Summary
Nitrophorin 2 (NP2) is a 20 kDa lipocalin identified in the salivary gland of the blood sucking insect, Rhodnius prolixus. It functions as a potent inhibitor of the intrinsic pathway of coagulation upon binding to factor IX (FIX) or FIXa. Herein we have investigated the in vivo antithrombotic properties of NP2. Surface plasmon resonance assays demonstrated that NP2 binds to rat FIX and FIXa with high affinities (KD = 43 and 47 nM, respectively), and prolongs the aPTT without affecting the PT. In order to evaluate NP2 antithrombotic effects in vivo two distinct models of thrombosis in rats were carried out. In the rose Bengal/laser induced injury model of arterial thrombosis, NP2 increased the carotid artery occlusion time by ≈35 and ≈155%, at doses of 8 and 80 µg/kg, respectively. NP2 also inhibited thrombus formation in an arterio-venous shunt model, showing ≈60% reduction at 400 µg/kg (i.v. administration). The antithrombotic effect lasted for up to 48 h after a single i.v. dose. Notably, effective doses of NP2 did not increase the blood loss as evaluated by tail-transection model. In conclusion, NP2 is a potent and long-lasting inhibitor of arterial thrombosis with minor effects on hemostasis. It might be regarded as a potential agent for the treatment of human cardiovascular diseases.
Keywords: Animal models, Arterial thrombosis, Coagulation inhibitors, Haemostasis, Nitrophorin
Introduction
Factor IXa (FIXa) is a vitamin K-dependent coagulation serine protease, which upon binding to the non-enzymatic cofactor, factor VIIIa, on membrane surfaces in the presence of Ca2+ forms the intrinsic Xase complex and rapidly activates factor X to factor Xa (FXa) during the blood coagulation process (1–3). FIXa plays a key role in hemostasis, and its deficiency is associated with the life-threatening disease hemophilia B (4). The zymogen FIX circulates in plasma as a single-chain glycoprotein with 415 amino acids. It can be converted to FIXa by either one of two physiological activators, the factor VIIa(FVIIa)-tissue factor complex or factor XIa (FXIa), upon cleavage of Arg145-Ala and Arg180-Val peptide bonds (1, 3, 5).
Under pathologic conditions, excessive clot formation may occlude blood vessels, resulting in a variety of thromboembolic disorders, such as deep vein thrombosis, pulmonary embolism and stroke, which are the main causes of morbidity and mortality (6). Anticoagulation therapy with heparin and oral vitamin K antagonists is currently the mainstay of treatment and prevention of thrombosis (6–8). Unfortunately, both classes of drugs have well-documented limitations, pointing toward need for the development of novel efficacious and safe anticoagulants (8). In practice, this involves identifying drugs capable of preventing thrombus formation without increasing the risk of hemorrhage (9). In this context, it has been proposed that targeting the intrinsic pathway of coagulation would be advantageous for treating thromboembolic diseases (10, 11).
A number of specific inhibitors from exogenous sources have been identified from salivary glands of blood sucking arthropods (12, 13). Those molecules have been regarded as important tools for the study of blood clotting system, and provided a valuable source of antithrombotic compounds. Nitrophorin 2 (NP2), or prolixin S, is a 20 kDa lipocalin identified in the salivary gland of the blood sucking insect, Rhodnius prolixus (14). It has been characterized as a potent anticoagulant due to its ability to inhibit the intrinsic Xase complex (15–18). Further studies have shown that NP2 binds with high affinity to either FIXa or FIX (19, 20), most likely by targeting the γ-carboxyglutamic acid-containing Gla domain (19). Thus, the interaction between NP2 and FIXa may interfere with enzyme association with phospholipids, resulting in failure to assemble into the intrinsic Xase and reducing its catalytic activity. In addition, complex formation between NP2 and FIX decreases the zymogen activation by both the FVIIa-TF complex and FXIa, possibly due to decreased zymogen association with phospholipids (19).
In the present study, we evaluated the in vivo effects of NP2 on thrombus formation using two distinct thrombosis models in rats. After intravenous administration, NP2 showed effective and long-lasting antithrombotic activity. Remarkably, NP2 produced no excessive bleeding at effective antithrombotic doses.
We conclude that NP2 is a potent and long-lasting inhibitor of arterial thrombosis with minor effects on hemostasis. It might be regarded as a potential agent for the treatment of human cardiovascular diseases.
Materials and Methods
Materials
Recombinant NP2 was produced in Escherichia coli, purified, and quantified as previously described (21). Rat FIX and FIXa were purchased from Haematologic Technologies (Essex Junction, VT). Activated partial thromboplastin time (aPTT) (cephalin plus kaolin) and prothrombin time (PT) (thromboplastin with calcium) reagents were from BioMériaux (Rio de Janeiro, Brazil). Anasedan (Xylazin) and Dopalen (Ketamin) were from Agribrands (Rio de Janeiro, Brazil). Chromogenic substrate for thrombin (H-D-phenylalanyl-L-pipecolyl-L-arginine-p-nitroaniline dihydrochloride, S-2238) was purchased from Diapharma (Westchester, OH). Gly-Pro-Arg-Pro amide (GPRP) from Sigma Chemical Co. (St. Louis, MO). Rose bengal dye was from Fischer Scientific Co. (Fair Lawn, NJ).
Animals
Adult Wistar rats (both sexes) were housed under controlled conditions of temperature (24 ± 1°C) and light (12 h light starting at 7:00 a.m.), and all experiments were conducted in accordance with standards of animal care defined by the Institutional Committee (Institute of Medical Biochemistry, Federal University of Rio de Janeiro).
Surface plasmon resonance (SPR) analysis
All experiments were carried out in a T100 instrument (GE Healthcare Inc., Piscataway, NJ) following the manufacturer’s instructions. Sensor chip CM5, amine coupling reagents, and buffers were also purchased from GE Healthcare. HBS (10 mM Hepes, pH 7.4, 150 mM NaCl supplemented with 5 mM CaCl2) was used as the running buffer for all SPR experiments. Each experiment was carried out in duplicate. FIX or FIXa (1–3 µg/ml) in acetate buffer pH 4.0 containing 5 mM CaCl2 were immobilized on a CM5 sensor chip using amine coupling methodology resulting in a final immobilization of 300–500 RU. Blank flow cells were used to subtract the buffer effect on sensorgrams. Kinetic experiments were carried out with a contact time of 200–300 s at a flow rate of 30 µl/min at 25°C. NP2-FIX/FIXa complex dissociation was monitored for 600 s, and the sensor surface was regenerated by a pulse of 30 s of 10 mM EDTA at a flow rate of 30 µl/minute. After subtraction of the contribution of bulk refractive index and nonspecific interactions with the CM5 chip surface, the individual association (ka) and dissociation (kd) rate constants were obtained by global fitting of data using the two-state reaction (conformational change) interaction model using BIAevaluation™ (Biacore, Inc.) (22):
Values were then used to calculate the dissociation constant (KD).
