display increased systolic blood pressure (SBP) compared with CTL mice. Chronic treatment with SB 203580 for two week attenuated the increase of SBP.
| Group | SBP after 14 days |
|---|---|
| CTL | 114.8 ± 1.9 |
| AngII | 140.6 ± 2.1** |
| CTL+SB 203580 | 115.6 ± 3.9 |
| AngII+SB 203580 | 129.6 ± 2.0* # |
Data are the mean ± SEM (N = 6–8).
P < 0.05;
P < 0.01 compared with CTL mice;
P < 0.05, compared with AngII mice.
SB 203580 Ameliorates Angiotensin II-Induced Endothelial Dysfunction
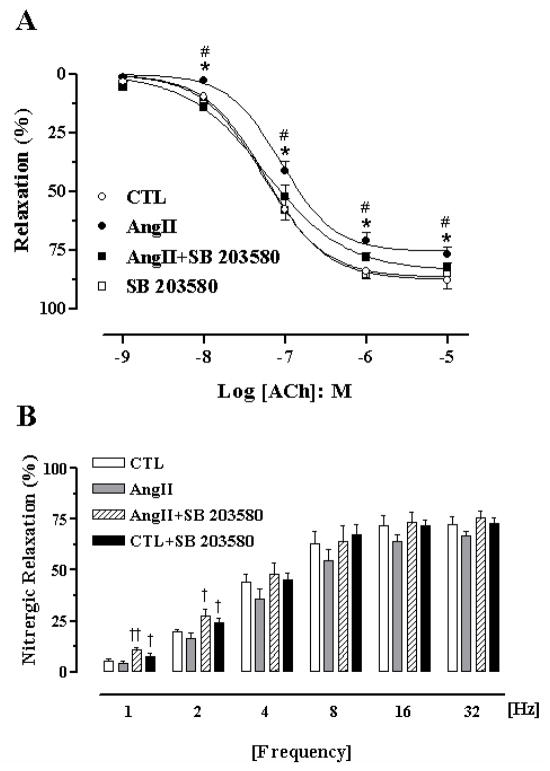
The cavernosal contraction induced by a high K+ concentration (KCl 80 mM) was not significantly different among the groups (CTL, 0.79 ± 0.08 mN; AngII, 0.86 ± 0.08 mN; CTL+SB 203580, 1.0 ± 0.12 mN; AngII+SB 203580, 0.78 ± 0.07 mN). Although there was no significant difference in the potency (pEC50) of ACh between the CTL (7.24 ± 0.03) and AngII (7.05 ± 0.04) group, the maximal relaxation response elicited by ACh in tissue from AngII-treated mice (76 ± 3%, P < 0.05) was significantly impaired compared to CTL mice (88 ± 3%, Figure 1A). Treatment with SB 203580 for two weeks significantly enhanced the endothelium-dependent relaxation elicited by ACh at all concentrations, and prevented endothelial dysfunction in AngII-treated mice (Figure 1A). In addition, there was a significant increase in sensitivity of CC relaxation responses to ACh in the AngII+SB 203580 compared to AngII-treated group, with pEC50 values of 7.41 ± 0.09 vs 7.05 ± 0.04, respectively. There was no difference in ACh-induced relaxation between strips from CTL+SB 203580 and the CTL mice. Incubation with L- NAME (10−4 M) fully blocked ACh-induced cavernosal relaxations in all groups (N = 4; not shown).
Figure 1. SB 203580 ameliorates AngII-induced endothelial dysfunction.
Endothelium-dependent NO-mediated relaxation to acetylcholine (ACh, 10−9 to 10−5 M, panel A) in cavernosal segments from control (CTL, open circle), AngII (closed circle), AngII+SB 203580 (closed square) and CTL+SB 203580 (open square) mice. Treatment with SB 203580 significantly prevented endothelial dysfunction in AngII mice. Panel B, Nitrergic relaxation induced by electrical field stimulation (EFS). Nitrergic relaxation induced by EFS (1–32 Hz) in cavernosal strips from CTL (open bars), AngII (grey bars), CTL+SB 203580 (solid bars) and AngII+SB 203580 (hatched bars). Data were calculated relative to the maximal changes from the contraction produced by phenylephrine (PE, 10−5 M) in each tissue, which was taken as 100%. Data represent the means ± S.E.M. of 5–7 experiments. *P < 0.05 compared with CTL mice; †P < 0.05 and ††P < 0.01, compared with the respective control group; #P < 0.05 compared with AngII+SB 203580. No differences were observed in CTL vs SB 203580 tissues.
