Abstract
Background
Biomarkers are needed that can distinguish between schizophrenia and schizoaffective disorder to inform the ongoing debate over the diagnostic boundary between these two disorders. Neuromorphometric abnormalities of the thalamus have been reported in individuals with schizophrenia and linked to core features of the disorder, but have not been similarly investigated in individuals with schizoaffective disorder. In this study, we examine whether individuals with schizoaffective disorder have a pattern of thalamic deformation that is similar or different to the pattern found in individuals with schizophrenia.
Method
T1-weighted magnetic resonance images were collected from individuals with schizophrenia (n=47), individuals with schizoaffective disorder (n=15), and controls (n=42). Large-deformation, high-dimensional brain mapping was used to obtain three-dimensional surfaces of the thalamus. Multiple analyses of variance were used to test for group differences in volume and measures of surface shape.
Results
Individuals with schizophrenia or schizoaffective disorder have similar thalamic volumes. Thalamic surface shape deformation associated with schizophrenia suggests selective involvement of the anterior and posterior thalamus, while deformations in mediodorsal and ventrolateral regions were observed in both groups. Schizoaffective disorder had distinct deformations in medial and lateral thalamic regions.
Conclusions
Abnormalities distinct to schizoaffective disorder suggest involvement of the central and ventroposterior medial thalamus which may be involved in mood circuitry, dorsolateral nucleus which is involved in recall processing, and the lateral geniculate nucleus which is involved in visual processing.
Keywords: Schizophrenia, Schizoaffective disorder, MRI, Neuroimaging, Thalamus
1. Introduction
There is ongoing debate as to whether the DSM-V should replace the distinction between schizophrenia and psychotic mood disorders with a dimensional approach that considers a continuum of psychotic illnesses, including schizophrenia, schizoaffective disorder and psychotic bipolar disorder (Laursen et al., 2009). This approach draws support from recent findings in the genetic, cognitive, and clinical literature that have challenged distinctions between schizophrenia and psychotic mood disorders.
The genetic literature suggests that schizophrenia and schizoaffective disorder share a common genetic risk where the risk of schizoaffective disorder is higher in relatives of schizophrenia patients and vice versa (Gershon et al., 1988; Kendler & Diehl, 1993). Also, some research suggests that schizophrenia and schizoaffective disorder share a number of equally severe cognitive deficits (Barch, 2009), while others have found the severity to be of a lesser magnitude in schizoaffective disorder (Heinrichs et al., 2008). Perhaps the most controversial distinction between schizophrenia and schizoaffective disorder is in the domain of psychopathology. Recent findings are disparate as some show that the severity of positive, negative and disorganized symptoms are similar between individuals with schizophrenia and schizoaffective disorder (Evans et al., 1999; Smith et al., 2009), while others show severity in positive and negative symptoms that rank individuals with schizoaffective disorder as intermediate between individuals with schizophrenia and controls (Peralta & Cuesta, 2008). Research examining the relationship of cognitive impairment to the psychopathology of individuals with schizophrenia or schizoaffective disorder suggests that both groups show moderate correlations between negative symptoms and cognitive deficits, with the magnitude of correlations in the individuals with schizoaffective disorder being somewhat smaller (Smith et al., 2009).
The debate as to whether schizophrenia and schizoaffective disorder are separable disorders would be greatly informed by determining whether or not schizophrenia and schizoaffective disorder shared similar neurobiological features. In this regard, studies of brain structures, known to play central roles in supporting key cognitive constructs, such as the thalamus, may be particularly helpful (Byne et al., 2009; Csernansky et al., 2004a; Harms et al., 2007).
The results of several studies suggest that the thalamus, a brain region that plays an integral role in cognitive processing and acts as a relay station between the basal ganglia and the cerebral cortex, has a significantly reduced volume in individuals with schizophrenia (Konick & Friedman, 2001; Sim et al., 2006). Multiple studies also examined whether the shape of the thalamus among individuals with schizophrenia has patterns of deformation that are characteristic of the disorder. Shape analysis can be used to identify subtle regional deformations within a structure, and thus can be complimentary to volumetry. Using computational anatomy to examine two independent samples of individuals with schizophrenia, our group has previously found significant inward deformations of the anterior, medial, and posterior regions of the thalamus; thus, implicating the anterior and mediodorsal nucleus and the pulvinar in the pathophysiology of schizophrenia (Csernansky et al., 2004a; Harms et al., 2007).
Although several studies have documented structural abnormalities of the thalamus in individuals with schizophrenia (for review see (Byne et al., 2009)), similar studies have not compared thalamic morphometry between individuals with schizophrenia and individuals with schizoaffective disorder. However, structural abnormalities of the thalamus were found in individuals who are at increased genetic risk for developing schizophrenia or schizoaffective disorder (Ettinger et al., 2007; Harms et al., 2007; Lawrie et al., 1999).
