Abstract
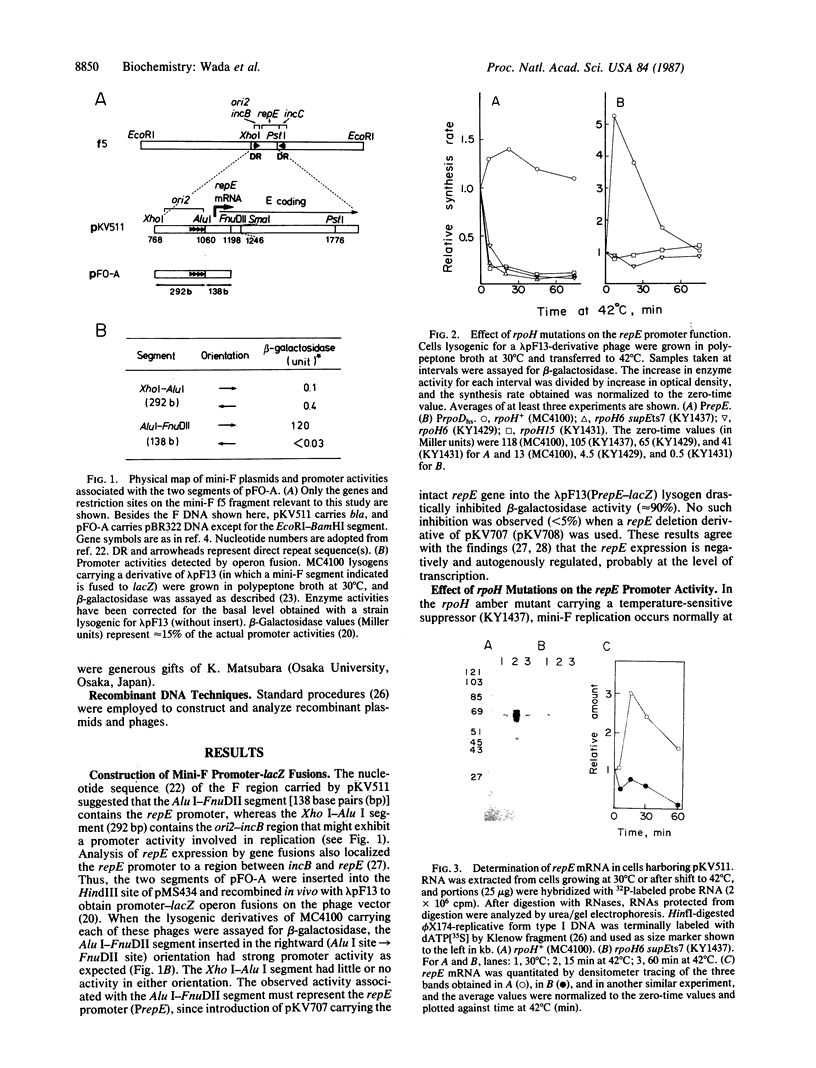
Replication of F factor or mini-F plasmid is strongly inhibited in the rpoH (htpR) mutants of Escherichia coli deficient in the sigma factor (sigma 32) known to be required for heat shock gene expression. Transcription of the mini-F repE gene encoding a replication initiator protein (E protein) was examined by operon fusion and by direct determination of repE mRNA. The synthesis rate and the level of repE mRNA were found to increase transiently upon temperature upshift (30 degrees C to 42 degrees C) in wild-type cells but to decrease rapidly in the rpoH mutants. Thus sigma 32 appeared to be directly involved in transcription of repE whose product, E protein, in turn activates DNA replication from the mini-F ori2 region. This scheme of host-controlled plasmid replication is further supported by the analysis of transcription in vitro: RNA synthesis can be initiated from the repE promoter by a minor form of RNA polymerase containing sigma 32 but not by the major polymerase containing the normal sigma factor sigma 70. The sigma 32-mediated transcription from the repE promoter is strongly inhibited by the E protein. We conclude that transcription of the mini-F repE gene is mediated by the host transcription factor sigma 32 and is negatively controlled by its own product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Bergquist P. L., Downard R. A., Caughey P. A., Gardner R. C., Lane H. E. Analysis of mini-F plasmid replication by transposition mutagenesis. J Bacteriol. 1981 Sep;147(3):888–899. doi: 10.1128/jb.147.3.888-899.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Bennett G. N., Lee F., Schweingruber M. E., Yanofsky C. RNA polymerase interaction at the promoter--operator region of the tryptophan operon of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):153–177. doi: 10.1016/s0022-2836(78)80003-5. [DOI] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Yarmolinsky M. B. Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4423–4427. doi: 10.1073/pnas.83.12.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M., Shigesada K., Imai M. Construction and characterization of plasmid and lambda phage vector systems for study of transcriptional control in Escherichia coli. Gene. 1987;57(1):89–99. doi: 10.1016/0378-1119(87)90180-6. [DOI] [PubMed] [Google Scholar]
- Kline B. C. A review of mini-F plasmid maintenance. Plasmid. 1985 Jul;14(1):1–16. doi: 10.1016/0147-619x(85)90027-7. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Kogoma T., Tam J. E., Shields M. S. Requirement of the Escherichia coli dnaA gene product for plasmid F maintenance. J Bacteriol. 1986 Oct;168(1):440–443. doi: 10.1128/jb.168.1.440-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R., Vaughn V., Lau E. T., VanBogelen R. A., Erickson J. W., Neidhardt F. C. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984 Aug;38(1):175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Lane H. E. Replication and incompatibility of F and plasmids in the IncFI Group. Plasmid. 1981 Jan;5(1):100–126. doi: 10.1016/0147-619x(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki S., Kuribayashi M., Miki T., Horiuchi T. An amber replication mutant of F plasmid mapped in the minimal replication region. Mol Gen Genet. 1983;191(2):231–237. doi: 10.1007/BF00334819. [DOI] [PubMed] [Google Scholar]