SB 203580 Augments Cavernosal Relaxation to Electrical-Field Stimulation (EFS) but not to Sodium Nitroprusside (SNP)
Electrical-field stimulation (EFS) of cavernosal tissues caused frequency-dependent relaxations in the four groups. Although there was a slight, non-significant decrease of nitrergic relaxation at all frequencies in CC from AngII-treated mice compared to CTL mice, treatment with SB 203580 significantly increased nitrergic relaxation at lower frequencies in both groups (CTL by 33% and 20%, and AngII by 150% and 65% at 1 and 2 Hz, respectively, Figure 1B). Incubation of cavernosal tissues with L-NAME (10−4 M), completely abolished the neurogenic relaxation evoked by EFS (1–32 Hz, N = 4, each, not shown).
The NO donor SNP (10−8 to 10−4 M) caused concentration-dependent cavernosal relaxations in all groups. No significant differences were observed in pEC50 and maximal response (Emax) values among the groups (pEC50 and Emax; CTL, 6.49 ± 0.06, 79 ± 7%; AngII, 6.34 ± 0.07, 85 ± 2%; CTL+SB 203580, 6.43 ± 0.08, 88 ± 5%; AngII+SB 203580 6.31 ± 0.06, 85 ± 8%, respectively).
SB 203580 Prevents Angiotensin II-Induced Augmented Responses to Phenylephrine (PE) and Adrenergic Nerve Stimulation
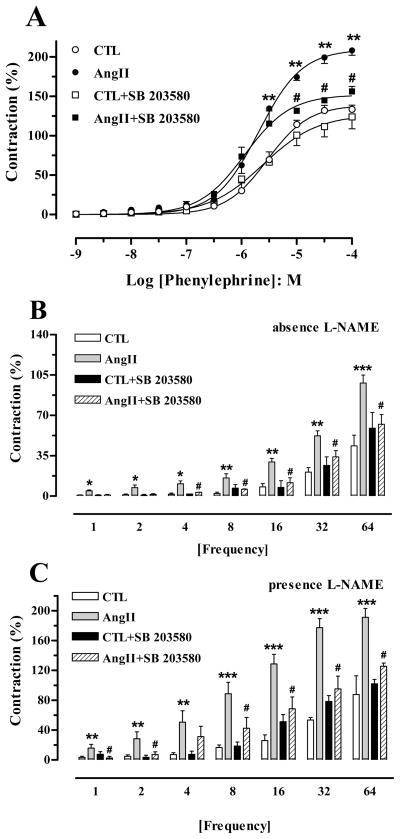
PE (10−9 to 10−4 M) caused concentration-dependent contractions in cavernosal preparations in all groups. Although no differences in pEC50 values were found between groups, the Emax elicited by PE from AngII-treated mice (208 ± 6%) was significantly augmented at the highest concentrations (10−4 M; P < 0.01) in comparison to that in CTL tissue (134 ± 5%). SB 203580 treatment significantly decreased the maximal contractions to PE at 10−5 M to 10−4 M from AngII-treated mice, and no effect was noted in the CTL group (Figure 2A).
Figure 2. SB 203580 treatment prevents AngII-induced augmented corporal contractile responses.
Contractile responses upon stimulation of α-1-adrenergic receptor phenylephrine (PE, 10−9 to 10−4 M, panel A) or adrenergic nerves (electric field stimulation, EFS 1–64 Hz) in cavernosal segments from control (CTL), AngII, CTL+SB 203580 and AngII+SB 203580 mice. Frequency-response curves elicited by EFS were performed in the absence (panel B) or in the presence of L-NAME (10−4 M) and atropine (10−6 M, panel C). Data were calculated relative to the maximal changes from the contraction produced by KCl (80 mM), which was taken as 100%. Data represent the mean ± S.E.M. of 6 animals. *P < 0.05; **P < 0.01, and ***P < 0.001 compared with CTL mice; #P < 0.05, compared to AngII mice.
Cavernosal segments from AngII-treated mice also exhibited increased frequency-dependent contractions, both in the absence and presence of L-NAME (10−4 M) and atropine (10−6 M) when compared to strips from CTL mice. SB 203580 did not affect the contractile responses in CC from CTL mice, but significantly decreased contraction in the absence (Figure 2B) and in the presence of L-NAME (Figure 2C) in CC from AngII-treated mice.