Based on prior research suggesting that schizophrenia and schizoaffective disorder share similar clinical and cognitive profiles (with a lesser magnitude in schizoaffective disorder), and that first-degree relatives are at increased genetic risk and share attenuated thalamic abnormalities, we hypothesized that individuals with schizoaffective disorder would have similar, but perhaps less marked, patterns of thalamic volume loss and shape deformation as compared to individuals with schizophrenia. Given that mood dysregulation is characteristic of schizoaffective disorder (Malhi et al., 2008) and that the thalamus is involved with the neurobiological circuitry associated with mood disorders (Price & Drevets, 2010), we also hypothesized that schizoaffective disorder would have abnormal neurobiological features of the thalamus that are distinct from the patterns associated with schizophrenia. We previously compared the clinical and cognitive profiles of individuals with schizophrenia or schizoaffective disorder using a sample that overlaps with the current one (Smith et al., 2009). Thus, we will report on the clinical and cognitive profiles of the groups in the current study to confirm our prior results, and explore whether there are neuromorphometric abnormalities that correlate to cognition and psychopathology.
2. Methods
2.1. Sample
Participants included 47 individuals with schizophrenia (SCZ), 42 controls (CON), and 15 individuals with schizoaffective disorder (SA). Recruitment methods by our group have been described previously (Smith et al., 2009). The SCZ and CON subjects in this study were selected from a larger sample of available participants from our previous work (Csernansky et al., 2004a; Harms et al., 2007; Smith et al., 2009), in order to match the SA subjects with respect to age, gender, parental SES, and race. Socioeconomic status was measured with the Hollingshead Four Factor Index of Social Status (Hollingshead, 1975).
Given that a longer duration of illness, mood stabilizing psychotropic and antipsychotic medication treatment, and a history of substance use disorders have been shown to affect thalamic morphometry (Dazzan et al., 2005; Frodl et al., 2008; Gur et al., 1998; Sassi et al., 2002; Wang et al., 2008) and that nicotine reduces gray matter density (McClernon, 2009), we examined whether between-group differences were present for these potential confounds. Between-group differences on demographic and clinical variables are summarized in Table 1. SCZ and SA did not have statistically different durations of illness and lifetime history of substance use disorders. Thus, these variables were not examined as potential confounds. SCZ and SA differed with respect to treatment with antidepressant medication (see Table 1). We also found that SCZ and SA had greater nicotine use than CON. Although the quantity of nicotine use and whether or not patients received treatment with first- and second generation antipsychotic (FGA, SGA) and antidepressant medications differed between groups, using them as covariates did not contribute a significant amount of variance to the model nor did they change the pattern of the results in volume and shape. Hence, nicotine use, and whether or not patients received FGA, SGA, and antidepressant treatment (for SCZ vs. SA comparisons only) were not used as covariates in the final analysis. However, based on recent evidence by Andreasen et al (2009), we will explore whether quantitative FGA and SGA measures (i.e., dose-years) are correlated with measures of volume and shape in SCZ and SA.
Table 1.
Demographic and Clinical Characteristics of Study Sample
| SCZ (n=47) |
SA (n=15) |
CON (n=42) |
χ2 / F Statistic |
|
|---|---|---|---|---|
| Demographic | ||||
| Age, years (SD) | 41.2 (12.3) | 42.6 (9.8) | 42.6 (12.1) | 0.18 |
| Duration of Illness, years (SD) | 18.0 (14.2) | 17.9 (12.6) | Na | 0.002 |
| Gender (% male) | 55.3% | 53.3% | 54.8% | 0.01 |
| Mean SES (SD) | 3.6 (0.9) | 3.4 (0.9) | 3.3 (1.0) | 0.80 |
| Race (% White) | 51.1% | 46.7% | 57.1% | 0.59 |
| Clinical | ||||
| Mean cigarette use, past year (SD)a | 4908 (4811) | 3750 (3971) | 1595 (2968) | 7.4*** |
| Substance Use Disorder (% present) | ||||
| Alcohol | 40.4% | 46.7% | 0.0% | 0.08 |
| Cannabis | 36.2% | 33.3% | 0.0% | 0.04 |
| Cocaine | 19.1% | 26.7% | 0.0% | 0.39 |
| Stimulants | 4.3% | 6.7% | 0.0% | 0.14 |
| Hallucinogen | 6.4% | 6.7% | 0.0% | 0.002 |
| Sedatives | 4.3% | 0.0% | 0.0% | 0.66 |
| Opioids | 6.4% | 0.0% | 0.0% | 1.01 |
| Medication Use | ||||
| First Generation Only | 2.1% | 13.3% | Na | 3.10 |
| Second Generation Only | 59.6% | 80.0% | Na | 2.07 |
| Both First & Second Gen. | 23.4% | 0.0%* | Na | 4.27* |
| First Generation Dose-Yearsb | 6.1 (7.9) | 4.9 (2.1) | Na | 0.2 |
| Second Generation Dose-Yearsc | 3.7 (3.5) | 4.3 (4.1) | Na | −0.5 |
| Mood Stabilizer | 14.9% | 26.7% | Na | 1.08 |
| Anti-Depressant | 29.8% | 80.0%* | Na | 11.77*** |
Note.
p<.05
p<.01
p<.001
SCZ>CON (p<.001); SA>CON (p=.081).
n=12 for SCZ, n=2 for SA.
n=38 for SCZ, n=13 for SA.