The values of average squared residual obtained were not significantly improved by fitting data to models that assumed other interactions. Conditions were chosen so that the contribution of mass transport to the observed values of KD was negligible. In addition, the models in the T100 evaluation software fit for mass transfer coefficient to mathematically extrapolate the true ka and kd.
Effect of NP2 on aPTT and PT
The effect of NP2 on coagulation tests aPTT and PT was evaluated on an Amelung KC4A coagulometer (Labcon, Heppenheim, Germany). Human blood samples were collected from healthy donors in 3.8% trisodium citrate (9:1, v/v), and platelet poor plasma were obtained by further centrifugation at 2000×g for 10 min. Rat plasma samples were obtained following the same procedure after blood collection by cardiac puncture. Plasma (50 µl) was incubated with NP2 (10 µl) for 2 min at 37°C, followed by addition of the aPTT reagent (50 µl, 1 min) and then 25 mM CaCl2 (100 µl) or the PT reagent (100 µl). Time for clot formation was then recorded. For ex vivo assays in rats, PBS, NP2 or heparin were given I.V. 30 min before cardiac puncture. Blood collection, plasma preparation and aPTT procedure were performed as above.
Measurement of thrombin generation in plasma
Thrombin formation in plasma was followed after activation of the intrinsic pathway, as previously described (23). Rat plasma (50 µL) was incubated with different concentrations of NP2 diluted in 50 µl TBS and 10 µL of APTT reagent (cephalin plus kaolin) for 2 min at room temperature. Reaction was started by addition of 100 µL of 12.5 mM CaCl2 and aliquots of 10 µL were transferred every 10 s into microplate wells containing 40 µL of TBS-EDTA buffer. In order to prolong plasma clotting, reactions were carried out in the presence of 10 mM GPRP. Thrombin generation was determined by the addition of 100 µM of the chromogenic substrate S-2238. Hydrolysis of the substrate was detected using a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA), equipped with a microplate mixer and heating system. Reactions were recorded continuously at 405 nm, for 20 min, at 37°C. The total volume of the reactions was 100 µL.
Thrombus formation in the arterio-venous shunt model
Antithrombotic activity in an extracorporeal shunt was investigated as described (24). Rats (both sexes, 200–250 g body weight) were anesthetized with intramuscular xylazine and ketamine (16 and 100 mg/kg, respectively). Through a longitudinal incision in the skin over the trachea along the midline, the left jugular vein and the right carotid artery were cautiously freed from the surrounding tissue. The right carotid artery and the left jugular vein were cannulated; a solution of NP2 was injected on the artery and allowed to circulate at various times (15 min to 72 h) before initiating the surgery for thrombosis induction.
In the meantime an extracorporeal shunt was prepared. This shunt consisted of two 6 cm pieces of polyethylene tubing (1.3 mm inner diameter) with both ends cut at an angle; one end of each piece of tubing was forced into the end of a central, 6 cm tube (1.6 mm inner diameter) with a 5 cm length of silk thread drawn through it, so as to leave 1.0 cm outside the tubing at one end and 4.0 cm inside the tubing. This shunt was filled with a heparin solution (50 U/mL). One end of the shunt was inserted into the left jugular vein, and subsequently the other end was inserted into the right carotid artery and the circulation of the blood was established. After 20 min of blood circulation through the shunt, the blood flow was stopped at the arterial side of the tubing with a pinch-clamp and the middle of the extracorporeal shunt was isolated. From this segment the silk thread coated with the thrombus was carefully pulled out with the help of the piece of thread that remained external to the shunt. The wet thrombus weight was immediately determined, subtracting the weight of the wet thread.
Photochemically-induced carotid artery thrombosis in rats
The effect of NP2 on arterial thrombosis was evaluated in a rat model of arterial thrombosis (25). Animals were anesthetized as described above. The right common carotid artery was isolated through a midline cervical incision, and the blood flow was monitored continuously using a 1PR doppler flow probe and a TS420 flowmeter (Transonic Systems, Ithaca, NY). Rose bengal dye (90 mg/kg body weight) was diluted to 60 mg/mL in PBS and administered via slow injection (over 2 min) through the cava vein. Just before injection, the common carotid artery was transilluminated with a 1.5-mV, 540 nm green laser (#25-LGR-193–249, Melles Griot Inc., Carlsbad, CA) immediately proximal to the doppler probe from a distance of 3 cm. Mean carotid artery blood flow was monitored until stable occlusion occurred (defined as a blood flow of 0 ml/min for ≥ 5 min), at which time the experiment was terminated. NP2 or PBS (control) was injected in the cava vein 15 min before initiating the rose bengal administration.
Bleeding effect
Rats (both sexes, ~100 g body weight) were anesthetized as described above and a cannula was inserted into the right carotid artery for administration of NP2. After 60 min, tail was cut at 2 mm of diameter and carefully immersed in 40 ml distilled water at room temperature. The hemoglobin content in water solution (absorbance at 540 nm) was used to estimate the blood loss (26). Appropriate controls (i.v. injection of PBS or heparin at 1mg/kg) were run in parallel.
Statistical analysis
Results were expressed as mean ± standard error. Statistical analysis was performed with one-way ANOVA, followed by a post-hoc test (Dunnett) using the statistical package GraphPad Prism 4.0 (GraphPad software, USA).