SB 203580 Treatment Prevent Angiotensin II-Induced Increased Corpora Cavernosa Arginase Activity/Expression
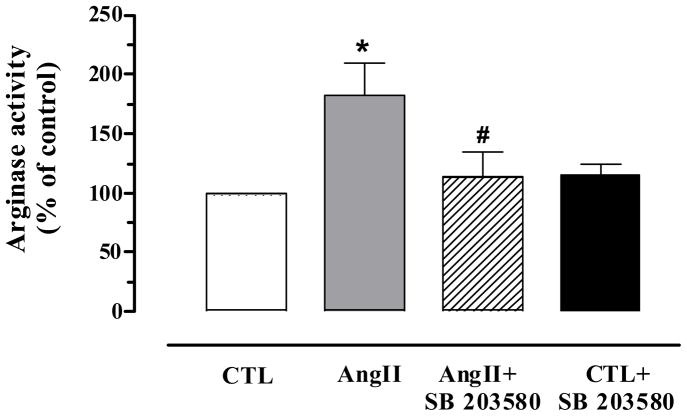
A significant increase of arginase activity was observed in the cavernosal tissue from AngII-treated mice compared with CTL (Figure 3). The AngII-induced increases in arginase activity were blocked by SB 203580 treatment (73%). However, SB 203580 did not change the basal levels of arginase activity in CTL mice.
Figure 3. Treatment with SB 203580 significantly attenuates AngII-induced increased corporal arginase activity.
Arginase activity in cavernosal tissues from CTL (open bar), AngII (grey bar), CTL+SB 203580 (closed bar) and AngII+SB 203580 (hatched bar) mice was determined by the conversion of L-arginine to urea and L-ornithine. AngII mice markedly increased arginase activity. Treatment with SB 203580 significantly attenuated arginase activity in AngII mice. Data are expressed as % of control. Data represents the mean ± S.E.M. of 5 experiments. *P < 0.05, compared to CTL mice; #P < 0.05, compared to AngII mice.
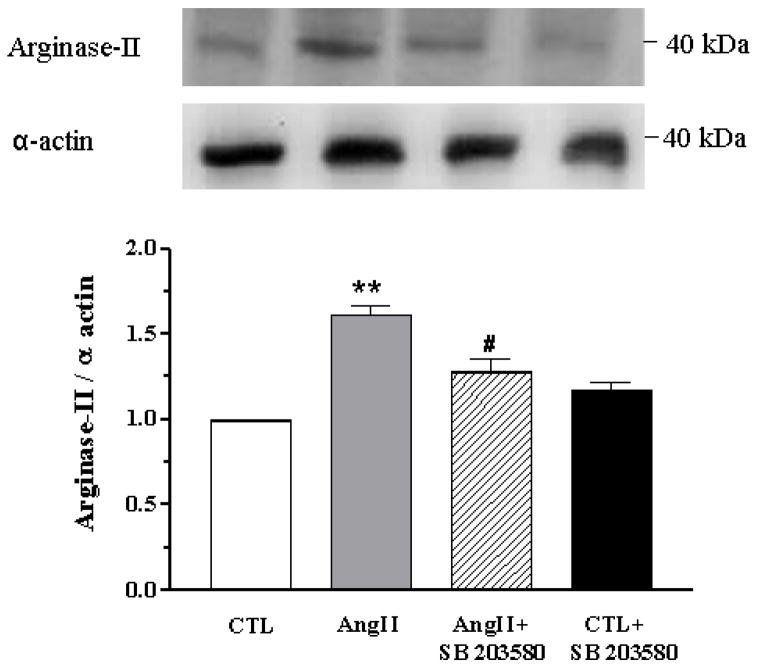
Arginase II corporal protein expression levels were increased in AngII-treated mice (61%) compared to control (P < 0.01). SB 203580 significantly attenuated arginase II expression in CC from AngII-treated mice (by 54%), and no change was observed in CTL mice CC (Figure 4). On the other hand, no differences were observed in arginase I protein expression levels in the corporal tissue from CTL or AngII-treated mice with or without SB 203580 treatment (data not shown).
Figure 4. AngII increases corporal expression of arginase II.
Western blot analysis of arginase II expression in cavernosal tissues of CTL (open bar), AngII (grey bar), AngII+SB 203580 (hatched bar) and CTL+SB 203580 mice (closed bar). SB 203580 treatment suppressed arginase II expression in CC from AngII mice. A representative blot is shown in the top panel. Results were quantified by densitometry. Protein expression of arginase II was normalized to α-actin levels and expressed as % of control. Data represent the mean ± S.E.M. of 6 experiments (each group). **P < 0.01, compared to CTL mice; #P <0.05, compared to AngII mice.
