SUMMARY
Autophagy is known to be important in presentation of cytosolic antigens on MHC class II (MHC II). However, the role of autophagic process in antigen presentation in vivo is unclear. Mice with dendritic cell (DC)-conditional deletion in Atg5, a key autophagy gene, showed impaired CD4+ T cell priming after herpes simplex virus infection and succumbed to rapid disease. The most pronounced defect of Atg5−/− DCs was the processing and presentation of phagocytosed antigens containing Toll-like receptor stimuli for MHC class II. In contrast, cross-presentation of peptides on MHC I was intact in the absence of Atg5. Although induction of metabolic autophagy did not enhance MHC II presentation, autophagic machinery was required for optimal phagosome-to-lysosome fusion and subsequent processing of antigen for MHC II loading. Thus, our study revealed that DCs utilize autophagic machinery to optimally process and present extracellular microbial antigens for MHC II presentation.
INTRODUCTION
Classical pathways of antigen presentation involve peptides derived from intracellular and extracellular antigens to be presented in the context of MHC class I (MHC I) and MHC II, which are recognized by cognate CD8+ and CD4+ T cells, respectively (Trombetta and Mellman, 2005). However, in specialized antigen-presenting cells such as dendritic cells (DCs), extracellular antigens are processed for presentation in the context of MHC I molecules via cross-presentation pathway (Heath et al., 2004). Conversely, several lines of evidence indicate that intracellular antigens can also be processed and presented on MHC II molecules (Brazil et al., 1997; Chen et al., 1998; Dörfel et al., 2005; Guéguen and Long, 1996; Jacobson et al., 1989; Jaraquemada et al., 1990; Lich et al., 2000; Nimmerjahn et al., 2003; Paludan et al., 2005; Qi et al., 2000; Zeng et al., 2001).
Recent studies suggested the involvement of autophagy in MHC II presentation of intracellular antigens (Levine and Deretic, 2007; Schmid and Münz, 2007). By using pharmacological inhibitors of the class III PI3 kinase, 3-methyladenine (3-MA) and Wortmannin, MHC II presentation of peptides derived from ectopically expressed complement C5 protein was shown to be impaired in mouse macrophage and B cell lines (Brazil et al., 1997). MHC II presentation of peptides derived from a tumor antigen, Mucin gene 1, and Neomycin phosphotransferase II, was also decreased when autophagy was inhibited by 3-MA and Wortmannin (Dörfel et al., 2005; Nimmerjahn et al., 2003). Another report demonstrated that the MHC II presentation of nuclear antigen 1 of EBV (EBNA1) is reduced by siRNA-mediated knockdown of Atg12, an essential protein involved in autophagosome formation. Electron microscopy analysis indicated that EBNA1 accumulated in autophagosomes in EBV-transformed B cell lines (Paludan et al., 2005). The same group demonstrated that delivery of a model antigen, influenza matrix protein 1 (MP1), to the autophagosome enhanced MHC II presentation. Upon fusion of MP1 to LC3, a mammalian homolog of Atg8 that is inserted into autophagosomal membrane, presentation of MP-1 on MHC class II to MP1-specific CD4+ T cells was enhanced by up to 20-fold, whereas MHC I presentation of the same antigen to MP-1-specific CD8+ T cells was not affected. This study demonstrated that cytosolic antigens could be delivered to the MHC II processing compartment via autophagy for enhanced presentation to CD4+ T cells (Schmid et al., 2007). Although these reports collectively demonstrated the importance of autophagy in vitro, contribution of autophagic delivery of antigens in CD4+ T cell priming in vivo remains unclear.
To examine the requirement for Atg5 in the initiation of immune responses in vivo, we generated Atg5−/− neonatal liver chimera that lack Atg5 gene in hematopoietic compartment, and CD11c-Cre × Atg5flox/flox conditional gene-deleted mice (DC-Atg5−/−) that selectively lack Atg5 in conventional DCs (cDCs). With these mice, we examined the requirement for autophagy and Atg5 in antigen presentation by cDCs leading to priming of CD4+ T cell responses in vivo. We showed that innate immune recognition, antigen capture, migration, maturation, and cytokine production remained intact in Atg5-deficient cDCs. However, Atg5 and other proteins required for autophagy were found to be essential for optimal processing and presentation of a variety of forms of phagocytosed antigens containing TLR agonists. Our data provide in vivo evidence of the requirement for Atg5-dependent process in antigen presentation to CD4+ T cells and revealed an important role of the autophagic machinery in processing and presentation of extracellular microbial antigens in DCs.
RESULTS
Impaired CD4+ T Cell Priming by Atg5-Deficient APCs
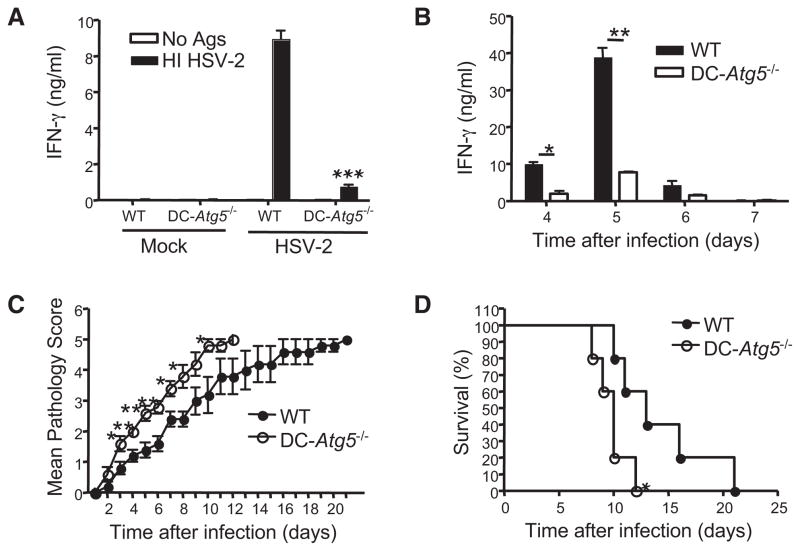
Atg5 is an essential protein required for autophagosomes formation (Mizushima et al., 2001). Because Atg5-deficient mice are incapable of surviving beyond 1 day of life (Kuma et al., 2004), we generated Atg5−/− hematopoietic cells from lethally irradiated wild-type (WT) mice reconstituted with liver cells from the Atg5−/− neonates. Upon full reconstitution, we confirmed that all hematopoietic cells lacked the Atg5 gene by quantitative PCR (Lee et al., 2007). Such mice reconstituted hematopoietic compartments including cDCs and T cells in the spleen, lymph nodes, and bone marrow (Figure S1 available online). After mucosal HSV-1 infection, robust T helper 1 (Th1) cell responses are induced in WT mice (Lee et al., 2009). We examined CD4+ T cell activation in Atg5−/− chimeras after intravaginal (ivag) infection with HSV-1. After 7 days, iliac draining lymph nodes were isolated and CD4+ T cells were purified and cocultured with WT antigen-presenting cells (APCs) in the presence of viral antigen. Interferon-γ (IFN-γ) secretion from CD4+ T cells, as well as the number of cells secreting IFN-γ, in the Atg5−/− chimeric hosts were significantly reduced compared to the WT control (Figure 1A). It has been reported that T cells have a survival defect in Atg5−/− mice (Pua et al., 2007; Stephenson et al., 2009). In order to isolate the effect of Atg5 deficiency on cDCs, we purified cDCs from the draining lymph nodes of HSV-1-infected WT or Atg5−/− chimeric mice at day 3 postinfection (p.i.) and assessed their ability to stimulate HSV-specific CD4+ T cells in the absence of exogenously added antigens. We found a significant reduction in the ability of Atg5−/− DCs to elicit IFN-γ production from WT effector Th1 cells ex vivo compared to the DCs from the WT → WT chimera (Figure 1B). The number of CD4+ T cells secreting IFN-γ was also significantly reduced when Atg5−/− DCs were used as APCs (Figure 1B).