Results
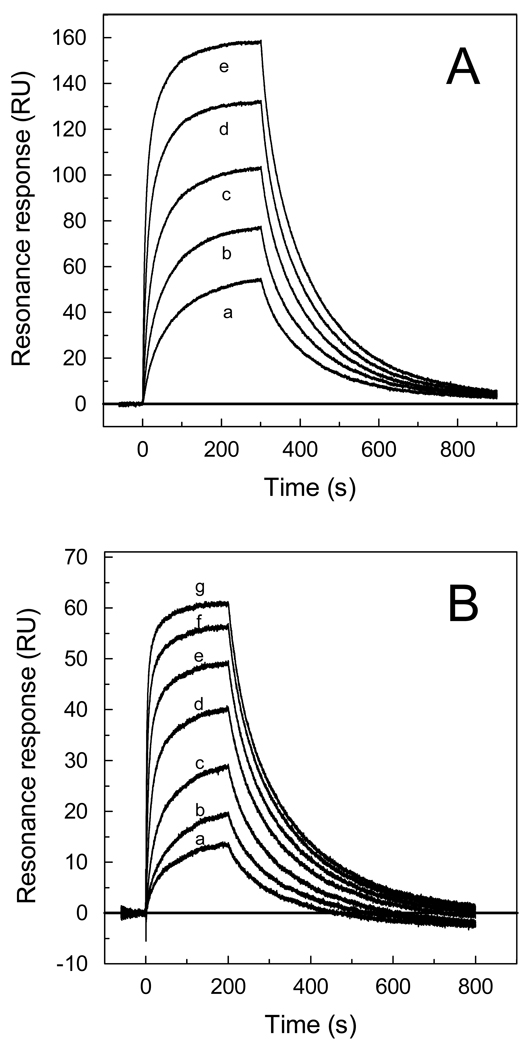
Previous studies have demonstrated that NP2 interacts with human FIXa and FIX with high affinity, as demonstrated by SPR analysis (19, 20). To further investigate binding kinetics of NP2-rat FIX/FIXa interactions, SPR experiments were performed. Typical sensorgrams obtained upon NP2 interaction with rat FIX and FIXa are shown in Figs 1A and 1B, respectively. In both cases, the best fit was attained using a two-state reaction model (Table 1) (22). Using this model, KD values of 43 nM for FIX and 47 nM for FIXa were calculated (Table 1).
Figure 1. NP2 binds to rat Factor IX and Factor IXa.
Typical sensorgrams of NP2 interaction with Factor IXa (A) and Factor IX (B). Different concentrations of FIXa (in nM:a, 20; b, 40; c, 80; d, 160; and e, 320) or FIX (in nM: a, 10; b, 20; c, 40; d, 80; e, 160; f, 320; g, 640) were injected over immobilized NP2 for 180 s. Dissociation of NP2-FIXa and NP2-FIX complex was monitored for 900 s and 800 s, respectively, and a global two-state binding model was used to calculate kinetic parameters. Representative sensorgrams are shown.
Table 1.
Kinetics of NP2 interaction with rat FIX and FIXa estimated by SPR.*
|
Kα1 (M−1s−1 × 105) |
Kd1 (s−1) |
Kα2 (s−1) |
Kd2 (s−1) |
KD (nM) |
|
|---|---|---|---|---|---|
| FIX | 5.70 | 0.069 | 0.015 | 0.009 | 43.0 |
| FIXa | 2.86 | 0.026 | 0.075 | 0.080 | 47.0 |
Responses were obtained by injecting FIX or FIXa over immobilized NP2 for 180 s at a flow rate of 30 µl/min.
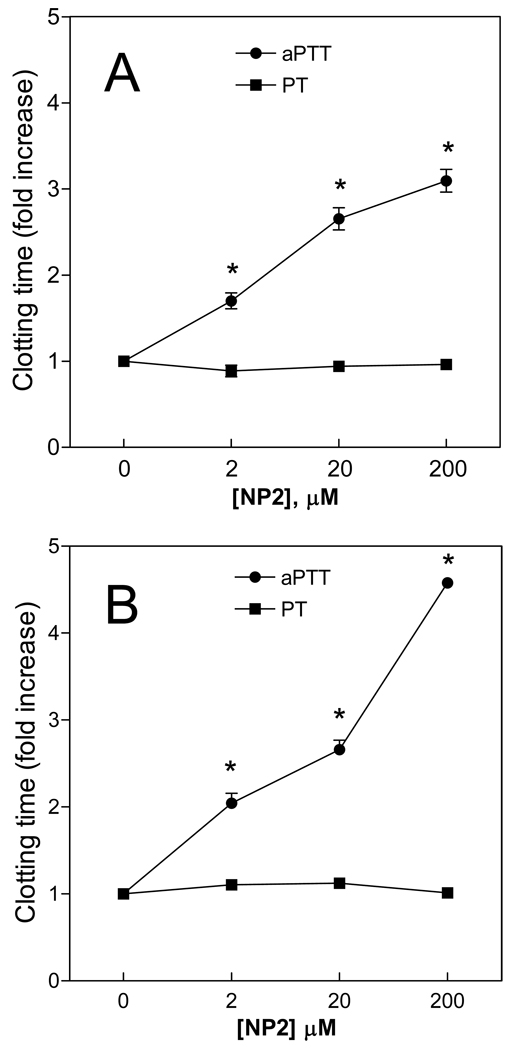
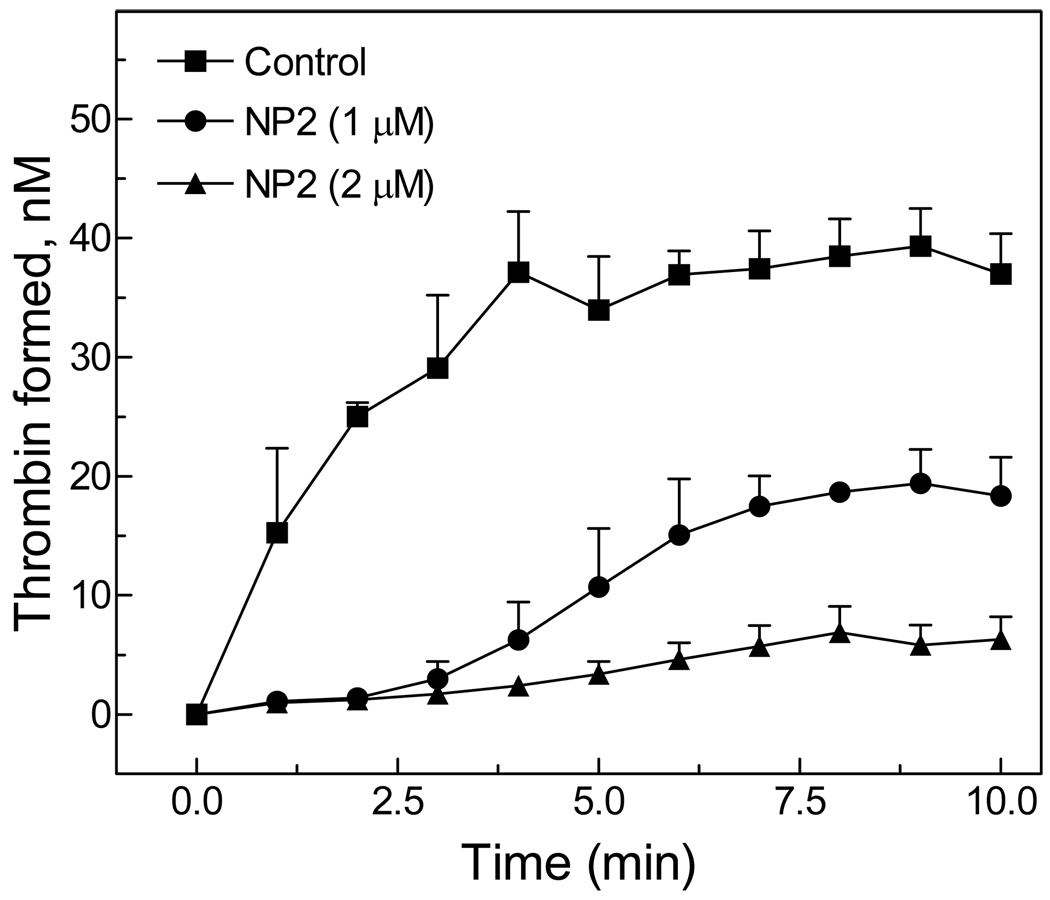
Consistent with its mechanism of action, Fig. 2A shows that NP2 prolonged aPTT in human plasma in a concentration-dependent manner. No effect was observed in the extrinsic pathway, as evaluated by PT. We have also tested the anticoagulant properties of NP2 in rat plasma. Fig. 2B shows that aPTT was prolonged at NP2 concentrations above 0.04 µg/ml without changes in the PT. In order to verify the effects of NP2 in thrombin generation in plasma, experiments were performed as described in Methods. Fig. 3 shows that NP2 (20–40 µg/ml) inhibited thrombin formation by 50–80%, as compared to the control. Overall, data presented on Figures 1–3 and Table 1 confirm that inhibitory properties of NP2 towards rat FIX(a) are similar to that previously observed for the human counterparts (16–20).
Figure 2. In vitro anticoagulant activity of NP2.
