1. Structure
The collagens are a family of 28 extracellular matrix glycoproteins. Collagens are composed of 3 polypeptides, called alpha chains. Each α chain contains at least one stiff, rod-like domain of varying length with a glycine at every third residue that can form a triple helix, i.e., collagenous domain. Collagens V and XI are members of the fibril-forming class of collagens, along with collagens I, II, III, XXIV and XXVII. Collagens V and XI comprise a subclass of regulatory fibrillar collagens that co-assemble with collagens I, II and III. All collagens are trimers composed of 3 α chains with at least one triple helical or collagen (COL) domain. Alpha chains of collagens V (P20908 and P25940) and XI (P12107 and P13942) are encoded by genes with different phylogenetic origins, belonging to different clades or `ancestral groups' (clade 2) from collagens I, II and III (clade 1) or XXIV and XXVII (clade 3). Clade 1 also contains the α2(V) gene (P05997; i.e., the α2 chain of collagen V) and the α1(II) gene, termed α3(XI) when part of a collagen XI trimer (P02458). These genes code for multi-domain polypeptide chains with features common to all fibrillar collagens: a signal peptide; an N-terminal non-collagenous region (NC3); a short N-terminal triple helical domain of interrupted Gly-X–Y repeats (COL2), where X and Y can be any amino acid, but are often proline and hydroxyproline, respectively; a short non-collagenous domain (NC2 or `hinge'); the main triple helical or collagenous domain of continuous (Gly-X–Y)n repeats (COL1); and a non-collagenous C-propeptide (NC1) (Fig.1).
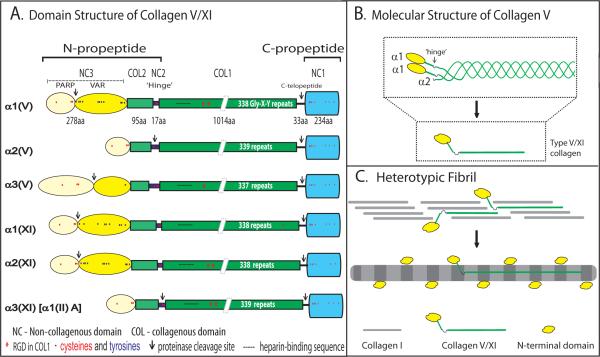
Structure of collagens V and XI.
(A) Collagens V/XI are trimers of 3 α chains. The α chain domain structures are illustrated, along with amino acid numbers (#aa) for the prototype α1(V) chain (α1 chain of collagen V). All have a C-terminal propeptide (NC1 [ ]) domain. A short stretch of non-collagenous sequence (C-telopeptide) remains at the C-terminal end after cleavage of the non-collagenous C-propeptide. The major collagenous domain (COL1) is composed of uninterrupted (Gly-X–Y)337–339 repeats (1011 to 1017 amino acids). This domain forms a triple helix with 2 other α chains. COL2 is the N-terminal collagenous domain separated from the main collagenous domain (COL1) by a non-collagenous (NC2) `hinge' region. The domains N-terminal to this hinge contain the major differences among the different chains and also direct the functional properties of these regulatory collagens. In all chains, except α2(V) and α3(XI), the N-terminal NC3 domain is composed of a PARP (proline- and arginine-rich protein) domain [
]) domain. A short stretch of non-collagenous sequence (C-telopeptide) remains at the C-terminal end after cleavage of the non-collagenous C-propeptide. The major collagenous domain (COL1) is composed of uninterrupted (Gly-X–Y)337–339 repeats (1011 to 1017 amino acids). This domain forms a triple helix with 2 other α chains. COL2 is the N-terminal collagenous domain separated from the main collagenous domain (COL1) by a non-collagenous (NC2) `hinge' region. The domains N-terminal to this hinge contain the major differences among the different chains and also direct the functional properties of these regulatory collagens. In all chains, except α2(V) and α3(XI), the N-terminal NC3 domain is composed of a PARP (proline- and arginine-rich protein) domain [ ] and a variable domain (VAR [
] and a variable domain (VAR [ ]). The α2(V) and α3(XI) chains are more similar to collagens I, II, III and are clade (ancestral group) 1 genes versus clade 2 for the others. Enzymatic processing of the chains occurs between the PARP and VAR domains for clade 2 and in the hinge region for the clade 1 gene products. The N- and C-proteinase cleavage sites are indicated (↓). (B) The structure of the [α1(V)]2α2(V) isoform of collagen V is presented. The C-propeptide was removed, the COL1 domains formed a triple helix and the partially processed N-terminal domains are illustrated. While processing of the α2(V) and α3(XI) chains results in cleavage at the hinge region, the domain remains non-covalently associated with the partially processed NC3 domains (not shown). (C) Structure of heterotypic collagen I/V fibrils. Collagens V/XI co-assemble with the quantitatively major fibril-forming collagens I, II and III to form heterotypic fibrils. The COL1 domains assemble into a staggered pattern. The partially processed NC3 domains of collagens V/XI will not fit into the staggered pattern and must be localized to the fibril surface. The hinge (NC2) domain is flexible and allows the COL2 domain to project perpendicular to the fibril axis in the gap region with the NC3 domain on the fibril surface.