Figure 1. Atg5-Deficient DCs Induce Impaired CD4+ T Cell Responses In Vivo.
(A and B) WT → WT (WT) and Atg5−/− → WT (Atg5−/−) chimeras were infected with 106 PFU of HSV-1 ivag.
(A) Seven days later, purified CD4+ T cells from the draining lymph nodes were cocultured with splenocytes in the presence or absence of heat-inactivated (HI) HSV-1 for 48 hr (right) or for 72 hr (left).
(B) Three days later, the draining lymph node DCs were purified and cocultured for 72 hr with CD4+ T cells isolated from day 7 ivag WT HSV-1-infected mice. No exogenous viral antigens were added.
(A and B) IFN-γsecretion was measured by ELISA (left) and IFN-γ-secreting T cells were enumerated by ELISPOT (right).
(C and D) 1,000,000 CFSE-labeled CD45.1+ OT-II CD4+ T cells were adoptively transferred to WT or Atg5−/− chimeras 1 day prior to infection. Five days after (C) ivag infection with 106 PFU of HSV-2-OVA or (D) i.v. infection with 5 × 105 CFU of Listeria-OVA, OT-II cells from the draining iliac lymph nodes (HSV-2-OVA) and spleen (Listeria-OVA) were analyzed for CFSE dilution by flow cytometry.
(E) Sorted WT or Atg5−/− splenic DCs (5 × 105) along with 106 PFU of HSV-1 were injected intradermally into naive WT mice. A third group of mice received HSV-1 intradermally in the absence of DCs. Seven days later, CD4+ T cells from draining brachial lymph nodes were isolated and cocultured with splenic APCs in the presence of HI HSV-1 for 72 hr. IFN-γ production by CD4+ T cells was assessed by ELISA.
**p ≤ 0.01; *p < 0.05 relative to WT (Student’s t test). These results are representative of three similar experiments. Error bars indicate SD; n = 3.
Next, the ability of WT T cells to be primed by Atg5−/− APCs was examined in vivo after adoptive transfer of WT OT-II cells into Atg5−/− chimeras. These mice were infected with OVA-expressing HSV-2 (Dobbs et al., 2005) intravaginally (Figure 1C) or with OVA-expressing Listeria monocytogenes (Pope et al., 2001) intravenously (Figure 1D), and proliferation of OT-II cells were monitored by CFSE dilution. These data demonstrated that T cells failed to undergo appropriate priming and proliferation upon stimulation by Atg5−/− APCs in vivo. To examine the DC-intrinsic effect of Atg5 deficiency, splenic cDCs from naive WT and Atg5−/− chimeras were sorted by flow cytometry and injected along with HSV-1 intradermally into WT hosts. Seven days later, CD4+ T cells response in the draining lymph nodes was assessed. Although intradermal injection of HSV-1 alone resulted in weak Th1 cell responses through presentation by endogenous DC population, a 5-fold increase in Th1 cell induction was seen when WT cDCs were injected together (Figure 1E). In contrast, only a moderate increase in Th1 cell responses was detected in mice receiving Atg5−/− cDCs (Figure 1E). Thus, these results suggested that Atg5 is required for cDCs to prime CD4+ T cell responses in vivo after HSV-1 and Listeria infections.
DC-Specific Atg5−/− Mice Fail to Prime Antiviral Th1 Cells and Succumb to HSV-2 Infection
To provide definitive evidence for the in vivo role of autophagic machinery in antigen presentation by cDCs, and to gain insights into the physiological importance of T cell priming via this pathway, we generated CD11c-Cre × Atg5flox/flox conditional gene-deleted mice (DC-Atg5−/−), which are specifically defective in autophagy in DCs. We have confirmed that CD11c-Cre is expressed specifically in cDCs (Figure S2; Stranges et al., 2007). After intravaginal infection with HSV-2, iliac draining lymph node was isolated 7 days later, and CD4+ T cells were purified and cocultured with WT APCs in the presence of viral antigen. Th1 cell responses in the DC-specific Atg5−/− mice were significantly reduced compared to WT mice (Figure 2A). Immune protection against HSV-2 is mediated in a CD4+ T cell-dependent manner (Harandi et al., 2001; Milligan et al., 1998). To determine the importance of the Atg5-dependent pathway in DCs in the generation of protective Th1 cell immunity, we next challenged the DC-Atg5−/− mice with a lethal dose of HSV-2 and followed their survival and pathology. In the absence of Atg5 in DCs, IFN-γ secreted from CD4+ T cells between days 4 and 7 after infection (Milligan and Bernstein, 1997) was virtually absent in the vaginal secretion (Figure 2B), and mice sustained more severe disease (Figure 2C) and succumbed to HSV-2 infection more rapidly compared to WT mice (Figure 2D). Thus, taken together, these data indicated a critical in vivo role of Atg5-dependent process in antigen presentation by DCs to prime protective antiviral Th1 cell responses.
Figure 2. DC-Specific Atg5−/− Mice Fail to Mount Protective Immunity against HSV-2 Challenge.
(A) CD11c-Cre × Atg5flox/flox (DC-Atg5−/−) mice were infected with 106 PFU of TK− HSV-2 ivag. Seven days later, IFN-γ secretion from draining lymph node CD4+ T cells was analyzed.
(B–D) DC-Atg5−/− mice were infected with 103 PFU of WT HSV-2 ivag. IFN-γ in the vaginal secretion on the indicated days after infection (B), mean clinical scores (C), and survival (D) are shown.
Error bars represent SEM of five mice per group. **p ≤ 0.01; *p < 0.05 relative to WT (Student’s t test for A–C and Logrank test for D). These results are representative of three similar experiments.
Intact Migration and Innate Responses by Atg5−/− DCs
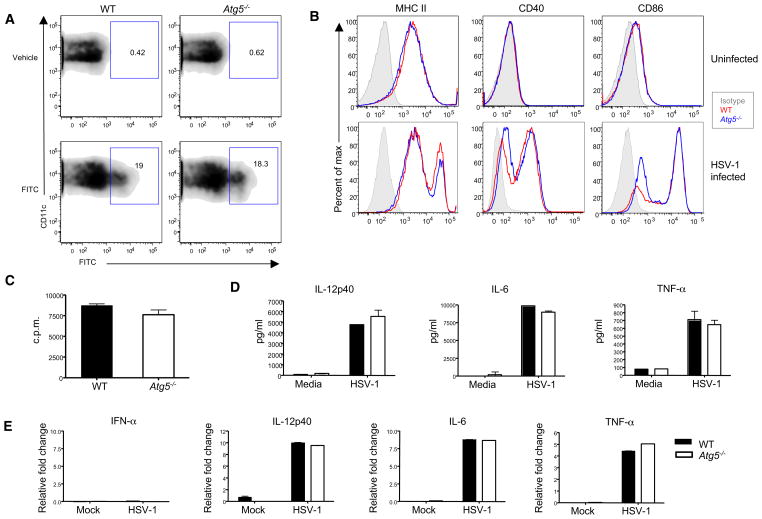
Next, we examined the requirement for Atg5 in pathways involved in T cell activation by DCs. DC migration is pivotal for the initiation of cellular immune responses. Activated DCs upre-gulate CCR7 and migrate into secondary lymphoid organs in order to prime naive lymphocytes (Dieu et al., 1998; Förster et al., 1999). To examine the contribution of Atg5 in DC migration in vivo, we examined the ability of endogenous skin DC populations to migrate to the draining lymph nodes after a standard FITC painting protocol. Thus, WT or Atg5−/− chimeras were painted with 1% FITC solution (acetone:dibutyl phthalate = 1:1) and at 72 hr, migration of FITC+ DCs in the draining lymph nodes was analyzed by flow cytometry. These analyses revealed no defects in the ability of Atg5−/− DCs to migrate from the skin to the lymph nodes (Figure 3A).
Figure 3. Intact Migration and Innate Responses in Atg5−/− DCs.