NP2 at the indicated concentrations was incubated with human (A) or rat (B) citrated plasma for 2 min. Coagulation aPTT (●) and PT (▪) were determined as described in Materials and methods. Each point represents mean ± SE of 5 independent determinations. Asterisk, P < 0.01, as compared to values observed in the absence of NP2.
Figure 3. Effect of NP2 on thrombin generation in plasma.
Thrombin generation in human plasma was followed after initiation of the intrinsic pathway. Curves show thrombin formed in plasma in the absence or in the presence of NP2 at the indicated concentrations. Assay conditions are described in the Materials and methods section. Each point represents mean ± SE of 5 independent determinations. Asterisk, P < 0.01, as compared to values observed in the absence of NP2.
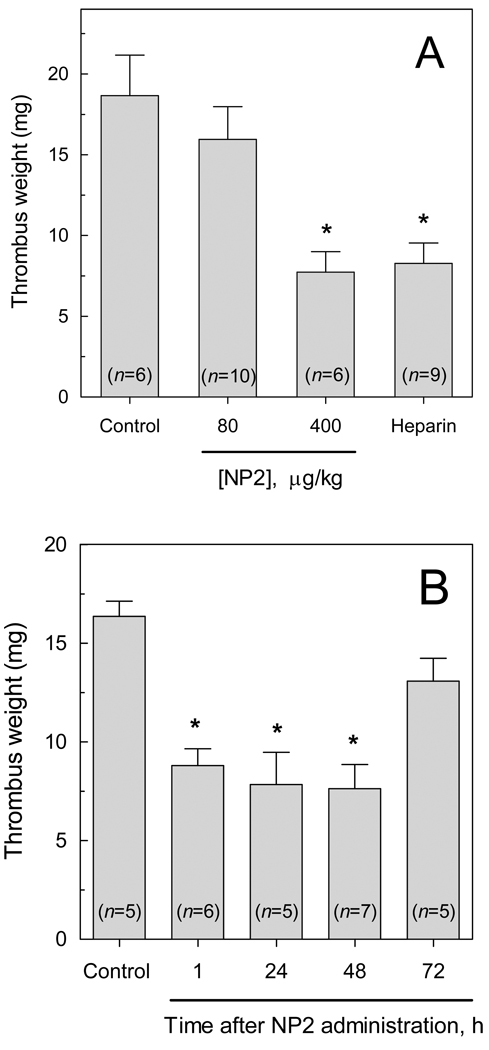
To determine whether NP2 exerts antithrombotic action in vivo, we employed two distinct models of thrombosis in rats. In an arterio-venous shunt model (24), NP2 at 400 µg/kg (i.v., single bolus administered 15 min before thrombus induction) significantly reduced thrombus weight (7.73 ± 1.27 mg versus 18.67 ± 2.50 mg in controls) (Fig 4A). The extent of the antithrombotic effect of NP2 was similar to that observed with 1 mg/kg of heparin (8.28 ± 1.23 mg). In order to demonstrate that the antithrombotic action of NP2 is dependent on FIX(a) interaction, we performed control experiments employing NP1, a recombinant lipocalin which shows no anticoagulant activity despite presenting great sequence similarity to NP2 (14). No antithrombotic activity was observed (data not shown). We have also evaluated the time-dependence of the antithrombotic activity of NP2. Fig 4B shows that thrombosis inhibition produced by a single dose of NP2 (400 µg/kg) remained for up to 48 h, returning to control values after 72 h.
Figure 4. Antihrombotic activity of NP2 in an arterio-venous shunt model.
The antithrombotic activity of NP2 was investigated using an arterio-venous shunt model, as described in Materials and methods. (A) NP2 at indicated doses or PBS (control) were injected intravenously. Asterisk, P < 0.01, as compared to values observed in the absence of NP2. (B) NP2 (400 µg/kg) was administered i.v. to animals, and thrombosis was induced after the indicated times. Each bar represents mean ± SE, and the number of animals tested for each condition is shown in the Figure. Asterisk, P < 0.01, as compared to values observed in the control.