Angiotensin II-Treated Mice Exhibit Increased Corporal Levels of Phosphorylated p38 MAPK and Decreased Phosphorylated eNOS at Ser-1177
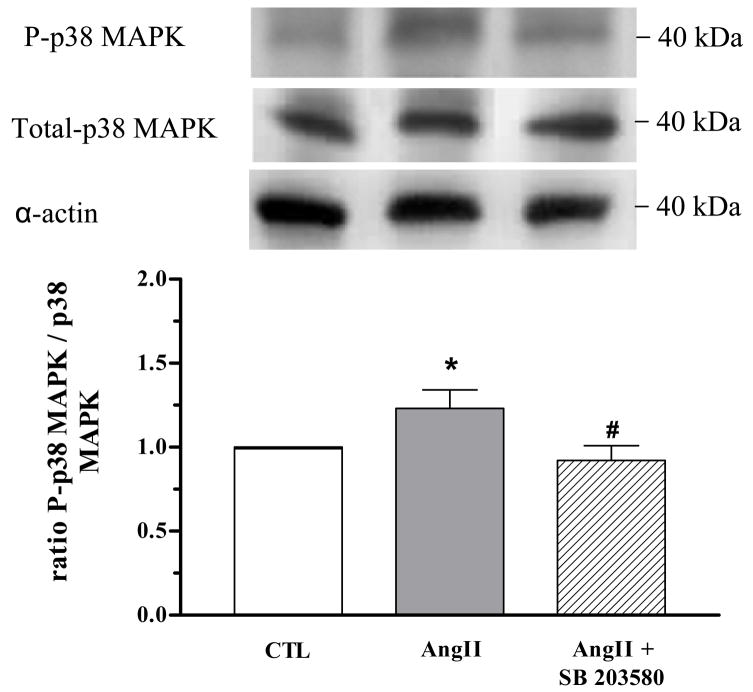
Protein levels of total p38 MAPK were not different among CC samples from all groups. However, phosphorylated p38 MAPK expression was significantly increased in CC from AngII-treated mice (by 45%) compared with CTL mice, and treatment with SB 203580 significantly attenuated this increase (Figure 5).
Figure 5. AngII increases corporal p38 MAPK activity.
Western blot analysis of phophorylated (P)-p38 MAPK and total p38 MAPK expression in cavernosal tissue of CTL (open bar), AngII (grey bar) and AngII+SB 203580 mice (hatched bar). SB 203580 treatment significantly attenuated increased P-p38 MAPK expression in AngII mice. Representative blot of P-p38 MAPK and total p38 MAPK is shown in the top panel. P-p38 MAPK and total p38 MAPK were normalized by α-actin levels and expressed as % of control. A summarized bar graph shows that increased P-p38 MAPK was detected in tissues from AngII mice when normalized to total p38 MAPK (N = 5, each group).*P < 0.01, compared to CTL mice; #P < 0.05, compared to AngII mice.
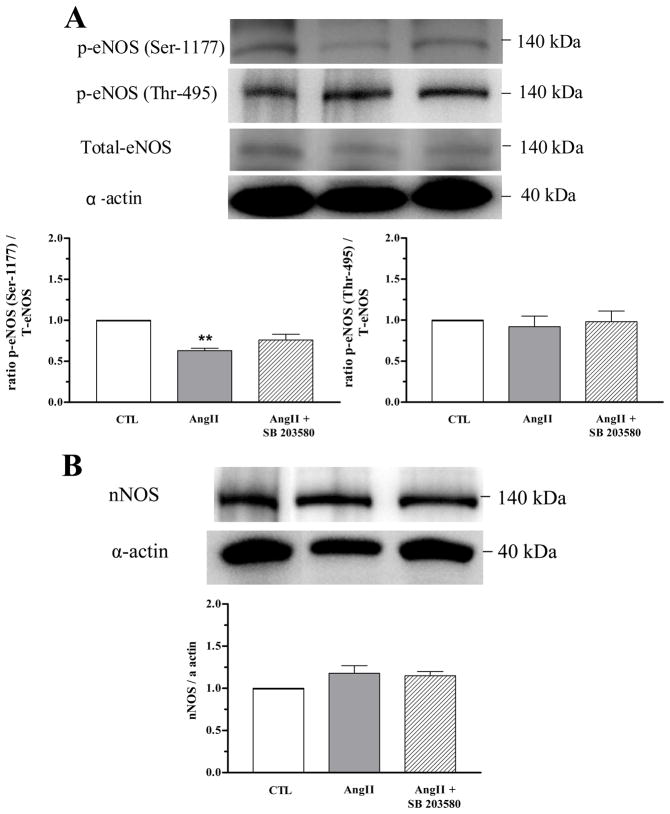
Decreased phosphorylated eNOS expression (at the regulatory site Ser-1177, but not at Thr-495) was observed in CC from AngII-treated mice as compared with the CTL mice. No differences were observed in total eNOS and nNOS protein levels between CC from CTL or AngII-treated mice with or without SB 203580 treatment (Figure 6A and 6B).
Figure 6. SB 203580 prevents decreased corporal expression of eNOS-Ser1177.