- Masson L., Ray D. S. Mechanism of autonomous control of the Escherichia coli F plasmid: different complexes of the initiator/repressor protein are bound to its operator and to an F plasmid replication origin. Nucleic Acids Res. 1986 Jul 25;14(14):5693–5711. doi: 10.1093/nar/14.14.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Ohmori H., Yura T., Nagata T. Requirement of the Escherichia coli dnaA gene function for ori-2-dependent mini-F plasmid replication. J Bacteriol. 1987 Apr;169(4):1724–1730. doi: 10.1128/jb.169.4.1724-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Tsutsui H., Matsubara K. Identification of the minimal essential region for the replication origin of miniF plasmid. Mol Gen Genet. 1984;196(2):373–378. doi: 10.1007/BF00328075. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Nomura T., Fujita N., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase: alteration by fMet-tRNAfMet. Nucleic Acids Res. 1986 Sep 11;14(17):6857–6870. doi: 10.1093/nar/14.17.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokeach L. A., Søgaard-Andersen L., Molin S. Two functions of the E protein are key elements in the plasmid F replication control system. J Bacteriol. 1985 Dec;164(3):1262–1270. doi: 10.1128/jb.164.3.1262-1270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Rokeach L. A., Molin S. Regulated expression of a gene important for replication of plasmid F in E. coli. EMBO J. 1984 Feb;3(2):257–262. doi: 10.1002/j.1460-2075.1984.tb01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacon W., Carey N., Emtage S. The construction and characterisation of plasmid vectors suitable for the expression of all DNA phases under the control of the E. coli tryptophan promoter. Mol Gen Genet. 1980 Feb;177(3):427–438. doi: 10.1007/BF00271481. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokino T., Murotsu T., Matsubara K. Purification and properties of the mini-F plasmid-encoded E protein needed for autonomous replication control of the plasmid. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4109–4113. doi: 10.1073/pnas.83.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Separation of the minimal replication region of the F plasmid into a replication origin segment and a trans-acting segment. Mol Gen Genet. 1982;186(3):372–377. doi: 10.1007/BF00729456. [DOI] [PubMed] [Google Scholar]
- Trawick J. D., Kline B. C. A two-stage molecular model for control of mini-F replication. Plasmid. 1985 Jan;13(1):59–69. doi: 10.1016/0147-619x(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Wada C., Akiyama Y., Ito K., Yura T. Inhibition of F plasmid replication in htpR mutants of Escherichia coli deficient in sigma 32 protein. Mol Gen Genet. 1986 May;203(2):208–213. doi: 10.1007/BF00333956. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Control of F plasmid replication by a host gene: evidence for interaction of the mafA gene product of Escherichia coli with the mini-F incC region. J Bacteriol. 1984 Dec;160(3):1130–1136. doi: 10.1128/jb.160.3.1130-1136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L. A., Phua S. H., Bergquist P. L., Lane H. E. An Mr 29000 protein is essential for mini-F maintenance in E. coli. Gene. 1982 Sep;19(2):173–178. doi: 10.1016/0378-1119(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Imai M., Yura T. The use of operon fusions in studies of the heat-shock response: effects of altered sigma 32 on heat-shock promoter function in Escherichia coli. Mol Gen Genet. 1987 Apr;207(1):24–28. doi: 10.1007/BF00331486. [DOI] [PubMed] [Google Scholar]
- Yura T., Tobe T., Ito K., Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]