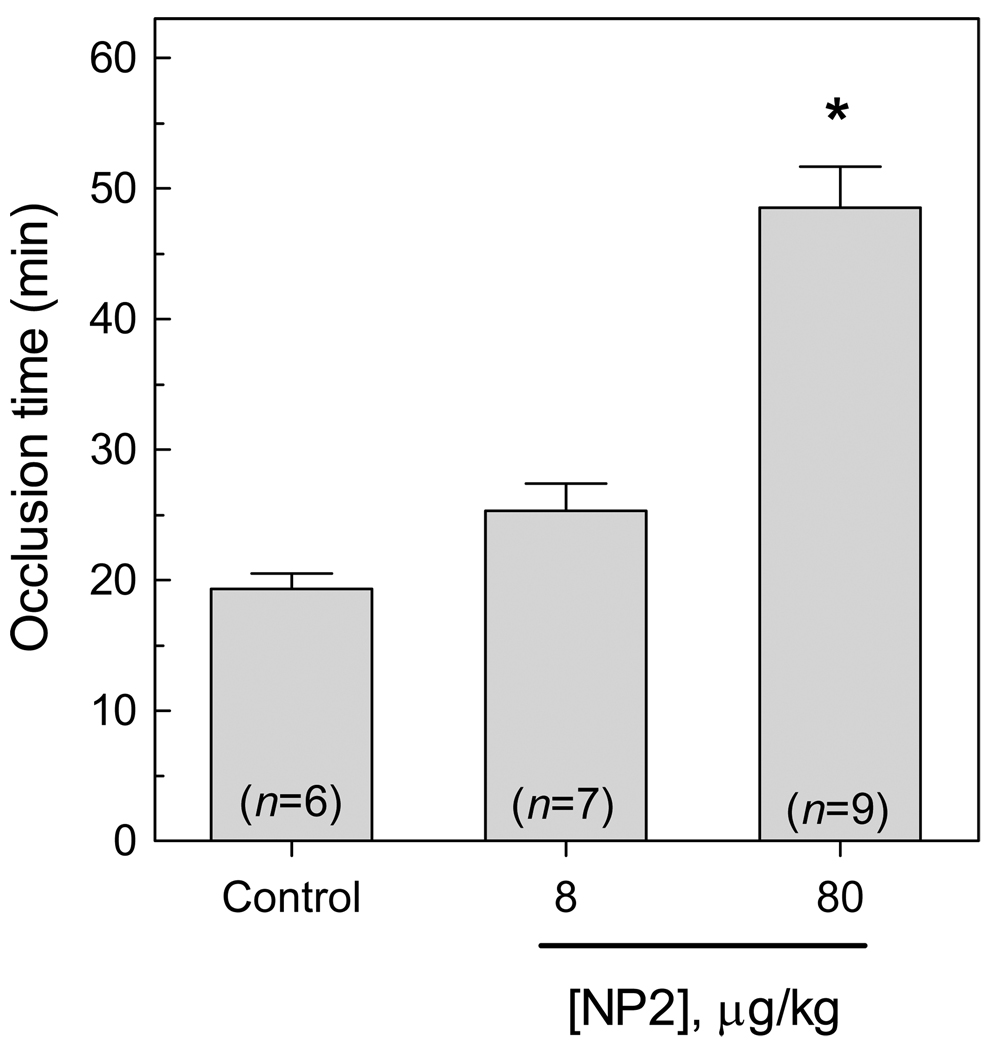
Next, antithrombotic action of NP2 was tested in the rose Bengal/laser injury model of arterial thrombosis. This model is based on a photochemically-induced endothelial injury that decreases the carotid artery blood flow, attributable to the development of stable thrombus (24). Figure 5 shows that the blood flow of control (PBS injected) animals stopped in ≈ 19 minutes. In contrast, NP2 delayed thrombus formation, increasing the carotid artery occlusion time by ≈ 35% (25.3 ± 5.6 min) and ≈ 155% (48.6 ± 9.4 min), at doses of 8 and 80 µg/kg body weight, respectively.
Figure 5. NP2 prevents thrombus formation in a rose bengal/laser induced model.
NP2 (8 or 80 µg/kg) or PBS (control) was injected in the cava vein of rats and thrombosis was induced by slow injection (over 2 min) of 90 mg/kg body weight of rose bengal dye into the cava vein at a concentration of 60 mg/mL. Before injection, green light laser was applied to the desired site of injury from a distance of 3 cm and remained on until stable occlusion occurred. Each bar represents mean ± SE, and the number of animals tested for each condition is shown in the Figure. Asterisk, P < 0.01, as compared to values observed in control.
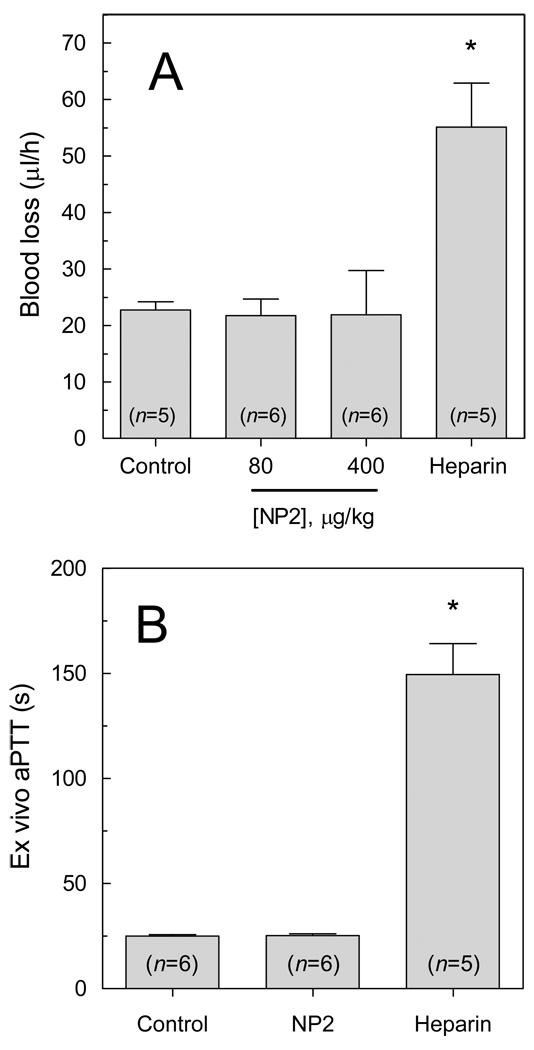
Finally, the effect of NP2 on hemostasis was evaluated in a tail-transection model (26). Fig. 6A shows that bleeding was not significantly increased in the presence of antithrombotic concentrations of NP2, as compared with rats receiving PBS. In contrast, heparin at 1 mg/kg - an effective, but not excessive antithrombotic dose (data not shown), significantly increased blood loss. Remarkably, NP2 did not affect the aPTT ex vivo, as shown in Fig. 6B. Again, heparin produced a significant change in this hemostatic parameter. Considering a blood volume of ~7.5 mL/100 g, it is possible to estimate a concentration of ~250 nM NP2 in the blood of 200 g animals that received 400 µg/kg doses. According to Fig. 2B, this may explain why such dose does not alter aPTT ex-vivo nor increase tail bleeding.