(A) To track endogenous DC migration, 1% FITC solution (acetone:dibutyl phthalate = 1:1) was painted on the back skin of the indicated chimera. At 72 hr, draining brachial lymph nodes were isolated and FITC content within the CD11c+ MHC II+ DCs was analyzed.
(B) MACS-purified DCs from WT or KO chimera were left untreated or infected with HSV-1 (MOI = 1) for 18 hr. The expression of MHC II, CD40, and CD86 molecules were analyzed by flow cytometry.
(C) OT-II T cells were cultured with the HSV-1-infected splenic DCs (as in A) in the presence of 10 ng/ml OVA323-339 peptides for 72 hr at 37°C. T cell proliferation was measured by 3H thymidine incorporation.
(D and E) Sorted splenic WT and Atg5−/− DCs (105) were infected with HSV-1 (MOI = 5). After 18 hr, IL-12p40, IL-6, and TNF-α levels were measured from supernatants by ELISA (D) or by quantitative RT-PCR (E). These results are representative of three similar experiments. Error bars indicate SD; n = 3.
After their migration into the lymph node, DCs induce differentiation of naive T cells into effector cells. This process requires at least three signals: cognate peptide presented in the context of MHC molecules, costimulatory signals, and cytokines. Expression of costimulatory molecules CD86 and CD40 molecules as well as MHC II was comparable in the Atg5−/− and WT DCs at steady state (Figure 3B). In addition, both MHC II and costimulatory molecules were upregulated in response to HSV-1 infection comparably between Atg5−/− and WT DCs (Figure 3B). To examine whether HSV-infected WT and Atg5−/− DCs have similar capacity to present antigens on MHC II, we pulsed HSV-infected DCs with exogenous peptide OVA323-339 and used them to stimulate OT-II cells. OT-II cells underwent similar extent of proliferation when WT or Atg5−/− DCs were used as APCs (Figure 3C), indicating that maturation of DCs is not affected by the absence of Atg5 in HSV-infected DCs. Both WT and Atg5−/− cDCs were equally infected by HSV-1 in vitro (Figure S3). In addition, secretion of cytokines, IL-12p40, IL-6, and TNF-α by HSV-infected Atg5−/− cDCs was similar to those of WT cDCs (Figure 3D). No difference in the mRNA expressions corresponding to these cytokines was detected (Figure 3E). These results indicated that Atg5 is not necessary for cytokine production upon innate recognition of HSV-1 in cDCs, consistent with our previous study (Lee et al., 2007). In addition, Atg5 deficiency does not affect the ability of cDCs to migrate or to upregulate costimulatory molecules and MHC II.
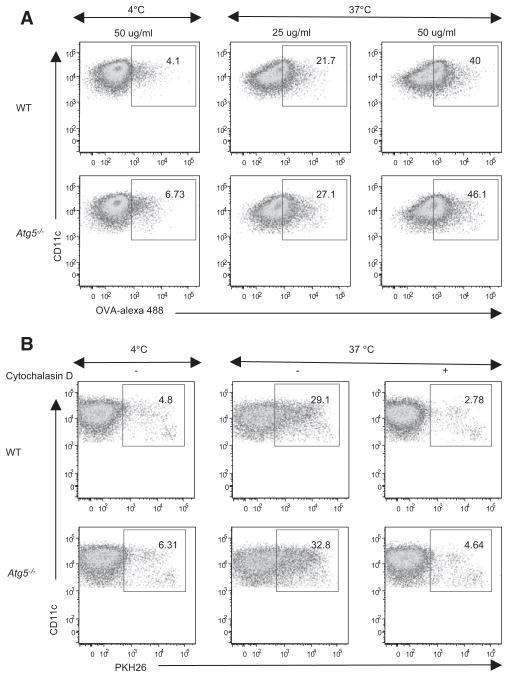
Atg5-Deficient DCs Have Normal Endocytic and Phagocytic Capacity
Thus far, our data indicate that Atg5−/− DCs have no defect in innate recognition, migration, costimulation, MHC expression, or cytokine secretion in response to HSV-1 infection. Yet, these cells had an impaired capacity to stimulate CD4+ T cells in vivo. Our previous study indicated that DCs responsible for CD4+ T cell priming in the lymph node after vaginal HSV-2 infection are not infected by the virus, but rather take up viral antigens from the infected vaginal keratinocytes in vivo (Zhao et al., 2003). Thus, to test the possibility that Atg5 is required for uptake of antigens, we examined the endocytic ability of Atg5−/− DCs for different forms of antigens ex vivo. To probe the pinocytosis-mediated uptake of soluble antigen, Atg5−/− splenic cDCs were incubated with OVA conjugated to the pH-insensitive fluorochrome Alexa Fluor 488. Both WT and Atg5−/− DCs captured comparable amounts of OVA-Alexa 488 in a dose-dependent manner (Figure 4A). Next, to examine the phagocytic ability of DCs from Atg5−/− mice, we incubated splenic cDCs with syngeneic apoptotic MHC II-deficient splenocytes labeled with the membrane dye PKH26. After 3 hr incubation at 37°C, both WT and Atg5−/− splenic cDCs showed similar uptake of splenocytes (Figure 4B). Nonspecific binding, as demonstrated either by incubation at 4°C or by treatment with cytochalasin D, an inhibitor of actin polymerization, was less than 7% in both WT and Atg5−/− splenic cDCs. These data indicated that cDCs do not require Atg5 for endocytic or phagocytic uptake of exogenous antigens.
Figure 4. Unimpaired Endocytosis and Phagocytosis in Atg5−/− DCs.
Purified splenic DCs from WT or Atg5−/− chimeras were incubated with the indicated amounts of OVA-Alexa 488 for 1 hr at 4°C or 37°C (A) or irradiated PKH26-labeled MHC II−/− splenocytes on ice or at 37°C in the presence or absence of 10 μM cytochalasin D for 3 hr (B). Uptake of OVA (A) or PKH26-labeled apoptotic cells (B) by DCs was determined by flow cytometry. The results shown are representative of three independent experiments. Numbers indicate percentages.
Atg5 Is Required for Optimal Processing and Presentation of Antigens for MHC II by DCs
Autophagy has been shown to contribute to the processing and presentation of cytosolic antigens (Ags) (Brazil et al., 1997; Dörfel et al., 2005; Nimmerjahn et al., 2003; Paludan et al., 2005; Schmid et al., 2007). Thus, to examine the importance of autophagy in presentation of cytosolic Ag, OVA was introduced into the cytosol of DCs after infection with OVA-expressing Listeria monocytogenes (Pope et al., 2001). A significant decrease in OT-II T cell proliferation was seen when T cells were stimulated by Atg5−/− DCs compared to WT DCs (Figure 5A) despite the similar levels of bacterial infection in these groups (Figure S4A). This reduced ability of the Atg5−/− DCs to present OVA peptide from Listeria-OVA in vitro was consistent with the lower proliferation of OT-II cells induced in the Atg5−/− host upon infection with Listeria-OVA in vivo (Figure 1D). Therefore, DC presentation of peptides derived from a cytosolic bacteria-derived Ag was moderately impaired in the absence of Atg5. These data indicated that canonical autophagy contributes to presentation of cytosolic microbial antigens on MHC II by DCs.
Figure 5. Atg5 Is Necessary for Antigen Processing for MHC II, but Not MHC I.
(A) WT or Atg5−/− DCs infected with Listeria-OVA were cocultured with OT II T cells for 72 hr at 37°C.
(B and C) OT-II (B) or OT-I (C) cells were incubated in the presence of purified splenic DCs loaded with indicated amounts of soluble OVA.
(D) Purified splenic DCs were loaded with the indicated numbers of OVA-coated MHC II-deficient splenocytes and incubated with OT-II cells for 72 hr at 37°C.
(E) Purified splenic DCs were loaded with the indicated numbers of OVA-coated MHC I-deficient splenocytes and incubated with OT-I cells for 72 hr at 37°C. T cell proliferation was measured by 3H incorporation.
**p ≤ 0.01; *p < 0.05, relative to WT (Student’s t test). These data are representative of three to six similar experiments. Error bars indicated SD.