Western blot analysis of total eNOS, phosphorylated eNOS in the regulatory site at Ser-1177, at Thr-495 (panel A) and nNOS expression (panel B) in cavernosal tissues of CTL (open bar), AngII (grey bar) and AngII+SB 203580 (hatched bar). Representative blot is shown in the top of each panel. Protein expression of constitutive NOS were normalized by α-actin levels and expressed as % of control. A summarized bar graph shows phosphorylated eNOS at Ser-1177 was decreased in tissues from AngII mice when normalized to total eNOS (N = 5, each group) **P < 0.01, compared to CTL mice.
Discussion
Our findings show that isolated CC from AngII-treated mice display decreased endothelium-dependent relaxation, increased contractile responses to an α1-adrenergic receptor agonist and adrenergic nerve stimulation, enhanced arginase activity and activation of p38 MAPK, all favoring penile detumescence and dysfunction. Our study also shows altered expression of key enzymes involved in the erectile responses, such as decreased phospho eNOS expression in the positive regulatory site (Ser-1177) and increased arginase II expression, in cavernosal strips from AngII-treated mice. In addition, we demonstrate for the first time that in vivo treatment with the p38 MAPK inhibitor, SB 203580, attenuates AngII-induced activation of p38 MAPK, suppresses arginase activity as well as arginase II expression, and blocks AngII-induced endothelial dysfunction in mice CC.
Activation of p38 MAPK is a stress-sensitive mechanism triggered by AngII, diabetes, hyperglycemia and oxidative stress that has been linked to the pathogenesis of vasculopathy via increased endothelial cell proliferation with lesion formation and impairment of endothelial function [26, 28, 30, 31, 38]. We observed that cavernosal strips from AngII-treated mice display decreased endothelium-dependent relaxation compared to control mice. This impairment in ACh-induced relaxation was correlated with significantly elevated arginase activity and arginase II protein levels in the CC. Treatment with SB 203580 prevented the endothelial dysfunction in AngII-treated mice and attenuated augmented arginase activity/expression. Our results are consistent with previous studies showing that AngII induces endothelial dysfunction in multiple vascular tissues, including corpora cavernosa [19], and that p38 MAPK inhibition prevents endothelial dysfunction [28, 33, 34, 39]. Our present findings relate to earlier studies which have shown that p38 MAPK inhibition reduces superoxide anion production [33], and has cardiac protective actions [34, 35]. However, our study is the first to show that activation of p38 MAPK contributes to AngII-induced elevation of arginase activity/expression in CC. p38 MAPK is markedly activated in a variety of cellular stresses including AngII stimulation [29]. AngII-induced activation of p38 MAPK has been shown in vascular smooth muscle cells [40, 41], proximal tubular cells [42] and vascular tissues [28, 43]. In the present study, two-week infusion of AngII resulted in the activation of p38 MAPK. Indeed, administration of SB 203580 suppressed levels of phosphorylated p38 MAPK. Inhibition of p38 MAPK activation by SB 203580 may be due to a direct inhibition of p38 MAPK autophosphorylation [44]. Our findings also provide strong evidence that MAPK inhibition causes reduction of arginase activity and expression levels of arginase II. These actions are correlated with improved endothelium-dependent relaxation, suggesting that this preservation of penile function by SB 203580 is mediated by suppression of vascular arginase levels. Our results agree with a recent report that the p38 MAPK signaling pathway is involved in the elevation of arginase activity in macrophages [37].
Arginase is a key regulator of the nitric oxide signaling pathway, competing with NOS and limiting their shared substrate. Although two isoforms of arginase have been characterized in human CC, the predominant isoform responsible for limiting NO production is arginase II [8]. Increased arginase II expression and activity is associated with impotence in diabetic patients [8]. We have demonstrated that arginase II plays an important role in decreasing NO production since arginase II knockout diabetic mice maintain CC relaxation and prevent vascular dysfunction [6]. Furthermore, enhanced arginase expression and activity in pathological conditions may limit L-arginine bioavailability for constitutive NOS. The constitutive eNOS and nNOS isoforms are tightly regulated and produce physiologically relevant levels of NO in endothelial cells and autonomic nerve endings of the penis, respectively [45]. Our results indicate that AngII suppresses eNOS phosphorylation at its positive regulatory site Ser-1177 without any alteration in phosphorylation of eNOS at Thr-495 or in total expression of either eNOS or nNOS. These data suggest a reduction in NOS activity.
Our functional studies show that AngII treatment for two weeks reduced ACh-induced cavernosal relaxation. However, this two week treatment did not markedly alter the nitrergic relaxation responses compared with the control mice. Previous studies have shown that four week treatment of rats with AngII impairs erectile function by preventing the increase in intracavernosal pressure upon stimulation of the cavernous nerve in vivo [22]. A longer period of our treatment with AngII may have produced obvious impairment of nitrergic vasorelaxation and erectile dysfunction. We did observe that p38 MAPK inhibitor treatment increased nitrergic relaxation of both control and AngII treated tissues at low frequencies (1–2 Hz). The nitrergic relaxation responses at higher frequencies tended to increase with p38 MAPK inhibitor, but were not significant. Although this apparent difference may be directly related to inhibition of arginase function, other actions of p38 MAPK could be involved.