Figure 6. Hemostatic parameters are not changed by NP2.
(A) Bleeding effect by transection model. PBS (control), NP2 (80 or 400 µg/kg) or heparin (1 mg/kg) was administered i.v. and allowed to circulate for 15 min. Blood loss was determined as a function of hemoglobin in water (A540 nm), as described in the material and methods section. Data are reported as mean ± SE. Asterisk, P < 0.01, as compared to values observed in control group. (B) Ex vivo aPTT. PBS (control), NP2 (400 µg/kg) or heparin (1 mg/kg) was administered i.v. and allowed to circulate for 30 min. Blood collection, plasma preparation and aPTT measurement were performed as described in the Material and methods section.
Discussion
NP2 is a member of the nitrophorin (NP) family of heme-binding lipocalins which consists of at least four related molecules found in R. prolixus salivary gland. These molecules display distinct functions related to blood feeding such as carrying nitric oxide which serves as a vasodilator in the host, or binding histamine and attenuating anti-inflammatory events (14, 21, 27, 28). NP2 is the unique member of the NP group displaying potent anticoagulant activity which has been characterized as a FIX and FIXa-binding protein (16, 18–20). Accordingly, NP2 decreases FX activation by FIXa bound to either the phospholipid or activated platelet surface or to the cofactor FVIII, possibly by interfering with the assembly of FX-activating complex (18). In fact, kinetic analyses have demonstrated that NP2 decreases both Km and Vmax of FX activation by the intrinsic Xase complex. Moreover, complex formation between NP2 and FIX decreases the rate of zymogen activation by FVIIa/TF complex (19). Further, NP2 binding to FIX strongly inhibits FIXa generation by FXIa (19). Therefore, NP2 can be a unique tool to dissect the contribution of Factor IX(a) in coagulation in vitro, and thrombosis in vivo.
The aim of this study was to investigate how the blockade of FIX(a) affects thrombosis in rats. SPR experiments confirmed that NP2 binds to rat FIX(a) with an affinity comparable to human FIX(a) (KD ~ 34 nM for FIX and 16 nM for FIXa) (20). In addition, NP2 prolonged aPTT without affecting PT. These results indicated that NP2 is a suitable molecule to be tested as an anticoagulant in rats. Accordingly, our experiments demonstrate that NP2 decreases thrombus formation induced by extracorporeous arterio-venous shunt and also by laser-induced arterial injury. In both models, coagulation cascade has been implicated as a major player leading to vascular occlusion (24, 29). However, NP2 was less effective in the arterio-venous model possibly because the relative contribution of FVIIa/TF-induced FIX activation or the intrinsic Xase as a thrombotic stimulus differ. At this point, it is not clear whether the effects of NP2 occur because it prevents activation of FIX by TF/VIIa, or attenuates productive assembly of the intrinsic Xase, or because it interfered with FXIa-induced FIX activation in vivo. Likewise, it has been demonstrated that a sulfated galactan with antithrombotic properties, inhibits arterial thrombosis, whereas it is inactive in the arterio-venous shunt in rats (30). It is conceivable that NP2 effects are results of synergistic and redundant inhibition of distinct and sequential steps of coagulation cascade both in the extrinsic and intrinsic pathways (i.e., targeting FIX and FIXa directly, or FVIIa/TF complex and FXIa indirectly). This contention is supported by the prominent antithrombotic effects of NP2 in vivo. Most remarkably, antithrombotic doses of NP2 showed minor effects on hemostasis as assessed by tail-transection and aPTT measurements, while administration of a therapeutic dose of heparin was associated with bleeding and significant increase in aPTT.
Our results using a selective FIX(a) inhibitor are in line with results obtained with inhibition/genetic deficiency of IX(a) and other components of the intrinsic pathway in the preclinical animal models of thrombosis. Accordingly, an anti-FIX antibody display antithrombotic properties in a rat model of FeCl3-induced arterial thrombosis without affecting bleeding time (31). Likewise, inhibition of recurrent arterial thrombosis by active site-blocked FIXa has been effectively tested in a guinea pig model without impaired hemostasis (32). Further, the inhibition of the intrinsic coagulation cascade with catalytically inactive FIXa limits cerebral injury in stroke without increasing intracerebral hemorrhage (33). Finally, a tick salivary protein (Ir-CPI) which targets contact phase factors (FXIIa, FXIa, and kallikrein) inhibits thrombus formation without an increase in blood loss (34).
Clinical data in humans and genetically altered mice also suggest that components of the intrinsic pathway of coagulation may play distinct roles in thrombosis and hemostasis (35). FXI deficiency is often associated with mild to moderate bleeding in humans, while bleeding does not occur in FXII deficiency (33). In genetically modified mice, FXI and FXII-knockout are less susceptible to thrombus formation with negligible impact on hemostasis (36, 37). On the other hand, FIX deficiency is protective against experimental thrombosis but is associated with bleeding in humans and in mice (4, 36, 38). It is plausible that NP2 doses administered to rats in our study did not produce bleeding because it did not reach plasma levels to saturate FIX since its concentration is relatively high (90 nM) in the blood (4). This contention is consistent with no prolongation of aPTT ex vivo (Figure 6B). It is conceivable that tailoring FIXa activity or perhaps tailoring the intrinsic Xase assembly (through different mechanism) is the most appropriate approach when attempting to prevent thrombosis without impairing hemostasis.
Analysis of the time-dependence of the antithrombotic action of NP2 indicates a prolonged half-life which remained effective for up to 48h after a single i.v. dose. The long-lasting antithrombotic action of NP2 may derive from a stable complex formation with FIX in plasma. This is in accordance with the pharmacokinetic profile observed for an anti-FIX monoclonal antibody, which has a half-life of about 90 hours in primates (39). In addition, prolonged half-lives have been reported for NAPc2 and Ixolaris which are high-affinity ligands of FX (40, 41). In this context, future measurements of the pharmacokinetic profile of NP2 will be essential to understand its long-lasting antithrombotic effect. Finally, exogenous inhibitors, targeting multiple steps of coagulation, have been identified. Their activity towards zymogen is particularly interesting because it may represent an evolutionary strategy to effectively block coagulation at multiple points. For example, Ixolaris from tick saliva uses FX(a) as scaffolds for the inhibition of FVIIa-tissue factor complex (42) and at appropriate concentrations it decreases prothrombin activation by FXa (43) and FX activation by the intrinsic Xase complex (44). Bothrojaracin from snake venom is a high-affinity ligand of pro(thrombin) that decreases both the enzyme activity and zymogen activation (45, 46).