2.2. Clinical and Cognitive Assessments
Research participants were assessed by Master’s or Doctoral clinicians, blind to the diagnosis of the participant, who regularly participated in training and reliability sessions. DSM-IV Axis I diagnoses (i.e., schizophrenia or schizoaffective disorder) of each participant were determined by the consensus of a research psychiatrist and trained research clinicians who used the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 2002). The SCID also identified whether participants had a lifetime diagnosis of a substance use disorder for alcohol, cannabis, cocaine, stimulants, hallucinogens, sedatives, and opioids. Substance use disorders were defined as meeting DSM-IV-TR criteria for a lifetime rating (but not current) of abuse or dependence (present: yes=1, no=0). The participants’ duration of illness and treatment with psychotropic medication were assessed using self-report. Nicotine use was estimated using self-report and a semi-structured interview adapted from Sullivan et al., (Sullivan et al., 2000), and the Lifetime Alcohol Consumption Assessment Procedure (Skinner, 1982).
Participants completed a battery of neuropsychological tests. Based on prior research (Nuechterlein et al., 2004), we converted raw scores from the neuropsychological tests into standardized scores (based on current sample) for four domains: IQ, working memory, episodic memory, and executive functioning. Psychopathology (i.e., positive, negative, disorganized symptoms) in the patient groups (i.e., SCZ, SA) was assessed using global ratings from the Scale for the Assessment of Positive Symptoms (Andreasen, 1983b) and the Scale for the Assessment of Negative Symptoms (Andreasen, 1983a). All ratings were z-scored using the mean and standard deviation of the current sample and averaged within symptom clusters. A description of the specific neuropsychological tests and global symptom ratings used for each cognitive and psychopathological domain is reported in our prior work (Smith et al., 2009).
Antipsychotic medication use by SCZ and SA was assessed via self-report. Quantitative measures of FGA and SGA treatment were based on the type of medication (i.e., FGA or SGA), the dosage amount (in milligrams), the duration of use (in days over the past two years), and guidelines for chlorpromazine equivalents recently published by Andreasen and colleagues (Andreasen et al., 2010). Their measure of ‘dose-years’ is defined as “the product of the dose of a particular antipsychotic (converted into equivalents of a ‘standard medication such as chlorpromazine or haloperidol) and the time on that dose expressed in years” (Andreasen et al., 2010).
2.3. Image Acquisition
Details of image acquisition, surface mapping and analysis of SCZ subjects can be found in previous published reports (Csernansky et al., 2004a; Harms et al., 2007). These same methods were applied to the SA sample for the current analysis. Briefly, magnetic resonance (MR) scans were collected with a standard head coil on a Siemens Magnetom 1.5-Tesla (Erlangen, Germany) scanner using a turbo-FLASH sequence (repetition time=20msec, echo time=5.4msec, flip angle=30°, 180slices, FOV=256mm, matrix=356×256, time=13.5 min) that acquired 1mm3 isotropic whole-head image (Venkatesan & Haacke, 1997). Total brain volume was estimated using atlas scaling factor (ASF) (Buckner et al., 2004). The ASF is the reciprocal of the determinant of the alignment matrix to Talairach atlas space, and it signifies the extent that brain volume contracts or expands during alignment. The ASF did not show between-group differences (F=0.8, p=0.45) and thus, was not used as a covariate in statistical analyses.
2.4. Surface Mapping
The surface of the thalamus was transferred from a template scan (from a subject not included in this study) by applying Large-Deformation High-Dimensional Brain Mapping (HDBM-LD) (Csernansky et al., 2004b). Our group has previously established the validity and reliability of HDBM-LD for mapping the thalamus (Csernansky et al., 2004a; Harms et al., 2007). Prior to diffeomorphic transformations, anatomic landmarks were placed by expert raters who were blinded to the group status of the scan being landmarked. Detailed landmarking procedures can be found in previous publications from our group (Csernansky et al., 2004a; Mamah et al., 2007). We consulted with an atlas of the human brain to associate deformation patterns to particular thalamic nuclei (Mai et al., 1997).
2.5. Data Analysis
A two-way ANOVA with group and hemisphere as fixed effects was used to examine thalamic volume. For shape, a principal components analysis was used to diminish the high dimensionality of the thalamic surface, thus generating an orthonormal set of eigenvectors that represented variation in the shape of the left and right hemisphere of the thalamus. The thalamic surface was defined by the first 10 eigenvectors, yielding 10 eigenvector scores for the surface of each hemisphere. The eigenvector scores accounted for more than 80% of total shape variance (across subjects and hemispheres). The left and right scores were then averaged for each structure.