Downregulation of eNOS activity and increased arginase endothelial activity has been demonstrated in penes from aged mice [9]. Previous studies have reported that inhibition of p38 MAPK corrects endothelial dysfunction in vascular tissues like aorta [33–35], renal artery [39], and pulmonary artery [46] as well as in the CC in disease state [28]. Our findings also indicate that treatment with SB 203580 in AngII mice prevents downregulation of eNOS activity. New mechanisms whereby inhibition of p38 MAPK restores endothelial NOS-dependent function in AngII-treated mice may involve inhibition of arginase activity and suppression of arginase II expression in the CC. Because inhibition of p38 MAPK prevented enhancement of arginase activity/expression, it is an essential step in this action of AngII. Although the mechanism of its action is not clear, one may speculate that active p38 MAPK causes phosphorylation of transcription factors, leading to enhanced expression of arginase [38].
No differences in the cavernosal relaxing responses to SNP, an endothelium-independent NO donor, were observed in AngII-treated or control mice, either in the absence or in the presence of SB 203580. These findings indicate that AngII induces endothelial dysfunction, with no alteration in vascular smooth muscle function.
AngII and elevated levels of arginase activity and expression are also associated with oxidative stress and activation of the small GTPase RhoA and Rho kinase in endothelial cells and in vascular smooth muscle [16, 38]. Rho/ROCK pathway is considered as a central upstream regulator of MAPK activity [47], thus we expect that p38 MAPK activation is a downstream target of Rho/ROCK that is involved in increased arginase activity in corporal cavernosa tissue. Studies in our laboratory indicate that exposure of endothelial cells to reactive oxygen species or AngII activates RhoA/ROCK and p38 MAPK [38, 48].
Corpora cavernosa and penile vessels are contracted mainly via stimulation of α1-adrenoceptors. Increased sensitivity to α1-adrenergic agonist, phenylephrine, was reported in disease states [49]. In addition, AngII increases contractions to other agents in pathological processes, such as arterial hypertension [50]. Our results showed that phenylephrine- and EFS-induced corporal contractions are clearly greater in AngII mice, and these enhanced contractions are attenuated by SB 203580 treatment. Similar contractile responses to EFS were observed in the absence or presence of L-NAME. Our data suggest that increased contractile responses to EFS in the strips from AngII mice are due to changes in prejunctional events, most likely to enhanced sympathetic nerve function. Increased sympathetic nerve transmission associated with increased vasoconstrictor action mediated by α1-adrenoceptor activation contributes to detumescence and flaccidity state of the penis. Accordingly, these mechanisms may play a role in impaired erectile function in AngII-treated mice.
In conclusion, our study shows that CC tissue from AngII-treated mice exhibit increased arginase activity and arginase II expression, which can contribute to reduced endothelium-dependent relaxation and eNOS activity. Since Inhibition of p38 MAPK prevents vascular endothelial dysfunction through reduction of arginase activity/expression, this pathway could serve as a therapeutic target worthy of consideration for clinical trials.
Acknowledgments
This study was supported by grants from the National Institutes of Health (HL-70215 and EY-11766)
Footnotes
Conflict of Interest: None
References
- 1.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–50. [PubMed] [Google Scholar]
- 2.Champion HC, Bivalacqua TJ, Hyman AL, Ignarro LJ, Hellstrom WJ, Kadowitz PJ. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc Natl Acad Sci. 1999;96:11648–52. doi: 10.1073/pnas.96.20.11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park K, Kim SW, Rhu KS, Paick JS. Chronic administration of an oral Rho kinase inhibitor prevents the development of vasculogenic erectile dysfunction in a rat model. J Sex Med. 2006;3:996–1003. doi: 10.1111/j.1743-6109.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 4.Xie D, Odronic SI, Wu F, Pippen AM, Donatucci CF, Annex BH. A mouse model of hypercholesterolemia-induced erectile dysfunction. J Sex Med. 2007;4:898–907. doi: 10.1111/j.1743-6109.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 5.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–62. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 6.Toque HA, Iddings J, Xu Z, Tostes RC, Webb RC, Caldwell RB, Caldwell RW. Arginase II Deletion Increases Corpora Cavernosa Relaxation in Diabetic Mice. doi: 10.1111/j.1743-6109.2010.02098.x. (Experimental Biology, abstract, 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barassi A, Colpi GM, Piediferro G, Dogliotti G, D’Eril GV, Corsi MM. Oxidative stress and antioxidant status in patients with erectile dysfunction. J Sex Med. 2009;6:2820–5. doi: 10.1111/j.1743-6109.