Given that APCs responsible for priming T cell immunity to genital HSV-2 infection are tissue-migrant DCs that have acquired extracellular viral antigens (Zhao et al., 2003), we hypothesized that the phenotype of Atg5−/− DCs (Figures 1 and 2) reflect the deficiency of DCs to process and present extracellular viral antigens to CD4+ T cells. To dissect this possibility, we examined the ability of Atg5−/− DCs to process and present various forms of extracellular antigens to CD4+ T cells. We already ruled out an intrinsic and general defect in the ability of Atg5−/− DCs to present peptide on MHC II and activate CD4+ T cells (Figure 3C). First, to examine the ability of DCs to process and present soluble Ags, DCs were first incubated with OVA and then naive purified OVA-specific OT-II cells were added to the culture. We found a slight reduction in the capacity of Atg5−/− DCs to stimulate proliferation of OT-II cells compared to WT DCs (Figure 5B). However, this difference was not statistically significant except at the highest Ag doses in all repeated experiments. To determine whether this slight difference could be attributed to an unexpected toxicity of OVA on Atg5−/− DCs, we carried out a parallel experiment in which the same set of DCs were used to stimulate OT-I cells. In contrast to MHC II presentation, Atg5−/− DCs had no observable defect in MHC I presentation of soluble OVA (Figure 5C). These data excluded the possibility of OVA toxicity or intrinsic defects in Atg5−/− DCs in antigen uptake, survival, or costimulation.
Next, the ability of DCs to present peptide on MHC II after phagocytic uptake of antigens was assessed. We examined presentation of apoptotic cell-associated antigen. To restrict antigen presentation to DCs alone, we incubated WT and Atg5−/− DCs with irradiated OVA-loaded MHC II-deficient splenocytes. Presentation of cell-associated OVA was markedly diminished in the Atg5−/− DCs compared to WT DCs (Figure 5D). However, Atg5−/− DCs demonstrated no defects in cross-presentation of apoptotic cell-associated antigen on MHC I as detected by OT-I cells (Figure 5E). A previous study indicated that phagosomes containing TLR ligands utilize autophagic machinery to facilitate phagosome fusion with lysosomes, leading to rapid acidification and enhanced killing of the ingested organism (Sanjuan et al., 2007). Because most commercially available OVA is contaminated with LPS (Eisenbarth et al., 2002), we examined MHC II presentation of phagocytosed antigen devoid of TLR agonists in Atg5−/− DCs. Irradiated apoptotic splenocytes from C3H/HeN mice (I-Eα+), coated with LPS or not, were given to WT or Atg5−/− DCs and the extent of Eα presentation on I-Ab was assessed by the Y-Ae antibody (Figure S4B; Murphy et al., 1992). These data indicated that in the absence of TLR engagement, both WT and Atg5−/− DCs presented equivalent amounts of MHC II peptide complex on their cell surface, whereas MHC II presentation of LPS-coated apoptotic cells was enhanced only in WT but not Atg5−/− DCs. Collectively, these data indicated that whereas uptake of Ags, activation of DCs, and cross-presentation to MHC I occur normally in Atg5−/− DCs, these cells have a pronounced defect in MHC II processing pathways for phagocytosed antigens that contain TLR agonists.
Autophagic Machinery Facilitates MHC II Presentation of Phagocytosed Antigens by DCs
Because Atg5 protein has been reported to serve autophagy-independent functions (Jounai et al., 2007; Yousefi et al., 2006; Zhao et al., 2008), we examined whether other key components of autophagic machinery are required for antigen processing and presentation by DCs. To this end, we employed two strategies. First, we used well-known inhibitors of autophagy, 3 methyladenine (3MA) and Wortmannin (WM). At doses of 3MA (1–10 mM) and Wortmannin (10–100 nM) that are known to specifically inhibit autophagy (Blommaart et al., 1997; Seglen and Gordon, 1982), presentation of phagocytosed antigen was significantly reduced. In contrast, the same doses of these inhibitors did not affect exogenous peptide presentation on MHC II (Figure S5A). Second, to more specifically inhibit the functions of autophagic machinery, we used siRNA knockdown of Atg7 or Atg12 to block the function of autophagic machinery in primary DCs. We examined the ability of DCs that have reduced Atg7 or Atg12 to process and present viral Ag from primary keratainocytes that have been infected with HSV-2-expressing OVA (Dobbs et al., 2005), to mimic the situation of Ag presentation in vivo. Blockade of the function of autophagic machinery resulted in a significant reduction of MHC II peptide presentation from HSV-2-infected cells (Figure S5B). To determine whether signs of metabolic autophagy are detected in DCs after phagocytosis, WT and Atg5−/− DCs were fed LPS-coated latex beads, and at various time points, indicators of metabolic autophagy, lipidated LC3 and p62 (Klionsky et al., 2008), were assessed. Although lipidated LC3 was absent in Atg5−/− DCs, WT DCs underwent constitutive autophagy (Figure S5C; Schmid et al., 2007). However, phagocytosis of LPS-coated beads did not alter the total level of cytosolic autophagy as indicated by constant levels of LC3-II and p62 (Figure S5C). Next, we tested whether induction of canonical autophagy enhances MHC II presentation of phagocytosed antigens. To this end, we induced autophagy by rapamycin treatment or starvation by an established method (Sanjuan et al., 2007) prior to the addition of LPS-Eα-coated latex beads. Peptide presentation on MHC II as measured by Y-Ae antibody (Figure S5D) demonstrated that induction of metabolic autophagy by rapamycin or starvation failed to enhance MHC II peptide presentation. If anything, these treatments led to a reduction in MHC II presentation, even when Ag uptake was normalized by gating on DCs that have captured a single bead (Figure S5D).
Our data thus far indicated that TLR-phagosome might utilize autophagic machinery for processing of antigens for MHC II presentation in DCs without invoking canonical cytosolic autophagy. To directly address this possibility, we analyzed the membrane surrounding the TLR-phagosome by transmission electron microscopy (TEM). Lysosomal contents were labeled with magnetic beads and were visualized by the presence of electron-dense material (Figure 6, black arrowheads). DCs were given LPS-OVA-coated latex beads and chased for 20 min. This time point was chosen based on the fact that LC3 recruitment to the phagosome was reported to begin around 20 min postingestion (Sanjuan et al., 2007). At this time point, lysosomal membranes fusing with the bead-containing phagosome were clearly detected (Figure 6, white arrows). However, we failed to detect any double membrane structures surrounding the phagosome. These data are consistent with previously published report with RAW264.7 macrophage cell line (Sanjuan et al., 2007) and further emphasize that in DCs, phagosomal maturation requires Atg5-dependent process that does not involve double membrane structure around the phagosomes. Thus, these data suggested that a process that depends on the components of autophagic machinery, but not canonical autophagy, is required in DC presentation of peptide on MHC II.
Figure 6. Absence of Double Membrane Structure around LPS-Bead-Containing Phagosome in DCs.

Lysosomes were labeled with magnetic beads (electron-dense materials). MACS-purified CD11c+ BMDCs were pulsed with LPS-OVA-latex beads for 20 min. BMDCs were fixed and sectioned for transmission electronic microscopy. White arrows indicate lysosomal membranes fusing with the bead-containing phagosome. Black arrowheads indicate lysosomes (labeled with magnetic beads). Scale bars represent 2 μm in (A) and 1 μm in (B). Results are representative of three independent experiments.