To determine whether overall differences in the shapes of the structures existed across groups, we conducted a multivariate analysis of variance (MANOVA) with the 10 averaged eigenvectors of the thalamus as dependent variables with group as a fixed effect. Group consisted of SCZ, SA, and CON. Next, we conducted three additional MANOVAs to identify significant differences between each group (i.e., SCZ vs. CON, SA vs. CON, SCZ vs. SA). For the final step, we used a logistic regression (backward selection) guided by a significant likelihood ratio statistic to identify which eigenvectors contributed most to between-group differences for SCZ and SA when compared to CON. One-way ANOVA was also used to examine the main effect of group on the demographic and cognitive variables for between-group differences. This same approach was used to examine differences on measures of psychopathology between SCZ and SA. To account for smaller sample sizes, Spearman’s rho correlations (ρ) estimated the relationship between the volume and shape and the cognitive and psychopathological domains and dose-years of anti-psychotic medication.
To visualize the pattern of thalamic shape deformation, we constructed a map of the composite surface of the thalamus at every graphical surface point. The shape displacements were estimated at each surface point as the difference between the means of the group vectors in magnitude.
3. Results
3.1. Surface Shape Analyses
Thalamus Shape
Using all three study groups, MANOVA applied to the eigenvectors for the thalamus showed a significant main effect of group on shape (λ=.32, F2,104=6.98, p=.001) (Table 2). Post-hoc comparisons found significant differences between SCZ and CON (F1,87=2.7, p=.006), SA and CON (F1,55=11.2, p<.001), and SA and SCZ (F1,60=13.2, p<.001). Logistic regression (backward selection) found that eigenvectors 1, 3, 4, 6 and 10 discriminated SCZ from CON, while eigenvectors 1, 3, 5, and 9 discriminated SA from CON, and eigenvectors 1, 3, 5, 9, and 10 discriminated SA from SCZ.
Table 2.
Between-Group Comparisons of Shape Measures and Significant Eigenvectors for Thalamus
| Fdf1,df2 , p-value | 2-Group MANOVA |
|||
|---|---|---|---|---|
| 3-Group MANOVA |
SCZ vs. CON |
SA vs. CON |
SA vs. SCZ |
|
| Thalamus | F2,104=7.0 p<.001 |
F1,89=2.7 p=.006 |
F1,57=11.2 p<.001 |
F2,62=11.4 p<.001 |
| Eigenvectors | -- | 1,3,4,6,10 | 1, 3,5,9 | 1,3,5,9,10 |
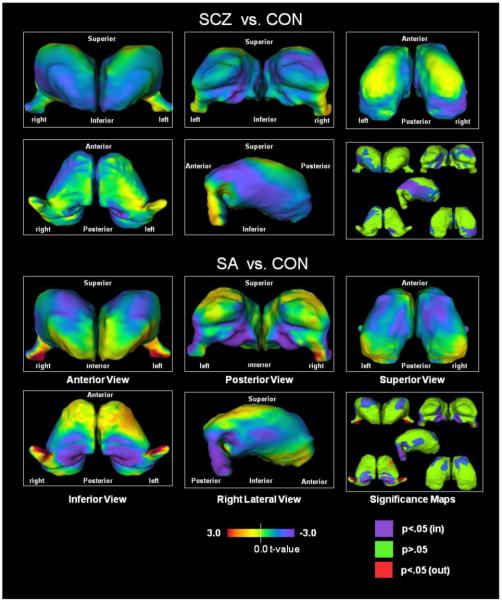
Figure 1 displays a visual representation of thalamic shape difference between SCZ and CON, and SA and CON. Both SCZ and SA had inward deformations in the medial regions corresponding to the mediodorsal nucleus and mediolateral regions corresponding to the ventrolateral nucleus. The foremost additional areas of shape difference in SA were inward deformations of medial regions corresponding to the central and ventroposterior medial nuclei and superior region corresponding to the dorsolateral nucleus. SA also had deformations in the regions corresponding to the lateral geniculate nucleus, which were characterized by a shift in position towards the lateral side. The most prominent additional areas of shape difference in SCZ were in the anterior and posterior regions corresponding to the anterior nucleus, pulvinar, and posterior lateral nucleus.
Fig. 1. Thalamus surface map abnormalities and significance maps.
3.2. Volume Analyses
We found a significant effect of group (F=3.07, p=.05) and hemisphere (F=20.4, p<.0001; R>L) on thalamic volume (Table 3). The interaction of group X hemisphere was not statistically significant (F=0.6, p=.53). Post-hoc comparisons indicated that thalamic volumes in SCZ and SA were smaller than CON, however, this difference only reached significance when comparing SCZ to CON (p=.02) and not SA to CON (p=.13). Both differences were characterized by similar medium effect sizes (d=.50 and .48, respectively, calculated as Cohen’s d).
Table 3.
Thalamic Volume (cm3) (SE)
| SCZ (n=47) |
SA (n=15) |
CON (n=42) |
|
|---|---|---|---|
| Mean Thalamusa | 7.2 (0.1) | 7.2 (0.2) | 7.5 (0.1) |
| L Thalamus | 7.1 (0.1) | 7.1 (0.2) | 7.4 (0.1) |
| R Thalamus | 7.2 (0.1) | 7.3 (0.2) | 7.6 (0.1) |
F2,101=3.1, p=.05: SCZ<CON (p=.02), SA<CON (p=.13), SCZ:SA (p=.89).