]). The α2(V) and α3(XI) chains are more similar to collagens I, II, III and are clade (ancestral group) 1 genes versus clade 2 for the others. Enzymatic processing of the chains occurs between the PARP and VAR domains for clade 2 and in the hinge region for the clade 1 gene products. The N- and C-proteinase cleavage sites are indicated (↓). (B) The structure of the [α1(V)]2α2(V) isoform of collagen V is presented. The C-propeptide was removed, the COL1 domains formed a triple helix and the partially processed N-terminal domains are illustrated. While processing of the α2(V) and α3(XI) chains results in cleavage at the hinge region, the domain remains non-covalently associated with the partially processed NC3 domains (not shown). (C) Structure of heterotypic collagen I/V fibrils. Collagens V/XI co-assemble with the quantitatively major fibril-forming collagens I, II and III to form heterotypic fibrils. The COL1 domains assemble into a staggered pattern. The partially processed NC3 domains of collagens V/XI will not fit into the staggered pattern and must be localized to the fibril surface. The hinge (NC2) domain is flexible and allows the COL2 domain to project perpendicular to the fibril axis in the gap region with the NC3 domain on the fibril surface.
The V/XI α chains are assembled as procollagens with terminal N- and C-propeptides that are cleaved by specific enzymes to produce the mature collagen molecule. The first half of the human α1(V) N-propeptide has 73% homology with the human α1(XI) chain; there is 83% homology between the COL1 domains and ~76% between the C-propeptides. These N- and C-propeptides differ from those of the other fibrillar collagens. It is based on these properties that collagens V and XI are grouped as a separate subclass. The C-propeptides are removed by cleavage of the NC1 domain. The N-propeptide is composed of the NC3, COL2 and NC2 domains. The NC3 domain of all V/XI α chains, except α2(V) and α3(XI), have a PARP (proline- and arginine-rich protein) domain and a variable (VAR) domain. Chain-specific differences in the N-propeptide result in differences in N-propeptide processing. Processing of the N-propeptide involves cleavage in the NC2 domain in the α2(V) and α3(XI) chains, and between the PARP and variable domains in the remaining chains, resulting in the retention of an N-terminal non-collagenous domain, a rigid COL2 domain and a hinge (NC2) region.
Different collagen V/XI α chains assemble to form distinct isoforms: [α1(V)]2α2(V); [α1(V)]3; α1(V)α2(V)α3(V); α1(XI)α2(XI)α3(XI); as well as collagen V/XI hybrids such as [α1(XI)]2α2(V). Although collagens V and XI were originally characterized as separate collagen types, it has become clear that they are a single collagen type with multiple tissue-specific isoforms (Hoffman et al. 2010).
2. Function
The major isoforms of collagen V/XI, [α1(V)]2α2(V) and α1(XI)α2(XI)α3(XI), co-assemble with collagens I, II and III to form heterotypic fibrils in a tissue-specific manner. Collagen V is expressed ubiquitously in the eye, with very high levels in Bowman's layer and the corneal stroma. Collagen XI isoforms are highly expressed in the vitreous. Full characterization of isoform expression throughout the eye remains to be determined. The V/XI class of regulatory fibrillar collagens is characterized by incomplete processing of the N-propeptide. After cleavage by N-proteinases, a large globular region of the terminal NC3 domain is retained in the collagen V/XI molecules (Fig. 1B). This partial processing requires heterotypic fibrils formed by collagens V/XI with collagens I, II and III to assemble in a manner whereby the large COL1 domains of collagens V/XI participate in the formation of a staggered array within the heterotypic fibril. However, the uncleaved, globular region in the NC3 domain is incompatible with the staggered organization of the COL1 domains, and the hinge and COL2 domains allow it to project outward in the gap region with the NC3 domain on the surface of the heterotypic fibril (Fig. 1C). Studies done to date show that the regulatory properties of collagens V/XI reside in the retained portion of the N-propeptides. The functions of other collagen V/XI isoforms have not been elucidated.
Collagens V and XI have been shown to regulate fibrillogenesis by nucleating collagen fibril formation. A targeted deletion in the Col5a1 gene is embryonic lethal in a mouse model due to a virtual lack of fibril formation in the mesenchyme (Wenstrup et al. 2004). This occurs despite the presence of normal type I collagen synthesis and secretion and demonstrates that collagen V is essential for the assembly of collagen I-containing fibrils. A similar situation occurs in two mouse models in which the production of normal collagen XI is compromised.