Impaired Phagosome-to-Lysosome Fusion in Atg5−/− DCs
In an effort to determine the mechanism by which the autophagic machinery facilitates processing of phagocytosed antigens, we first examined the kinetics and extent of peptide loading onto MHC II with a pulse-chase analysis after phagocytosis of Eα-LPS-bound-latex beads. These results demonstrated that the emergence of the Eα peptide-MHC II complex as detected by the Y-Ae antibody was delayed in both the intracellular compartment and at the cell surface in Atg5−/− DCs (Figure 7A). Second, to rule out the possibility that the defect in antigen presentation might be related to defective trafficking of antigens, we performed a confocal microscopy experiment to localize the phagocytosed antigen and MHC II. These analyses revealed that antigens (yeast particles) are taken up and delivered to the MHC II loading compartment normally in Atg5−/− DCs (Figure 7B). Third, we asked whether the decreased peptide presentation on MHC II in Atg5−/− DCs could be a result of enhanced or reduced degradation of antigens within the phagosome. If Atg5−/− DCs degrade phagocytosed antigens too rapidly and/or extensively for MHC II loading, we should be able to rescue their phenotype by adding increasing concentrations of protease inhibitors. To this end, we inhibited the activity of lysosomal proteases with a combination of leupeptin and pepstatin (Jancic et al., 2007). Addition of protease inhibitors failed to revert the defective presentation phenotype of Atg5−/− DCs (Figure 7C). In addition, the kinetics of degradation of phagocytosed antigens in Atg5−/− DCs was severely delayed compared to WT DCs (Figure S6A). These results indicated that the phagolysosomes of the Atg5−/− DCs have impaired, rather than excessive, degradative capacity, resulting in less peptide presented on MHC II.
Figure 7. Atg5 Is Required for Effective Delivery of Lysosomal Proteases to the Phagosome.
(A) CD11c+ splenic DCs isolated from WT or Atg5−/− mice were incubated with I-Eα-bound latex beads for the indicated time. Cell surface and intracellular levels of Eα52-68:MHC II complex on DCs that have taken up precisely one bead were analyzed by Y-Ae antibody.
(B) Localization of yeast expressing GFP in WT or Atg5−/− BMDCs was analyzed by immuofluorescence confocal microscopy. Percentages of DCs containing yeast in the MHC II compartment in DCs are plotted. Data are means ± SD.
(C) Presentation of OVA323-339 peptides on MHC II after uptake of irradiated OVA-coated MHC II-deficient splenocytes by WT or Atg5−/− BM DCs was assayed in the presence of increasing concentrations of the protease inhibitors (leupeptin and pepstatin).
(D) Kinetics of lysosomal and phagosomal pH in WT or Atg5−/− BM DCs was analyzed.
(E) Extracts of phagosomes isolated from WT and Atg5−/− BM DCs were incubated for the indicated duration of time in the presence of fluorogenic substrate for cathepsins B and L (B/L) or cathepsins S at the indicated pH. The optimal pH for Cathepsin B/L is pH 5.5, whereas that for cathepsin S is pH 7.4. Substrate degradation was assayed fluorometrically.
Data are representative of three independent experiments. Error bars indicate SD.
The defective proteolytic environment of the phagosomes in Atg5−/− DCs could result from (1) a defective lysosomal compartment, (2) a defect in trafficking of vacuolar ATPases to acidify the phagosomal compartment, or (3) a defect in delivery of lysosomal proteases to phagosomes. To test these possibilities, we first measured the pH of both the lysosomes and phagosomes in WT and Atg5−/− DCs (Figure 7D). These analyses indicated that lysosomal pH levels in the Atg5−/− DCs were indistinguishable from those of the WT DCs. Furthermore, both the kinetics and the extent of acidification was identical in the phagosomes of Atg5−/− DCs compared to WT DCs, indicating that trafficking of V-ATPase is intact in the absence of Atg5. To further examine the possibility that lysosomes might be defective in Atg5−/− DCs, we analyzed the amounts of lysosomal proteases. Immunoblot analysis of the whole cell lysates revealed that amounts of cathepsins B, D, L, and S were comparable in WT and Atg5−/− DCs (Figure S6B). Consequently, Ii chain processing was not affected at steady-state (Figure S6B). In addition, we tested the proteolytic activity of the lysosomal enzymes by using casein, a generic proteolysis substrate, in a fluorescence assay that relies on relief of intermolecular quenching as fluorescent peptides are released from a highly labeled, internally quenched protein substrate (Jones et al., 1997). This analysis revealed that lysosomal proteolytic activity was intact in the absence of Atg5 (Figure S6C). Thus, collectively, these data indicated that the lysosomes of Atg5−/− DCs are not impaired with regards to pH maintenance, cathepsins levels, and proteolytic capacity. In addition, acidification of phagosomes in DCs is unaffected by the absence of Atg5.
These results led us to examine the third potential explanation for the defective phagosomal proteolysis in Atg5−/− DCs, namely a defective delivery of lysosomal proteases to the phagosomes. To test this hypothesis, phagosomes from WT and Atg5−/− DCs were isolated at various time points after phagocytosis of LPS-coupled beads, and protease activities were measured against specific substrates for cathepsins S or B and L. These analyses revealed that Atg5−/− DCs have a significant defect in cathepsins S, B, and L activity in the phagosome (Figure 7E). However, the defect in phagolysosomal proteolysis was not complete and not enough to reduce the steady-state MHC II levels (Figure 3) or Ii chain processing (Figure S6B), indicating that the reduction in presentation of phagocytosed antigenic peptide is probably due to a specific defect in the proteolysis of the phagosomal antigens associated with TLR agonists (Figure S4B). Finally, to directly examine the rate of phagosome fusion to lysosome, confocal microscopic analyses of WT and Atg5−/− DCs were carried out at 5, 15, and 30 min chase after a 5 min pulse with LPS-OVA-coated latex beads. Although the rate of bead uptake was comparable, Atg5−/− DCs were significantly delayed in their ability to fuse phagocytosed beads to the lysosome compared to the WT DCs (Figures S6D and S6E). Collectively, these data indicated that in the absence of Atg5, DCs have an impaired capacity to process and present phagocytosed antigen on MHC II, and such defect is unrelated to the pH environments of the phagolysosome or lysosome, but rather, is a result of delayed fusion of phagosome to lysosome and defective delivery of cathepsins to the MHC II processing compartments of Atg5−/− DCs.
DISCUSSION
Our present study provides evidence that autophagy is required for processing and presentation of antigens for MHC II presentation to enhance CD4+ T cell activation in vivo. In the absence of Atg5 in DCs, the animal failed to mount proper Th1 cell immunity, succumbing to viral disease and death. We demonstrated that whereas innate recognition, Ag capture, migration, maturation, and cytokine secretion by DCs is unimpaired in the absence of Atg5, processing and presentation of phagocytosed Ags containing TLR agonists for MHC II is diminished in Atg5−/− DCs. In the absence of Atg5, DCs had a reduced capacity to process cytosolic antigens for MHC II presentation. This is consistent with the notion that canonical autophagy delivers cytosolic antigens to MHC II compartment (Schmid et al., 2007). More significantly, Atg5−/− DCs were impaired in their ability to process phagocytosed antigen for loading onto MHC II, and this defect was due to the impaired phagosome-to-lysosome fusion and delivery of lysosomal proteases to the phagosomes. Although machinery of autophagy (PI3K, Atg5, Atg7, and Atg12) was required, metabolic autophagy was neither induced nor required for enhanced processing of phagocytosed Ag for MHC II presentation in DCs. Therefore, this study revealed the importance of autophagic machinery in DCs for facilitating optimal processing of extracellular antigens containing microbial signatures and presentation to CD4+ T cells.
In addition to the impaired CD4+ T cell responses, we also observed significant decrease in the CD8+ T cell responses after HSV-1 infections in vivo. However, because the induction of CTL responses to HSV-1 requires CD4+ T help (Jennings et al., 1991), our data do not distinguish whether the reduced CD8+ T cell responses represent the requirement for T help, or whether presentation of antigenic peptide on MHC I also requires autophagy in vivo. Parallel in vitro experiments to probe antigen presentation of various forms of OVA revealed that presentation of peptides for MHC II, but not MHC I, required Atg5 in DCs. Because infected DCs are rendered incapable of T cell priming (Kruse et al., 2000; Mikloska et al., 2001; Pollara et al., 2003; Salio et al., 1999), the results of our current study reflect the requirement for autophagic machinery in uninfected DCs to prime naive CD4+ T cells. In addition, recent studies indicate that in infected cells, autophagy may enhance antigen presentation to MHC I (English et al., 2009) and MHC II (Leib et al., 2009) after HSV-1 infection.