3.3. Cognitive, Psychopathological, and Antipsychotic Medication Profiles
Cognition was compared across the three study groups using ANOVAs with group as a fixed factor. Table 4 illustrates the group-level means, standard deviations, and post hoc effect sizes. There was a significant main effect of group for IQ, working memory, episodic memory, and executive function. For all four cognitive domains, CON was significantly higher than SCZ (all p<.001), with large effect sizes. Similarly, CON were significantly higher than SA (all p<.05), with large effect sizes, for all cognitive domains except IQ where CON had a higher IQ than SA (medium effect size) but was non-significant (p=.19). Differences between SCZ and SA were non-significant (p>.10), with small effects for all domains except IQ, which had a medium effect size. SCZ and SA were compared on measures of psychopathology, and did not differ with respect to measures of positive and disorganized symptoms (both p>.10). There was a trend for SCZ to have higher negative symptoms than SA (p=.07) (Table 4).
Table 4.
Mean Standardized Scores (SD) and Effect Sizes (Cohen’s d) Between-Group Comparison of Cognition and Psychopathology
| Standardized Scores | Effect Sizes | ||||||
|---|---|---|---|---|---|---|---|
| ANOVA | SCZ (n=47) |
SA (n=15) |
CON (n=42) |
SCZ vs. CON |
SA vs. CON |
SA vs. SCZ |
|
| Cognition | |||||||
| IQa | F2,104=9.8, p<.001 | −.39(.96) | −.004 (.84) | .49 (.93) | .94 | .56 | .43 |
| Working Memoryb | F2,104=13.9, p<.001 | −.44 (.73) | −.24 (.51) | .36 (.77) | 1.07 | .92 | .32 |
| Episodic Memoryc | F2,104=39.1, p<.001 | −.50 (.71) | −.49 (.51) | .74 (.74) | 1.71 | 1.94 | .02 |
| Executive Functioningd | F2,104=24.2, p<.001 | −.58 (.70) | −.32 (.71) | .38 (.57) | 1.50 | 1.09 | .37 |
| Psychopathology | |||||||
| Positive Symptoms | F1,60=0.3, p=.57 | .12 (.78) | .26 (1.1) | -- | -- | -- | .15 |
| Negative Symptoms | F1,60=3.3, p=.07 | .25 (.69) | −.10 (.58) | -- | -- | -- | .55 |
| Disorganized Symptoms | F1,60=0.0, p=.99 | .16 (.61) | .16 (.64) | -- | -- | -- | .00 |
Note. Tukey correction for multiple comparisons was used.
CON>SCZ (p<.001) and SA (p=.19), SA>SCZ (p=.35)
CON>SCZ (p<.001) and SA (p=.02), SA>SCZ (p=.62)
CON>SCZ (p<.001) and SA (p<.001), SA>SCZ (p=.99)
CON>SCZ (p<.001) and SA (p=.002), SA>SCZ (p=.38).
Structural measures were correlated with the domains of cognition and psychopathology. By correcting for multiple comparisons, correlations must meet a cutoff of p<.007 (.05/7) to attain statistical significance. There were no significant correlations between volume and the cognitive and psychopathological domains for SCZ or SA. For shape scores in SCZ, we found that a decreasing executive functioning score was related to inward shape deformation in the region corresponding to the lateral geniculate nucleus captured by eigenvector 6 (ρ= .41, p=.005), after examining deformation maps. For SA, volume and shape scores did not correlate with cognition or psychopathology.
In addition, we correlated measures of volume and shape with dose-years of FGA or SGA medication. Given there are 12 morphometric measures, we corrected for multiple comparisons using a cutoff of p<.004 (.05/12) to attain statistical significance. Although the number of available subjects was small, there were no significant correlations between volume or shape, and dose-years of FGA medication in SCZ (n=12). Correlations between volume or shape, and FGA dose-years were not examined in the SA group due to a small sample (n=2).
There were no correlations between volume and dose years of SGA medication in SCZ or SA. For shape scores, we found that an increasing dose-years of SGA medication in SCZ was related to outward shape deformation in the regions corresponding to the dorsolateral and lateral geniculate nuclei captured by eigenvector 3 (ρ= .45, p=.004). SGA dose-years did not correlate with shape in SA.
4. Discussion
Our findings provide partial support for the hypothesis that schizoaffective disorder is characterized by similar volume and shape abnormalities of the thalamus as schizophrenia, but that they are less marked. We based our hypothesis on the genetic overlap between SCZ and SA (Gershon et al., 1988; Kendler et al., 1993), because these groups generally share a similar profile in their psychopathology and cognitive impairments (Barch, 2009; Smith et al., 2009), and because individuals at an increased genetic risk for these disorders have attenuated thalamic volume loss (Ettinger et al., 2007; Lawrie et al., 1999) and shape abnormalities (Harms et al., 2007). However, the findings also provide support for our hypothesis that SA would have neurobiological abnormalities distinct from SCZ.