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 8.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–7. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 9.Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007;292:H1340–51. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- 10.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–6. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, Chilian WM, Zhang C. TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2007;27:1269–75. doi: 10.1161/ATVBAHA.107.142521. [DOI] [PubMed] [Google Scholar]
- 12.Numao N, Masuda H, Sakai Y, Okada Y, Kihara K, Azuma H. Roles of attenuated neuronal nitric-oxide synthase protein expression and accelerated arginase activity in impairing neurogenic relaxation of corpus cavernosum in aged rabbits. BJU Int. 2007;99:1495–9. doi: 10.1111/j.1464-410X.2007.06860.x. [DOI] [PubMed] [Google Scholar]
- 13.Mori M, Gotoh T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem Biophys Res Commun. 2000;275:715–9. doi: 10.1006/bbrc.2000.3169. [DOI] [PubMed] [Google Scholar]
- 14.Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD. Cloning and characterization of the human type II arginase gene. Genomics. 1996;38:118–23. doi: 10.1006/geno.1996.0606. [DOI] [PubMed] [Google Scholar]
- 15.Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 16.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox JD, Kim NN, Traish AM, Christianson DW. Arginase-boronic acid complex highlights a physiological role in erectile function. Nat Struct Biol. 1999;6:1043–7. doi: 10.1038/14929. [DOI] [PubMed] [Google Scholar]
- 18.Touyz RM, Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res. 2002;35:1001–15. doi: 10.1590/s0100-879x2002000900001. [DOI] [PubMed] [Google Scholar]
- 19.Kifor I, Williams GH, Vickers MA, Sullivan MP, Jodbert P, Dluhy RG. Tissue angiotensin II as a modulator of erectile function. I. Angiotensin peptide content, secretion and effects in the corpus cavernosum. J Urol. 1997;157:1920–5. [PubMed] [Google Scholar]
- 20.Becker AJ, Uckert S, Stief CG, Truss MC, Machtens S, Scheller F, Knapp WH, Hartmann U, Jonas U. Possible role of bradykinin and angiotensin II in the regulation of penile erection and detumescence. Urology. 2001;57:193–8. doi: 10.1016/s0090-4295(00)00881-5. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–9. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin L, Lagoda G, Leite R, Webb RC, Burnett AL. NADPH oxidase activation: a mechanism of hypertension-associated erectile dysfunction. J Sex Med. 2008;5:544–51. doi: 10.1111/j.1743-6109.2007.00733.x. [DOI] [PubMed] [Google Scholar]
- 23.Touyz RM, Deschepper C, Park JB, He G, Chen X, Neves MF, Virdis A, Schiffrin EL. Inhibition of mitogen-activated protein/extracellular signal-regulated kinase improves endothelial function and attenuates AngII-induced contractility of mesenteric resistance arteries from spontaneously hypertensive rats. J Hypertens. 2002;20:1127–34. doi: 10.1097/00004872-200206000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima H, Suzuki H, Ohtsu H, Chao JY, Utsunomiya H, Frank GD, Eguchi S. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol. 2006;4:67–78. doi: 10.2174/157016106775203126. [DOI] [PubMed] [Google Scholar]
- 25.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–88. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 26.Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–14. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- 27.Costanzo A, Moretti F, Burgio VL, Bravi C, Guido F, Levrero M, Puri PL. Endothelial activation by angiotensin II through NFkappaB and p38 pathways: Involvement of NFkappaB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J Cell Physiol. 2003;195:402–10. doi: 10.1002/jcp.10191. [DOI] [PubMed] [Google Scholar]
- 28.Nangle MR, Cotter MA, Cameron NE. Correction of nitrergic neurovascular dysfunction in diabetic mouse corpus cavernosum by p38 mitogen-activated protein kinase inhibition. Int J Impot Res. 2006;18:258–63. doi: 10.1038/sj.ijir.3901414. [DOI] [PubMed] [Google Scholar]
- 29.Sugden PH, Clerk A. “Stress-responsive” mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res. 1998;83:345–52. doi: 10.1161/01.res.83.4.345. [DOI] [PubMed] [Google Scholar]
- 30.Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kübler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20:940–8. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- 31.Meloche S, Landry J, Huot J, Houle F, Marceau F, Giasson E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2000;279:H741–51. doi: 10.1152/ajpheart.2000.279.2.H741. [DOI] [PubMed] [Google Scholar]