HSV-1 encodes ICP34.5, which inhibits autophagy by two distinct manners—by binding to Beclin-1/Atg6 (Orvedahl et al., 2007) and by inhibiting the action of PKR by dephosphorylating eIF2a (Tallóczy et al., 2002). These anti-autophagic actions of ICP34.5 result in enhanced viral replication in vivo (Orvedahl et al., 2007). Our in vitro infection of Atg5−/− DCs by TK− HSV-2-OVA exhibited little difference in the extent of infection as measured by viral titer, albeit slight increase in glycoprotein expression detected in the Atg5−/− DCs at all MOIs tested. These data are consistent with a previous report indicating that elimination of the autophagic pathway in mouse embryonic fibroblast did not alter the replication of wild-type HSV-1 or ICP34.5 mutants (Alexander et al., 2007). In addition, despite the actions of ICP34.5, we observed a significant reduction in both CD4+ and CD8+ T cell priming to HSV-1 in mice deficient in Atg5 in the hematopoietic compartment or in DCs. These results indicate that DCs responsible for presenting the viral peptide in vivo are not directly infected by HSV, and for this reason, the virulence factor ICP34.5 was unable to inhibit the autophagic machinery within Ag-presenting DCs.
The precise nature by which Atg5 contributes to antigen presentation by DCs is unknown. Consistent with previous studies demonstrating the role of autophagy in delivery of cytosolic Ags to the MHC II loading compartment, our data also showed that cytosolic antigens are presented to MHC II more efficiently in the presence of Atg5. The requirement of Atg5 in presentation of cytosolic Ags probably reflects the requirement for autophagy in delivering antigens to the MHC II loading compartment (Levine and Deretic, 2007; Schmid and Münz, 2007). In addition to this well-accepted role of autophagy in MHC II presentation of cytosolic antigens, our data revealed an important role of Atg5 in presentation of phagocytosed Ags, and to a lesser extent, soluble Ags that are associated with PAMPs. This process depended on autophagic machinery (PI3K, Atg5, Atg7, and Atg12), but metabolic autophagy was neither induced nor required for enhanced processing of phagocytosed Ag for MHC II presentation in DCs. Our data ruled out that such an Atg5-dependent process relates to dysfunctional lysosomes, synthesis and maturation of cathepsins, or pH regulation in the lysosomes or phagosomes. Instead, autophagic machinery was required for the efficient phagolysosomal fusion and subsequent delivery of lysosomal proteases to the phagosomes containing TLR agonists. These data are consistent with the demonstration in macrophages that autophagic machinery promotes the recruitment of LC3 and Beclin-1 to the phagosome in which TLR is engaged, allowing rapid fusion with lysosome (Sanjuan et al., 2007). Our study also provides a potential mechanism by which TLR-engagement enables MHC II peptide presentation in DCs (Blander and Medzhitov, 2006). Collectively, these studies and our current study suggest a model in which TLR engagement in the phagosome leads to recruitment of autophagic machinery, allowing rapid fusion to the lysosome and delivery of proteases that cleave antigens efficiently for MHC II loading.
Whether a similar requirement for autophagic machinery exists for MHC II peptide presentation by other APCs is unknown. For example, B cells have been shown to present endogenous Ags readily in a process largely dependent on intact autophagosome formation (Brazil et al., 1997; Nimmerjahn et al., 2003; Paludan et al., 2005; Schmid et al., 2007). Several tissues, including thymic epithelial cells, podocytes, and gastric chief cells undergo high constitutive rate of autophagy in nutrient-rich conditions in vivo (Mizushima et al., 2004). A recent study indeed revealed the importance of autophagy in presentation of self-peptide on MHC II by thymic epithelial cells in shaping the CD4+ T cell repertoire (Nedjic et al., 2008). Studies in this area are expected to reveal the relative involvement of autophagy in Ag presentation in a variety of immunological contexts.
Our study demonstrated an important role for autophagic machinery in processing and presentation of phagocytosed Ags for MHC II by conventional DCs. These findings provide in vivo evidence for the importance of autophagic machinery in MHC II presentation of a variety of antigenic forms by DCs and have important implications in the design of vaccines. By utilizing the biology of autophagosome and its machinery, DCs can be targeted for both optimal innate activation as well as improved peptide presentation on MHC molecules.
EXPERIMENTAL PROCEDURES
Animals
6- to 8-week-old female C57BL/6 was obtained from National Cancer Institute (Frederick, MD). Atg5+/− (Kuma et al., 2004), Atg5flox/flox (Hara et al., 2006), CD11c-Cre Tg (Stranges et al., 2007) MHC II-deficient (H2-Ab1−/−), MHC I-deficient (H-2K−/−D−/−) (Taconic), C3H/HeN (Taconic), OT-I Rag1−/− (Taconic), and OT-II (Jackson) mice were bred in the Yale animal facility. All procedures used in this study complied with federal guidelines and institutional policies by the Yale Animal Care and Use Committee.
Neonatal Liver Chimera Generation
Atg5 chimeras were produced as described (Lee et al., 2007). In brief, 2–3 × 106 liver cells from less than 24-hr-old pups born to Atg5+/− parents were screened for the Atg5 genotype and injected into recipient C57BL/6 mice via tail vein. Prior to transfer of liver cells, recipient mice were lethally irradiated with 4.75 Gy of irradiation two times 3 hr apart. This procedure led to complete chimerism of hematopoietic cells by 8 weeks posttransplantation (Lee et al., 2007).
Virus, Bacteria, and Infection
HSV-1 KOS, 186syn+ HSV-2, and TK− HSV-2 strains were kindly provided by D. Knipe (Harvard Medical School, Boston, MA). HSV-1 was propagated and titered by a plaque assay on Vero cells. HSV-2-TK− OVA (Dobbs et al., 2005) and Listeria monocytogenes-OVA (Pope et al., 2001) were gifts from G. Milligan (University of Texas Medical Branch, Galveston, TX) and L. Lefrançois (University of Connecticut Health Center, Farmington, CT), respectively. Intravaginal virus infection was carried out as previously described with 1 ×106 PFU of HSV-1 or HSV-2 in 10 μl volume (Lee et al., 2009). Upon WT HSV-2 challenge, the severity of disease was scored (Morrison et al., 1998): 0, no sign; 1, slight genital erythema and edema; 2, moderate genital inflammation; 3, purulent genital lesions; 4, hind-limb paralysis; 5, premoribund. Because of humane concerns, the animals were euthanized prior to reaching moribund state. For intradermal infection, mice were inoculated in the back skin with 1 × 106 PFU of HSV-1 with or without 5 × 105 sorted DCs in 100 μl PBS. For Listeria-OVA infection in vivo, a total of 1 × 106 CFSE-labeled CD45.1+OT-II T cells were intravenously transferred into CD45.2+ congenic Atg5 chimeras. Twenty-four hours later, these mice were intravenously infected with 5 × 105 CFU of Listeria-OVA.
CD4+ T Cell Response Assay
Anti-HSV CD4+ T cells were analyzed as previously described (Zhao et al., 2003). To assess the ability of DCs to present viral Ag ex vivo, 5 × 104 DCs isolated from the draining lymph nodes of day 3 ivag HSV-1-infected mice were cocultured with 1 × 105 CD4+ T cells isolated from day 7 ivag HSV-1-infected mice for 72 hr at 37°C in the absence of viral antigens. IFN-γ production in supernatants was measured by enzyme-linked immunosorbent assay (ELISA). For ELISPOT assay, 105 CD4+ T cells were incubated with 105 splenocytes as antigen-presenting cells in the presence of 500 PFU-equivalent of heat-inactivated HSV-1 in 96-well PVDF membrane plates for ELISPOT (Milli-pore) that were precoated with IFN-γ antibody (AN-18, eBiosciences) for 48 hr at 37°C. The plates were developed with IFN-γ antibody (R4-6A2, eBiosciences). IFN-γ + spots were analyzed by ELISPOT reader (CTL Cellular Technology). To assess the ability of DCs to present viral Ag ex vivo, 5 × 104 DCs isolated from the draining lymph nodes of day 3 ivag HSV-1-infected mice were cocultured with 1 × 105 CD4+ T cells isolated from day 7 ivag HSV-1-infected mice for 48 hr at 37°C in the absence of viral antigens in PVDF membrane plates and followed as described above for ELISOPT assay.