In the examination of thalamic volume, our results suggest that SCZ and SA have similar thalamic volumes which were smaller than CON participants, with a right>left asymmetry. These findings are consistent with previous research suggesting that individuals with schizophrenia have reduced volume in the thalamus when compared to controls (Byne et al., 2009) and a right>left asymmetry (Byne et al., 2001; Csernansky et al., 2004a). In addition, this finding supports our hypothesis that SCZ and SA would share similar reductions in volume.
4.1. Structural Similarities between Schizophrenia and Schizoaffective disorder
Our findings also suggest that there were both similarities and differences in thalamic shape between SCZ and SA. In terms of similarities, SCZ and SA had similar inward deformations in regions corresponding to the mediodorsal nucleus. This finding replicates previous work on schizophrenia which found structural abnormalities of the mediodorsal nucleus (Csernansky et al., 2004a; Harms et al., 2007; Pakkenberg, 1992).
SCZ and SA also shared inward deformations corresponding to the ventrolateral nucleus. This finding also replicates results from prior work on SCZ (Wang et al., 2008). However, deformation in this region in SA were of a lesser magnitude than comparable deformations in SCZ (i.e., SCZ had a deeper bluish purple while SA had lighter bluish green; see Figure 1). This nucleus has connections to the basal ganglia and is involved with motor and premotor regions (Lehericy et al., 2006; Shinoda et al., 1993). Thus, these regions may be involved with deficits in motor activity, however, most research examining motor activity in SCZ reported on the thalamus as an entire complex rather than examining the influence of particular nuclei (Camchong et al., 2006). Further research is needed to examine group variation between SCZ and SA with respect to abnormalities of the lateral thalamus and potentially impaired motor functions. Also, due to the connections between the thalamus and the basal ganglia, future research could also examine the pattern of basal ganglia shape between individuals with schizoaffective disorder and individuals with schizophrenia.
4.2. Structural Differences between Schizophrenia and Schizoaffective Disorder
Shape changes in the thalamus unique to SA were inward deformations in regions corresponding to the central and ventroposterior medial nuclei of the thalamus. Medial regions of the thalamus have been implicated in the neurocircuitry associated with mood dysregulation (Price et al., 2010). SA also had inward deformations in regions proximal to the dorsolateral nucleus, which is noted for its involvement in the hippocampal circuit and recall processes (Edelstyn et al., 2006). SA was also characterized by outward deformations in regions approximating the anterior side of the lateral geniculate nucleus (LGN). The LGN is not a portion of the core thalamic mass but a component of the metathalamus; and separated from the primary thalamus by the internal capsule (Le Gros Clark, 1932). Hence, the change in the deflection of the LGN could be explained by differences in the width of the retrolenticular internal capsule. As such, abnormalities of the internal capsule could contribute to cognitive impairment. Alternatively, the LGN abnormalities could be explained by ontogenetic changes in more medial thalamic nuclei such as the medial pulvinar (Le Gros Clark, 1932). Also, the lateral geniculate nucleus receives direct input from the visual cortex (Lennie, 1980), however, a recent meta-analysis suggests that SCZ and SA do not differ on visual memory tasks (Bora et al., 2009). Hence, further research is needed to examine whether abnormalities of the lateral geniculate nucleus may have implications for abnormal brain activity in this region during performance on additional visual evaluations.
The shape change observed in the thalamus of SCZ subjects was consistent with findings previously reported by our group (Csernansky et al., 2004a; Harms et al., 2007) and others (Byne et al., 2002)– i.e., inward deformations were present at both the both anterior and posterior extremes of the thalamus, perhaps suggesting localized volume losses in regions proximal to the anterior nucleus and pulvinar. In addition, our findings suggest that the inward deformation of the pulvinar is more pronounced in the right hemisphere and involves the medial and lateral regions. This finding is consistent with postmortem studies of schizophrenia patients which suggested an absence of the normal R>L asymmetry in the pulvinar (Highley et al., 2003). Although the sample of schizophrenia and control participants in the current study largely overlaps with a prior analysis (Csernansky et al., 2004a), a number of new patients were included. As such, the current findings for SCZ should not be considered a full replication, but rather an extension that confirms our prior findings in an expanded sample size with an additional matched comparison to individuals with schizoaffective disorder.
The anterior nucleus and pulvinar lacked significant deformations in SA, and so, functional brain activation in these regions may be less likely to be impaired in SA. In prior studies, we found decreased functional activation within SCZ during working memory tasks in regions corresponding to the anterior and mediodorsal nuclei (Andrews et al., 2006), while others found reduced activation in these regions and the pulvinar (Hazlett et al., 2004; Heckers et al., 2000). Given the overlapping deformation between SCZ and SA in the region corresponding to the mediodorsal nucleus and the contrasting deformation between these groups in the regions corresponding to the anterior nucleus and pulvinar, an analysis of functional brain activity in these regions during cognitive performance in carefully matched samples of SCZ and SA would be needed to address the association between structure and function.