- 32.Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Abe Y. ROS during the acute phase of Ang II hypertension participates in cardiovascular MAPK activation but not vasoconstriction. Hypertension. 2004;43:117–24. doi: 10.1161/01.HYP.0000105110.12667.F8. [DOI] [PubMed] [Google Scholar]
- 33.Widder J, Behr T, Fraccarollo D, Hu K, Galuppo P, Tas P, Angermann CE, Ertl G, Bauersachs J. Vascular endothelial dysfunction and superoxide anion production in heart failure are p38 MAP kinase-dependent. Cardiovasc Res. 2004;63:161–7. doi: 10.1016/j.cardiores.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, Johns DG, Woods TN, Doe CP, Coatney RW, Ohlstein JF, Douglas SA, Willette RN, Yue TL. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol. 2007;49:362–8. doi: 10.1097/FJC.0b013e318046f34a. [DOI] [PubMed] [Google Scholar]
- 35.Olzinski AR, McCafferty TA, Zhao SQ, Behm DJ, Eybye ME, Maniscalco K, Bentley R, Frazier KS, Milliner CM, Mirabile RC, Coatney RW, Willette RN. Hypertensive target organ damage is attenuated by a p38 MAPK inhibitor: role of systemic blood pressure and endothelial protection. Cardiovasc Res. 2005;66(1):170–8. doi: 10.1016/j.cardiores.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Stempin CC, Tanos TB, Coso OA, Cerbán FM. Arginase induction promotes Trypanosoma cruzi intracellular replication in Cruzipain-treated J774 cells through the activation of multiple signaling pathways. Eur J Immunol. 2004;34:200–9. doi: 10.1002/eji.200324313. [DOI] [PubMed] [Google Scholar]
- 37.Liscovsky MV, Ranocchia RP, Gorlino CV, Alignani DO, Morón G, Maletto BA, Pistoresi-Palencia MC. Interferon-gamma priming is involved in the activation of arginase by oligodeoxinucleotides containing CpG motifs in murine macrophages. Immunology. 2009;128:159–69. doi: 10.1111/j.1365-2567.2008.02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shatanawi A, Romero MJ, Iddings JA, Caldwell RB, Caldwell RW. Angiotensin II elevates endothelial arginase activity via RhoA/MAPK pathways. Experimental Biology. abstract 2010. [Google Scholar]
- 39.Komers R, Schutzer W, Xue H, Oyama TT, Lindsley JN, Anderson S. Effects of p38 mitogen-activated protein kinase inhibition on blood pressure, renal hemodynamics, and renal vascular reactivity in normal and diabetic rats. Transl Res. 2007;150:343–9. doi: 10.1016/j.trsl.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–9. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 41.Jiang B, Xu S, Hou X, Pimentel DR, Cohen RA. Angiotensin II differentially regulates interleukin-1-beta-inducible NO synthase (iNOS) and vascular cell adhesion molecule-1 (VCAM-1) expression: role of p38 MAPK. J Biol Chem. 2004;279:20363–8. doi: 10.1074/jbc.M314172200. [DOI] [PubMed] [Google Scholar]
- 42.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–8. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 43.Beltrán AE, Briones AM, García-Redondo AB, Rodríguez C, Miguel M, Alvarez Y, Alonso MJ, Martínez-González J, Salaices M. p38 MAPK contributes to angiotensin II-induced COX-2 expression in aortic fibroblasts from normotensive and hypertensive rats. J Hypertens. 2009;27:142–54. doi: 10.1097/hjh.0b013e328317a730. [DOI] [PubMed] [Google Scholar]
- 44.Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–4. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 45.Musicki B, Burnett AL. eNOS function and dysfunction in the penis. Exp Biol Med (Maywood) 2006;231:154–65. doi: 10.1177/153537020623100205. [DOI] [PubMed] [Google Scholar]
- 46.Weerackody RP, Welsh DJ, Wadsworth RM, Peacock AJ. Inhibition of p38 MAPK reverses hypoxia-induced pulmonary artery endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1312–20. doi: 10.1152/ajpheart.00977.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou S, Bachem MG, Seufferlein T, Li Y, Gross HJ, Schmelz A. Low intensity pulsed ultrasound accelerates macrophage phagocytosis by a pathway that requires actin polymerization, Rho, and Src/MAPKs activity. Cell Signal. 2008;20:695–704. doi: 10.1016/j.cellsig.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Chandra S, Romero MJ, Shatanawi A, Caldwell RB, Caldwell RW, editors. Experimental Biology. Peroxynitrite and Hydrogen Peroxide Increase Arginase Activity through the RhoA/Rho Kinase (RAK) Pathway. abstract 2010. [Google Scholar]
- 49.Okon EB, Szado T, Laher I, McManus B, van Breemen C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res. 2003;40:520–30. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- 50.Jin LM. Angiotensin II Signaling and Its Implication in Erectile Dysfunction. J Sex Med. 2009;3:302–10. doi: 10.1111/j.1743-6109.2008.01188.x. [DOI] [PubMed] [Google Scholar]