In conclusion, NP2 displays potent antithrombotic effect in vivo, with prolonged half-life and no major bleeding. The absence of major hemostatic changes at effective antithrombotic concentrations of NP2 may indicate an advantage in terms of therapeutic window when compared to antibodies against FIX and catalytic site blocked FIXa, which have been evaluated experimentally or in clinical trials (11, 31, 32). Overall, NP2 appears to be a promising anticoagulant, or as a prototype to develop anticoagulants for the prevention and treatment of thrombosis or other pathologies associated with dysregulated coagulation (47, 48).
What is known about this topic?
-
-
Factor IXa plays a central role in thrombin generation during initiation and amplification of the coagulation cascade.
-
-
Genetic disruption of components of the intrinsic pathway of coagulation (factor XII and factor XI) protects against thrombosis with minor effects on hemostasis.
-
-
Nitrophorin 2 has been characterized in vitro as an inhibitor of the intrinsic pathway of coagulation upon binding to factor IX(a).
What does this paper add?
-
-
Nitrophorin 2 is a potent antithrombotic agent in vivo, as demonstrated in rat models of thrombosis.
-
-
Effective antithrombotic doses of nitrophorin 2 do not cause excessive bleeding in the rat tail-transection model.
-
-
Factor IX(a) might be a potential target for the treatment of thrombosis with minor hemorrhagic effects.
-
-
Nitrophorin 2 is a useful tool to understand the contributions of FIX(a) in hemostasis or thrombosis.
Acknowledgments
We thank Dr. A.R. Rezaie (St. Louis University School of Medicine) for careful revision of the manuscript. Leonardo M. Nascimento and Natalia C. Rochael for technical assistance. This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro Carlos Chagas Filho (FAPERJ) and from the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. Because J.F.A. and I.M.B.F. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
References
- 1.Mann KG. Biochemistry and physiology of blood coagulation. Thromb Haemost. 1999;82:165–174. [PubMed] [Google Scholar]
- 2.Owens AP, 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost. 2010;104 doi: 10.1160/TH09-11-0771. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dieijen G, van Rijn JL, Govers-Riemslag JW, et al. Assembly of the intrinsic factor X activating complex--interactions between factor IXa, factor VIIIa and phospholipid. Thromb Haemost. 1985;53:396–400. [PubMed] [Google Scholar]
- 4.Lozier JN, Kessler CM. Hematology: Basic Principles and Practice. 4th ed. New York: Churchill Livingstone; 2005. Clinical aspects and therapy of hemophilia; pp. 2047–2069. [Google Scholar]
- 5.Gailani D. Activation of factor IX by factor XIa. Trends Cardiovasc Med. 2000;10:198–204. doi: 10.1016/s1050-1738(00)00070-0. [DOI] [PubMed] [Google Scholar]
- 6.Weitz JI. Emerging anticoagulants for the treatment of venous thromboembolism. Thromb Haemost. 2006;96:274–284. doi: 10.1160/TH06-05-0234. [DOI] [PubMed] [Google Scholar]
- 7.Gray E, Mulloy B, Barrowcliffe TW. Heparin and low-molecular-weight heparin. Thromb Haemost. 2008;99:807–818. doi: 10.1160/TH08-01-0032. [DOI] [PubMed] [Google Scholar]
- 8.Ahrens I, Lip GY, Peter K. New oral anticoagulant drugs in cardiovascular disease. Thromb Haemost. 2010;104:49–60. doi: 10.1160/TH09-05-0327. [DOI] [PubMed] [Google Scholar]
- 9.Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:287S–310S. doi: 10.1378/chest.126.3_suppl.287S. [DOI] [PubMed] [Google Scholar]
- 10.Gailani D, Renné T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 11.Howard EL, Becker KC, Rusconi CP, et al. Factor IXa inhibitors as novel anticoagulants. Arterioscler Thromb Vasc Biol. 2007;27:722–727. doi: 10.1161/01.ATV.0000259363.91070.f1. [DOI] [PubMed] [Google Scholar]
- 12.Koh CY, Kini RM. Molecular diversity of anticoagulants from haematophagous animals. Thromb Haemost. 2009;102:437–453. doi: 10.1160/TH09-04-0221. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 14.Andersen JF, Gudderra NP, Francischetti IM, et al. The role of salivary lipocalins in blood feeding by Rhodnius prolixus. Arch Insect Biochem Physiol. 2005;58:97–105. doi: 10.1002/arch.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellmann K, Hawkins RI. Prolixins-S and prolixin-G; two anticoagulants from Rhodnius prolixus Stål. Nature. 1965;207:265–267. doi: 10.1038/207265a0. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro JM, Schneider M, Guimarães JA. Purification and characterization of prolixin S (nitrophorin 2), the salivary anticoagulant of the blood-sucking bug Rhodnius prolixus. Biochem J. 1995;308:243–249. doi: 10.1042/bj3080243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Yamaguchi M, Yuda M, et al. Purification, characterization and cDNA cloning of a novel anticoagulant of the intrinsic pathway, (prolixin-S) from salivary glands of the blood sucking bug, Rhodnius prolixus. Thromb Haemost. 1996;75:573–577. [PubMed] [Google Scholar]
- 18.Zhang Y, Ribeiro JM, Guimarães JA, et al. Nitrophorin-2: a novel mixed-type reversible specific inhibitor of the intrinsic factor-X activating complex. Biochemistry. 1998;37:10681–10690. doi: 10.1021/bi973050y. [DOI] [PubMed] [Google Scholar]
- 19.Isawa H, Yuda M, Yoneda K, et al. The insect salivary protein, prolixin-S, inhibits factor IXa generation and Xase complex formation in the blood coagulation pathway. J Biol Chem. 2000;275:6636–6641. doi: 10.1074/jbc.275.9.6636. [DOI] [PubMed] [Google Scholar]
- 20.Gudderra NP, Ribeiro JM, Andersen JF. Structural determinants of factor IX(a) binding in nitrophorin 2, a lipocalin inhibitor of the intrinsic coagulation pathway. J Biol Chem. 2005;280:25022–25028. doi: 10.1074/jbc.M504386200. [DOI] [PubMed] [Google Scholar]
- 21.Andersen JF, Ding XD, Balfour C, et al. Kinetics and equilibria in ligand binding by nitrophorins 1–4: evidence for stabilization of a nitric oxide-ferriheme complex through a ligand-induced conformational trap. Biochemistry. 2000;39:10118–10131. doi: 10.1021/bi000766b. [DOI] [PubMed] [Google Scholar]