4.3. Associations with Cognition, Psychopathology and Antipsychotic Medication
Our findings on cognition suggest that impairments in working memory, episodic memory, and executive functioning between SCZ and SA were comparable, which is consistent with prior work suggesting that SA and SCZ shared similar cognitive deficits in a number of domains (Barch, 2009). We also found evidence for differences in the pattern of correlations between cognition and shape scores in SCZ. We found that lower executive functioning scores in SCZ were correlated with inward shape deformation located in regions corresponding to the lateral geniculate nucleus. We did not find any significant correlations between shape and cognition in SA. Findings on psychopathology were consistent with prior work suggesting similarities in disorganized symptoms (Smith et al., 2009) across SCZ and SA. Similar to previous research (Peralta et al., 2008), we also found that SA had fewer negative symptoms than SCZ.
In addition, we found that more exposure to SGAs (i.e., higher dose-years) was correlated with outward shape deformations in regions corresponding to the dorsolateral and lateral geniculate nuclei in SCZ. Although this doesn’t suggest an overall increase in thalamic volume, such shape changes could represent more localized volume gains. Thus, this finding is at least partially consistent with current reviews indicating that SGAs are correlated with increased thalamic volume (Navari & Dazzan, 2009; Smieskova et al., 2009).
4.4. Limitations
There were notable limitations to this study. First, although the sample of 15 individuals with schizoaffective disorder may have been underpowered to detect small effects, there were clear between-group differences with respect to our shape analysis. Second, we were able to examine correlations between SGAs and FGAs, and thalamic morphology in SCZ and SA using data on dose and duration for the two years prior to our assessment. However, we cannot account for the influence of FGA and SGA treatment that preceded this time period. Future research should take into account a more extended medication history and collect a larger sample of individuals with schizoaffective disorder being treated with FGAs. CON had no history of substance use disorders, thus, the presence of lifetime diagnoses of substance use disorders in the patient groups were likely to have some neurobiological effect on neuromorphometry that cannot be controlled as a potential confound, however, rates of diagnoses did not differ between the patient groups. Future research would benefit from a comparison group with a similar history of substance use disorders to examine as potential confounds.
5. Conclusions
We found evidence that SCZ and SA shared similar thalamic volume reduction and shape deformations in the mediodorsal and ventrolateral nuclei. These findings generally support our hypothesis that SA and SCZ have similar deformations of the thalamus. However, although present, we did find that abnormalities in the ventrolateral nucleus were less severe in SA when compared to SCZ. SA is also characterized by deformations that are distinct from those found in SCZ. These areas include medial regions of the thalamus, which is associated with mood circuitry, as well as regions corresponding to the dorsolateral and lateral geniculate nuclei. Lastly, we replicated prior research suggesting that SCZ have structural impairments in regions corresponding to the anterior nucleus and pulvinar. Future research needs to examine whether individuals with schizophrenia and schizoaffective disorder have neuroanatomical differences in regions with connections to the thalamus (e.g., basal ganglia), and determine whether there are specific functional correlations with these structural abnormalities.
Acknowledgement
We thank the research participants for volunteering their time and the research staff at the Conte Center for the Neuroscience of Mental Disorders and the Northwestern University Schizophrenia Research Group for clinical and neurocognitive assessments, and for database management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms. The University of Iowa; Iowa City, IA: 1983a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms. The University of Iowa; Iowa City, IA: 1983b. [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry. 2006;163:463–9. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- Barch DM. Neuropsychological abnormalities in schizophrenia and major mood disorders: similarities and differences. Curr Psychiatry Rep. 2009;11:313–9. doi: 10.1007/s11920-009-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195:475–82. doi: 10.1192/bjp.bp.108.055731. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry. 2001;58:133–40. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–68. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE. Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biol Psychiatry. 2006;60:235–41. doi: 10.1016/j.biopsych.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, Rastogi-Cruz D, Posener JA, Thompson PA, Miller MI. Abnormalities of thalamic volume and shape in schizophrenia. Am J Psychiatry. 2004a;161:896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi SC, Ratnanather JT, Miller MI. Computational anatomy and neuropsychiatric disease: probabilistic assessment of variation and statistical inference of group difference, hemispheric asymmetry, and time-dependent change. Neuroimage. 2004b;23(Suppl 1):S56–68. doi: 10.1016/j.neuroimage.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Morgan KD, Orr K, Hutchinson G, Chitnis X, Suckling J, Fearon P, McGuire PK, Mallett RM, Jones PB, Leff J, Murray RM. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30:765–74. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- Edelstyn NM, Hunter B, Ellis SJ. Bilateral dorsolateral thalamic lesions disrupts conscious recollection. Neuropsychologia. 2006;44:931–8. doi: 10.1016/j.neuropsychologia.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Landau S, Matsumoto K, van Haren NE, Marshall N, Hall MH, Schulze K, Toulopoulou T, Davies N, Ribchester T, McGuire PK, Murray RM. Magnetic resonance imaging of the thalamus and adhesio interthalamica in twins with schizophrenia. Arch Gen Psychiatry. 2007;64:401–9. doi: 10.1001/archpsyc.64.4.401. [DOI] [PubMed] [Google Scholar]