A number of fibril assembly assays modulating corneal collagen V in culture and mouse models have demonstrated that reducing the percentage of collagens V/XI relative to collagens I and II results in larger diameter fibrils. In cell based analyses this also results in decreased numbers of collagen fibrils assembled (Birk 2001; Segev et al. 2006; Wenstrup et al. 2004). This, coupled with the lack of fibril assembly in collagen V null embryonic mice, indicates a major regulatory role in nucleation of fibril assembly.
The relative amounts of collagens V/XI varies with tissue type. Tissues with less collagen V/XI, such as sclera, have larger diameter fibrils than those with more, such as the cornea or vitreous. The levels of collagens V/XI vary throughout the tissues of the eye and controlling the number of nucleation events in a tissue-specific manner translates into tissue-specific regulation of fibril diameter. The regulatory heterotypic interactions also occur closely associated with cell surfaces. A direct or indirect association of the cell with collagen V/XI would permit cellular control of fibril deposition and organization. However, the large amount of type V collagen incorporated into corneal fibrils relative to other tissues suggests that the N-terminal domain may have an additional regulatory role on the corneal fibril surface. This domain contains sulfated tyrosines and there may be steric and/or electrostatic interactions modulating the fibril surface.
3. Disease Involvement
There are two well-characterized genetic diseases linked to collagen V/XI and involving ocular pathology. Mutations in collagen V have been identified in more than half of the cases of classic (types I and II) Ehlers Danlos syndrome (EDS). Mutations in collagen XI (α1 and α3) result in Stickler Syndrome with ocular involvement. Both diseases are generalized connective tissue disorders with broad tissue involvement. Patients with these disorders have dysfunctional regulation of collagen fibrillogenesis. In the classic form of EDS with haploinsufficiency in collagen V, corneas were steep and 23% thinner, with irregular/asymmetric surfaces compared to unaffected patients. Eyelids were floppy, causing eyelid eversion with minimal applied force. Patients with Stickler syndrome with defects in type XI collagen present with abnormal vitreous (partly due to abnormally thick fiber bundles), cataracts, glaucoma, myopia and retinal tears and/or detachment. There are also leaky retinal vessels, lens dislocation and lens opacity.
There are mouse models for both of these genetic connective tissue disorders. The haploinsufficient collagen V (Col5a1+/−) mouse demonstrated abnormal regulation of fibril assembly in various tissues. The cornea has collagen fibrils with diameters twice that of wild type, ~25% lower fibril density, and a thinner corneal stroma comparable to that observed in human patients with similar mutations. Structural defects in the lens and vitreous of an α1(II)/α3(XI) mouse model mirror ocular features found in the human patients. The ocular phenotype in the collagen α1(XI) models has not been fully evaluated, but the animals display severe chondrodysplasia.
Most of the work has focused on the role(s) of collagen V/XI in regulation of fibrillogenesis. However, in the past several years there have been numerous studies indicating a role for collagen V immunity in lung transplant rejection and bronchiolitis obliterans syndrome. Data are beginning to accumulate indicating a role for collagen V auto-immunity in a variety of diseases.
4. Future Studies
The regulatory roles of the more ubiquitous isoforms in regulation of fibril nucleation are being studied. However, do these collagens interact with the cell surface directly or as part of a complex? This would provide a mechanism for cell-directed matrix assembly and requires further examination. It is clear that there are developmental changes in isoform expression; understanding the associated functional changes will contribute to our understanding of development and regeneration. One key area for future study involves the elucidation of the tissue-specific or developmental stage-specific functions of the different collagen V/XI isoforms. Do all isoforms form heterotypic fibrils or is there a non-fibrillar function? If there is a non-fibrillar function of a specific isoform, the suprastructural organization and specific interactions need to be elucidated. Are there critical regions in each chain that confer specific functions, or is it the recruitment of non-collagenous molecules (e.g. proteoglycans, glycoproteins, growth factors) that interact with one chain and not the other? In addition, further study of mouse models deficient in different isoforms (or combinations of isoforms) will contribute to our understanding of isoform-specific functions and their roles in development, repair and pathobiology of the eye and other tissues.
Acknowledgments
This work was supported by NIH grants EY05129, AR44745 (DEB) and NIH/NIAMS NRSA Post Doctoral Fellowship AR056937 (SMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Hoffman GG, Branam AM, Huang G, Pelegri F, Cole WG, Wenstrup RM, Greenspan DS. Characterization of the six zebrafish clade B fibrillar procollagen genes, with evidence for evolutionarily conserved alternative splicing within the pro-alpha1(V) C-propeptide. Matrix Biol. 2010;29:261–275. doi: 10.1016/j.matbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev F, Heon E, Cole WG, Wenstrup RJ, Young F, Slomovic AR, Rootman DS, Whitaker-Menezes D, Chervoneva I, Birk DE. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci. 2006;47:565–573. doi: 10.1167/iovs.05-0771. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]