- 22.Morton TA, Myszka DG, Chaiken IM. Interpreting complex binding kinetics from optical biosensors: a comparison of analysis by linearization, the integrated rate equation, and numerical integration. Anal Biochem. 1995;227:176–185. doi: 10.1006/abio.1995.1268. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes RS, Kirszberg C, Rumjanek VM, et al. On the molecular mechanisms for the highly procoagulant pattern of C6 glioma cells. J Thromb Haemost. 2006;4:1546–1552. doi: 10.1111/j.1538-7836.2006.01985.x. [DOI] [PubMed] [Google Scholar]
- 24.Umetsu T, Sanai K. Effect of 1-methyl-2-mercapto-5-(3-pyridyl)-imidazole (KC-6141), an anti-aggregating compound, on experimental thrombosis in rats. Thromb Haemost. 1978;39:74–83. [PubMed] [Google Scholar]
- 25.Matsuno H, Uematsu T, Nagashima S, et al. Photochemically induced thrombosis model in rat femoral artery and evaluation of effects of heparin and tissue-type plasminogen activator with use of this model. J Pharmacol Methods. 1991;25:303–317. doi: 10.1016/0160-5402(91)90030-9. [DOI] [PubMed] [Google Scholar]
- 26.Herbert JM, Hérault JP, Bernat A, et al. Biochemical and pharmacological properties of SANORG 32701. Comparison with the "synthetic pentasaccharide' (SR 90107/ORG 31540) and standard heparin. Circ Res. 1996;79:590–600. doi: 10.1161/01.res.79.3.590. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro JM, Hazzard JM, Nussenzveig RH, et al. Reversible binding of nitric oxide by a salivary heme protein from a bloodsucking insect. Science. 1993;260:539–541. doi: 10.1126/science.8386393. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro JM, Walker FA. High affinity histamine-binding and antihistaminic activity of the salivary nitric oxide-carrying heme protein (nitrophorin) of Rhodnius prolixus. J Exp Med. 1994;180:2251–2257. doi: 10.1084/jem.180.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters RF, Lees CM, Mitchell KA, et al. The characterisation of thrombus development in an improved model of arterio-venous shunt thrombosis in the rat and the effects of recombinant desulphatohirudin (CGP 39393), heparin, and iloprost. Thromb Haemost. 1991;65:268–274. [PubMed] [Google Scholar]
- 30.Melo FR, Mourão PA. An algal sulfated galactan has an unusual dual effect on venous thrombosis due to activation of factor XII and inhibition of the coagulation proteases. Thromb Haemost. 2008;99:531–538. doi: 10.1160/TH07-10-0649. [DOI] [PubMed] [Google Scholar]
- 31.Feuerstein GZ, Patel A, Toomey JR, et al. Antithrombotic efficacy of a novel murine antihuman factor IX antibody in rats. Arterioscler Thromb Vasc Biol. 1999;19:2554–2562. doi: 10.1161/01.atv.19.10.2554. [DOI] [PubMed] [Google Scholar]
- 32.Himber J, Refino CJ, Burcklen L, et al. Inhibition of arterial thrombosis by a soluble tissue factor mutant and active site-blocked factors IXa and Xa in the guinea pig. Thromb Haemost. 2001;85:475–481. [PubMed] [Google Scholar]
- 33.Choudhri TF, Hoh BL, Prestigiacomo CJ, et al. Targeted inhibition of intrinsic coagulation limits cerebral injury in stroke without increasing intracerebral hemorrhage. J Exp Med. 1999;190:91–99. doi: 10.1084/jem.190.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decrem Y, Rath G, Blasioli V, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206:2381–2395. doi: 10.1084/jem.20091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gailani D, Renné T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Cheng Q, Xu L, Feuerstein GZ, et al. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 37.Renné T, Pozgajová M, Grüner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gui T, Reheman A, Funkhouser WK, et al. In vivo response to vascular injury in the absence of factor IX: examination in factor IX knockout mice. Thromb Res. 2007;121:225–234. doi: 10.1016/j.thromres.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 39.Benincosa LJ, Chow F-S, Tobia LP, et al. Pharmacokinetics and pharmacodynamics of a humanized monoclonal antibody to factor IX in cynomolgus monkeys. J Pharmacol Exp Ther. 2000;292:810–816. [PubMed] [Google Scholar]
- 40.Nazareth RA, Tomaz LS, Ortiz-Costa S, et al. Antithrombotic properties of Ixolaris, a potent inhibitor of the extrinsic pathway of the coagulation cascade. Thromb Haemost. 2006;96:7–13. doi: 10.1160/TH06-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlasuk GP, Bradbury A, Lopez-Kinninger L, et al. Pharmacokinetics and anticoagulant properties of the factor VIIa-tissue factor inhibitor recombinant Nematode Anticoagulant Protein c2 following subcutaneous administration in man. Dependence on the stoichiometric binding to circulating factor X. Thromb Haemost. 2003;90:803–812. doi: 10.1160/TH03-05-0265. [DOI] [PubMed] [Google Scholar]
- 42.Francischetti IM, Valenzuela JG, Andersen JF, et al. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood. 2002;99:3602–3612. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- 43.Monteiro RQ, Rezaie AR, Ribeiro JM, et al. Ixolaris: a factor Xa heparin-binding exosite inhibitor. Biochem J. 2005;387:871–877. doi: 10.1042/BJ20041738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteiro RQ, Rezaie AR, Bae JS, et al. Ixolaris binding to factor X reveals a precursor state of factor Xa heparin-binding exosite. Protein Sci. 2008;17:146–153. doi: 10.1110/ps.073016308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zingali RB, Bianconi ML, Monteiro RQ. Interaction of bothrojaracin with prothrombin. Haemostasis. 2001;31:273–278. doi: 10.1159/000048073. [DOI] [PubMed] [Google Scholar]
- 46.Monteiro RQ, Zingali RB. Bothrojaracin, a proexosite I ligand, inhibits factor Va-accelerated prothrombin activation. Thromb Haemost. 2002;87:288–293. [PubMed] [Google Scholar]
- 47.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–4838. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]