Dendritic Cell Isolation and Sorting
The DCs were prepared from spleen and the draining lymph nodes based on previously described methods (Lee et al., 2009) with slight modifications. To obtain sufficient DCs from each chimeric mouse for in vitro experiments, 1 × 106 B16 melanoma cells expressing Flt3L (Mach et al., 2000) were injected into mouse neck ruff 14 days prior to harvest. The spleen and draining lymph nodes were removed from mice infected for the indicated time periods. To obtain FACS-sorted DCs, enriched CD11c+ cells were stained with anti-MHC II-FITC (M5/114.15.2, eBiosciences), anti-PDCA-1-PE (Miltenyi Biotec), anti-B220-PE-Cy5 (RA3-6B2, eBiosciences), and anti-CD11c-APC (HL3, BD Biosciences) after incubating for 10 min at 4°C in the presence of anti-CD16/32 (2.4G2, BD Biosciences) antibody to block Fc receptors. Stained cells were sorted by FACS Aria (BD Biosciences, Mountain View, CA). Sorted splenic CD11c+MHC II+B220−PDCA-1− cDCs were more than 98% pure as assessed by post-sort analyses. Sorted DCs were cultured in RPMI 1640 containing 10% FBS (Invitrogen), 50 μM β-mercaptoethanol, 100 unit/ml penicillin (Invitrogen), and 100 μg/ml streptomycin (Invitrogen) in 96-well U-bottom plates (BD Biosciences).
Tracking of Migrant DCs after FITC Painting
Migration of endogenous DC populations was analyzed according to a standard FITC painting protocol (Macatonia et al., 1987). In brief, mice were anesthetized, shaved with hair clipper, and depilated. The depilated areas of the skin were painted with 1% FITC solution in acetone:dibutyl phthalate (1:1 ratio, Sigma-Aldrich). The brachial lymph nodes were isolated at 72 hr and single-cell suspensions were prepared as described above. Isolated lymph node cells were stained with anti-CD11c (N418, eBioscience)-PE-Cy7 and MHC II (eBioscience)-Alexa 680 and FITC uptake was analyzed by FACS.
Flow Cytometric Analyses of T Cells and DCs
Enriched DCs were pretreated with anti-CD16/32 (2.4G2) antibody to block Fc receptors and stained with anti-CD40 (1C10, eBioscience), anti-CD86 (GL1, eBioscience), anti-CD8α (53-6.7, BD Biosciences), and anti-MHC II (I-Ab, BD Biosciences). T cells were stained with anti-CD4 (GK1.5, eBioscience) and anti-CD8α antibodies. To detect Eα52-68: MHC II complex on the surface of DCs that have taken up LPS-coated Eα-latex bead (4 μm, Invitrogen) (purified Eα protein with his tag from E. coli was kindly provided by I. Mellman [Genentech]), DCs were stained with Y-Ae-biotin (eBioscience)/SA-PE, anti-MHC II Alexa 680, and anti-CD11c APC (HL3, BD Biosciences). For intracellular staining, cell surface Y-Ae was first blocked by purified unlabeled Y-Ae antibody and DCs were intracellularly stained with anti-Y-Ae-biotin or anti-his-biotin (abcam) followed by SA-PE after fixation and permeabilization with cyto-fix/cyto-perm kit (BD Biosciences).
Detection of Cytokines
Levels of IL-12p40, IL-6, and TNF-α were measured with an ELISA kit (eBiosciences) according to manufacturer’s instruction. For RT-qPCR, total RNA was extracted by RNA isolation kit (QIAGEN) and cDNA was synthesized (Invitrogen). The qPCR was performed with SYBR green kit (QIAGEN) on MX4000 QPCR System according to manufacturer’s instruction (Stratagene, La Jolla, CA) as previously described (Lee et al., 2007).
Endocytosis and Phagocytosis Assays
To examine endocytic uptake of soluble Ags, MACS-purified DCs were incubated with OVA conjugated to Alexa Fluor 488 for 1 hr at 37°C or on ice. Cells were washed and stained with anti-CD11c and analyzed by LSR II. To assess the ability of DCs to phagocytose apoptotic cells, MHC II-deficient splenocytes were labeled with 2 μM PKH26 (Sigma) for 5 min at room temperature, washed, and irradiated (1500 rads). MACS-purified DCs (2.5 × 105) were stained with anti-CD11c Ab and subsequently cocultured with 1 × 106 PKH26-labeled splenocytes in the presence or absence of 10 μM cytochalasin D (MP Biomedicals) for 3 hr at 37°C. Cells were washed and analyzed by LSR II.
In Vitro Antigen Presentation Assays
Apoptotic cell-associated OVA was prepared according to a previous report (Schnorrer et al., 2006). In brief, MHC II-deficient or KbDb double knockout splenocytes were irradiated (1500 rads), washed, and incubated with 10 mg/ml OVA for 15 min at 37°C. To assess the ability of DCs to present OVA peptide on MHC II, 1 × 105 DCs were incubated with indicated amounts of OVA-coated MHC II-deficient splenocytes, soluble OVA, or OVA323-339 peptides. The Ag-loaded DCs were cocultured with 1 × 105 naive OT-II T cells for 72 hr 37°C. T cell proliferation was measured by 3H incorporation after adding 0.5 μCi of thymidine per well starting at 48 hr of incubation. In some experiments, inhibitors of autophagy, 3MA (0.5–1.0 mM) or Wortmannin (50–100 nM), were added to the DC-T cell coculture. To measure cross-presentation of OVA on MHC I, 1 × 105 DCs were incubated with indicated amounts of OVA-coated KbDb double knockout splenocytes or soluble OVA. The Ag-loaded DCs were cocultured with 5 × 104 naive OT-I/Rag1−/− T cells for 72 hr 37°C. T cell proliferation was measured by 3H thymidine incorporation after adding 0.5 μCi of thymidine per well starting at 48 hr of incubation.
Immunofluorescence Assay and Transmission Electron Microscopy
WT or Atg5−/− BM DCs were mounted on 0.5% alcian blue-coated coverslip and were pulsed with yeast expressing GFP for 15 min. DCs were washed and chased for 3 hr. For the analysis of the fusion of phagosomes and lysosomes, CD11c+ BM DCs were attached on coverslips and were labeled with LysoTracker (Invitrogen). DCs were pulsed with LPS-OVA-coated latex beads conjugated with Alexa 488 (Invitrogen) for 5 min and then chased for 5, 15, and 30 min. DCs were fixed with 4% paraformaldehyde for 20 min, permeabilized in PBS/0.05% saponin/1% BSA for 30 min, washed, and then stained for MHC II. Cells were analyzed by confocal microscopy. Immunofluorescence images were acquired with Zeiss confocal microscope (LSM 510). For transmission electron microscopy, CD11c+ BM DCs were first labeled with anti-CD11c magnetic beads (Miltenyi Biotec) to label lysosomes (5 hr chase), then incubated with LPS-OVA-coated latex beads (4 μm) for 20 min. Cells were washed with cacodylate buffer (pH 7.4) and then fixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.4). Cells were rinsed three times in 50 mM sodium maleate buffer (pH 5.2) and then stained in 2% uranyl acetate in 50 mM sodium maleate buffer for 1 hr in the dark. Cells were then rinsed three times in HPLC-grade water and then dehydrated in 50%, 70%, 90%, and 100% ethanol. The ethanol was replaced with propylene oxide and then the coverslips were embedded with Embed-812 (Electron Microscopy Sciences). Thin sections were cut with a Reichert Ultracut E ultramicrotome, placed on copper grids, and then stained for 10 min in 5% uranyl acetate in 50% methanol and 2 min in Reynold’s lead citrate solution. Cells were analyzed on a Tecnai 12 Biotwin transmission electron microscope. Images are analyzed with iTEM (Olympus Soft Imaging Solutions).
siRNA Knockdown
Primary mouse keratinocytes cultured in 6-well plates were infected with HSV-OVA (MOI = 5) for 20 hr. Infected keratinoctyes were UV irradiated and cocultured with 1 × 106 WT or Atg5−/− CD11c+ DCs that were transfected with 10 μl/well siRNA specific for Lamin A/C (irrelevant control), Atg7, or Atg12 (Santa Cruz) in 5 μl/well lipofectamine on 24-well plate for 18 hr. DCs were enriched by LSM lymphocyte separation medium. The purified 1 × 105 DCs were cocultured with 1 × 105 OT-II T cells for 72 hr. 3H thymidine was added for extra 24 hr to measure T cell proliferation.