- Evans JD, Heaton RK, Paulsen JS, McAdams LA, Heaton SC, Jeste DV. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60:874–82. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, Born C, Jager M, Morgenthaler M, Scheuerecker J, Zill P, Baghai T, Schule C, Rupprecht R, Bondy B, Reiser M, Moller HJ, Meisenzahl EM. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry. 2008;13:1093–101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- Gershon ES, DeLisi LE, Hamovit J, Nurnberger JI, Jr., Maxwell ME, Schreiber J, Dauphinais D, Dingman CW, 2nd, Guroff JJ. A controlled family study of chronic psychoses. Schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 1988;45:328–36. doi: 10.1001/archpsyc.1988.01800280038006. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–7. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27:13835–42. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Kemether E, Bloom R, Platholi J, Brickman AM, Shihabuddin L, Tang C, Byne W. Abnormal glucose metabolism in the mediodorsal nucleus of the thalamus in schizophrenia. Am J Psychiatry. 2004;161:305–14. doi: 10.1176/appi.ajp.161.2.305. [DOI] [PubMed] [Google Scholar]
- Heckers S, Curran T, Goff DC, Rauch SL, Fischman AJ, Alpert NM, Schacter DL. Abnormalities in the thalamus and prefrontal cortex during episodic object recognition in schizophrenia. Biological Psychiatry. 2000;48:651–657. doi: 10.1016/s0006-3223(00)00919-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Ammari N, McDermid Vaz S, Miles AA. Are schizophrenia and schizoaffective disorder neuropsychologically distinguishable? Schizophr Res. 2008;99:149–54. doi: 10.1016/j.schres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Crow TJ, Esiri MM, Harrison PJ. Low medial and lateral right pulvinar volumes in schizophrenia: a postmortem study. Am J Psychiatry. 2003;160:1177–9. doi: 10.1176/appi.ajp.160.6.1177. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19:261–85. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Agerbo E, Pedersen CB. Bipolar disorder, schizoaffective disorder, and schizophrenia overlap: a new comorbidity index. J Clin Psychiatry. 2009;70:1432–8. doi: 10.4088/JCP.08m04807. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353:30–3. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. A morphological study of the lateral geniculate body. British Journal of Opthamology. 1932;16:264–284. doi: 10.1136/bjo.16.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Bardinet E, Tremblay L, Van de Moortele PF, Pochon JB, Dormont D, Kim DS, Yelnik J, Ugurbil K. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb Cortex. 2006;16:149–61. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res. 1980;20:561–94. doi: 10.1016/0042-6989(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Mai J, Assheuer J, Paxinos G. Atlas of the Human Brain. Academic Press; San Diego: 1997. [Google Scholar]
- Malhi GS, Green M, Fagiolini A, Peselow ED, Kumari V. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215–30. doi: 10.1111/j.1399-5618.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr Res. 2007;89:59–71. doi: 10.1016/j.schres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ. Neuroimaging of Nicotine Dependence: Key Findings and Application to the Study of Smoking-Mental Illness Comorbidity. J Dual Diagn. 2009;5:168–178. doi: 10.1080/15504260902869204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–77. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res. 1992;7:95–100. doi: 10.1016/0920-9964(92)90038-7. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Exploring the borders of the schizoaffective spectrum: a categorical and dimensional approach. J Affect Disord. 2008;108:71–86. doi: 10.1016/j.jad.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–5. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Futami T, Kakei S. Input-output organization of the ventrolateral nucleus of the thalamus. Stereotact Funct Neurosurg. 1993;60:17–31. doi: 10.1159/000100587. [DOI] [PubMed] [Google Scholar]
- Sim K, Cullen T, Ongur D, Heckers S. Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm. 2006;113:907–28. doi: 10.1007/s00702-005-0363-8. [DOI] [PubMed] [Google Scholar]
- Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Addiction Research Foundation; Toronto, Canada: 1982. [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rossler A, Borgwardt SJ. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?--a systematic review. Curr Pharm Des. 2009;15:2535–49. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Barch DM, Csernansky JG. Bridging the gap between schizophrenia and psychotic mood disorders: Relating neurocognitive deficits to psychopathology. Schizophr Res. 2009;107:69–75. doi: 10.1016/j.schres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000;14:178–88. [PubMed] [Google Scholar]
- Venkatesan R, Haacke EM. Role of high resolution in magnetic resonance (MR) imaging: Applications for MR angiography, intracranial T1-weighted imaging, and image interpolation. International Journal of Imaging Systems Technology. 1997;8:529–543. [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, Thompson PA, Barch DM, Miller MI, Csernansky JG. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol Psychiatry. 2008;64:1060–8. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]