Phagosome Purification
DCs were incubated with 2.8 μm magnetic beads conjugated with streptavidin (Invitrogen) bound to biotin-LPS (Invivogen) for the indicated duration of time. DCs were then disrupted in homogenization buffer (250 mM sucrose, 3 mM imidazol [pH 7.4]) and 200 μg/ml DNase (Roche), as described (Jancic et al., 2007). After homogenization, phagosomes were isolated by a magnet and were washed three times in cold PBS. The purified phagosomes were pelleted and extracts were prepared in lysis buffer (1% Triton X-100, 25 mM NaCl in PBS). After 15 min at 4°C, the lysates were centrifuged to remove the magnetic beads. Phagosomal extracts were used for measurement of cathepsin activity.
pH Measurement
Levels of pH of the lysosomes and phagosomes were measured as previously described (Steinberg et al., 2007) with minor modifications. For lysosomal pH measurement, WT or Atg5−/− BM DCs were plated on cover slides. The DCs were chased either for 1 or 2 hr to adhere and spread on the poly-lysine-coated glass after pulsing with Oregon green 488-dextran. pH was calculated by the ratio of 490 nm/440 nm fluorescence intensity. For phagosomal pH measurement, WT or Atg5−/− BM DCs were pulsed with FITC-conjugated zymosan-beads for 4 min and chased for the indicated time periods. pH was calculated by the ratio of 490 nm/440 nm intensity of FITC compared to standard pH solution.
Immunoblot Analysis
BM DCs were purified with CD11c MACS beads. Cell lysates (50 μg total proteins) were run on SDS-PAGE and blotted to PVDF membrane and developed with the antibodies against cathepsin B (Fl-339), D (R-20), L (C-18), S (M-19), and Ii (FL-296) (Santa Cruz) and β actin (Sigma). For monitoring cytosolic autophagy, rabbit anti-LC3 antibody (Novus) and mouse anti-p62 (2C11, Abnova) were used.
Protein Degradation Assay
Protease activity was measured with a previously described in vitro degradation assay (Jancic et al., 2007). In brief, 1 μg of phagosome extract was incubated at 37°C for 5 hr in the presence of 40 μM fluorogenic substrate (Bachem, Weil am Rheim, Germany) for cathepsin B/L (Z-Phe-Arg-AMC) or for cathepsin S (Z-Val-Val-Arg-AMC). The enzymatic reactions were performed in 200 μl PBS with 0.5% Triton X-100 and 2 mM DTT at pH 5.5 or at pH 7.4 in a 96 flat-well plate. Substrate degradation was assayed fluorometrically with a VICTOR 2 plate reader (Perkin Elmer).
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute of Allergy and Immunology (AI054359, AI062428, and AI064705 to A.I. and AI72627 to A.C.). H.K.L. is a recipient of an Anna Fuller cancer research fellowship and Richard K. Gershon predoctoral training fellowship. A.I. is a recipient of the Burroughs Wellcome Investigators in Pathogenesis of Infectious Disease.
Footnotes
Supplemental Information includes six figures and can be found with this article online at doi:10.1016/j.immuni.2009.12.006.
References
- Alexander DE, Ward SL, Mizushima N, Levine B, Leib DA. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J Virol. 2007;81:12128–12134. doi: 10.1128/JVI.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelárová H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- Brazil MI, Weiss S, Stockinger B. Excessive degradation of intracellular protein in macrophages prevents presentation in the context of major histocompatibility complex class II molecules. Eur J Immunol. 1997;27:1506–1514. doi: 10.1002/eji.1830270629. [DOI] [PubMed] [Google Scholar]
- Chen M, Shirai M, Liu Z, Arichi T, Takahashi H, Nishioka M. Efficient class II major histocompatibility complex presentation of endogenously synthesized hepatitis C virus core protein by Epstein-Barr virus-transformed B-lymphoblastoid cell lines to CD4(+) T cells. J Virol. 1998;72:8301–8308. doi: 10.1128/jvi.72.10.8301-8308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol. 2005;79:14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörfel D, Appel S, Grünebach F, Weck MM, Müller MR, Heine A, Brossart P. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105:3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippé R, Desjardins M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Guéguen M, Long EO. Presentation of a cytosolic antigen by major histocompatibility complex class II molecules requires a long-lived form of the antigen. Proc Natl Acad Sci USA. 1996;93:14692–14697. doi: 10.1073/pnas.93.25.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Sekaly RP, Jacobson CL, McFarland HF, Long EO. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989;63:1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC, Amigorena S. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–378. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D, Marti M, Long EO. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990;172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SR, Bonneau RH, Smith PM, Wolcott RM, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- Jones LJ, Upson RH, Haugland RP, Panchuk-Voloshina N, Zhou M, Haugland RP. Quenched BODIPY dye-labeled casein substrates for the assay of protease activity by direct fluorescence measurement. Anal Biochem. 1997;251:144–152. doi: 10.1006/abio.1997.2259. [DOI] [PubMed] [Google Scholar]
- Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, Okuda K. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci USA. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M, Rosorius O, Krätzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, Iwasaki A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol. 2009;83:12164–12171. doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- Mikloska Z, Bosnjak L, Cunningham AL. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J Virol. 2001;75:5958–5964. doi: 10.1128/JVI.75.13.5958-5964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- Murphy DB, Rath S, Pizzo E, Rudensky AY, George A, Larson JK, Janeway CA., Jr Monoclonal antibody detection of a major self peptide. MHC class II complex. J Immunol. 1992;148:3483–3491. [PubMed] [Google Scholar]
- Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, Mautner J. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Tallóczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Münz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, Coffin RS, Katz DR, Chain B. Herpes simplex virus infection of dendritic cells: Balance among activation, inhibition, and immunity. J Infect Dis. 2003;187:165–178. doi: 10.1086/367675. [DOI] [PubMed] [Google Scholar]
- Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrançois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Rojas JM, Ostrand-Rosenberg S. Tumor cells present MHC class II-restricted nuclear and mitochondrial antigens and are the predominant antigen presenting cells in vivo. J Immunol. 2000;165:5451–5461. doi: 10.4049/jimmunol.165.10.5451. [DOI] [PubMed] [Google Scholar]
- Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Schmid D, Münz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D, Pypaert M, Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. 3-Methyladenine: Specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BE, Touret N, Vargas-Caballero M, Grinstein S. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc Natl Acad Sci USA. 2007;104:9523–9528. doi: 10.1073/pnas.0700783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LM, Miller BC, Ng A, Eisenberg J, Zhao Z, Cadwell K, Graham DB, Mizushima NN, Xavier R, Virgin HW, Swat W. Identification of Atg5-dependent transcriptional changes and increases in mitochondrial mass in Atg5-deficient T lymphocytes. Autophagy. 2009;5:625–635. doi: 10.4161/auto.5.5.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallóczy Z, Jiang W, Virgin HW, 4th, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci USA. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci USA. 2001;98:3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.