Summary

The switch from mitosis to meiosis is a unique feature of germ cell development. In mammals, meiotic initiation requires retinoic acid (RA), which activates meiotic inducers including Stra8, but how the switch to meiosis is controlled in male germ cells (spermatogonia) remains poorly understood. Here we examine the role of the Doublesex-related transcription factor DMRT1 in adult spermatogenesis using conditional gene targeting in the mouse. Loss of Dmrt1 causes spermatogonia to precociously exit the spermatogonial program and enter meiosis. Dmrt1 therefore determines whether male germ cells undergo mitosis and spermatogonial differentiation or meiosis. Loss of Dmrt1 in spermatogonia also disrupts cyclical gene expression in Sertoli cells. DMRT1 acts in spermatogonia to restrict RA responsiveness, directly repress Stra8 transcription, and activate transcription of the spermatogonial differentiation factor Sohlh1, thereby preventing meiosis and promoting spermatogonial development. By coordinating spermatogonial development and mitotic amplification with meiosis, DMRT1 allows abundant, continuous production of sperm.
Introduction
Germ cells are the agents of heredity, providing the genetic link between generations. To perform this role germ cells must be able to undergo mitotic divisions to maintain and expand the stem/progenitor cell population and meiotic divisions to halve the chromosome number for gametogenesis. In mammals the timing of meiotic entry and number of gametes are sex-specific (Handel and Schimenti, 2010). Meiosis in females begins in the fetus and each fetal germ cell can produce a maximum of just one oocyte by meiotic division. Meiosis in males begins at puberty and sperm are produced throughout reproductive life. Spermatogenesis has three phases: a mitotic proliferative phase, two reductive divisions of meiosis, and then a postmeiotic phase of spermiogenesis. The proliferative phase involves spermatogonia, stem/progenitor cells maintained and highly amplified by the mitotic divisions preceding meiosis. This amplification lets a tiny population of spermatogonial stem cells support continuous production of staggering numbers of sperm (>1000/second in humans).
In seminiferous tubules of the testis, undifferentiated spermatogonia reside in a seminiferous epithelium adjacent to the surrounding basement membrane. The undifferentiated spermatogonia consist of single cells (As spermatogonia, forming the principal stem cell component in steady state spermatogenesis) and chains of two to sixteen linked spermatogonia (termed Apr and Aal) (de Rooij and Russell, 2000; Nakagawa et al., 2010).
Spermatogonial differentiation starts when undifferentiated spermatogonia form A1 spermatogonia, a transition occurring in cycles with a species-specific period, 8.6 days in mice (de Rooij, 1998). A1 spermatogonia undergo five additional rounds of mitotic division and differentiate to form B spermatogonia. The B spermatogonia divide and differentiate into preleptotene spermatocytes that enter meiosis (de Rooij and Russell, 2000). While the seminiferous epithelial cycle initiates every 8.6 days, mature spermatids take 35 days to differentiate, encompassing four rounds of initiation. As a result, developing germ cells accumulate in layers above the basal population of spermatogonia. The precise cellular complement of these layers varies during the cycle, allowing its division into distinct stages (I to XII in the mouse) (Hess and de Franca, 2008; Oakberg, 1956; Russell et al., 1990). Of particular significance is stage VII, when undifferentiated spermatogonia enter the cycle of the seminiferous epithelium and begin differentiation (de Rooij, 1998). The cycle initiates asynchronously along the length of the tubules; as a result, distinct stages are present at different tubule positions, allowing continuous production of functional sperm.
Sertoli cells are the only somatic cells in the seminiferous tubules and surround the germ cells throughout differentiation, supporting spermatogenesis. During the cycle of the seminiferous epithelium, Sertoli cells exhibit cyclical gene expression (Yomogida et al., 1994), possibly matching their metabolism to the requirements of nearby germ cells and/or directing cyclical germ cell development.
In mammals the switch from mitosis to meiosis requires retinoic acid (RA). Regulation of this switch is best understood in the fetal gonad: in the fetal ovary accumulation of RA triggers meiosis by activating retinoic acid receptor-dependent transcription of meiotic inducers including Stra8 (Bowles and Koopman, 2007). Fetal males avoid meiosis by testicular expression of CYP26B1, a P450 enzyme that oxidizes RA to an inactive metabolite (Bowles et al., 2006; Koubova et al., 2006).
RA signaling regulates all three phases of male spermatogenesis. Vitamin A (the dietary precursor of RA) is essential for fertility, and vitamin A depletion (VAD) arrests spermatogonia prior to differentiation (McCarthy and Cerecedo, 1952; Thompson et al., 1964). Readministration of vitamin A to VAD mice synchronously reinitiates the seminiferous epithelial cycle at stage VII (van Pelt and de Rooij, 1990).
RA promotes meiosis in males by activating Stra8, as in females, and STRA8 is robustly expressed during stage VII of the seminiferous epithelial cycle in preleptotene spermatocytes entering meiosis (Oulad-Abdelghani et al., 1996; Vernet et al., 2006a; Zhou et al., 2008b). In Stra8 mutant mice, the majority of preleptotene spermatocytes fail to enter meiosis (Anderson et al., 2008; Mark et al., 2008) suggesting that STRA8 and RA control the switch from spermatogonial differentiation to meiosis. Weaker RA-dependent STRA8 expression occurs in spermatogonia, consistent with RA regulation of spermatogonial differentiation (Ghyselinck et al., 2006; Koubova et al., 2006; Zhou et al., 2008b). These observations suggest that variation in RA levels and/or RA responsiveness controls spermatogonial differentiation and meiotic initiation, and highlight stage VII as a likely period of high RA signaling activity.
Germ cells likely play a key role in controlling the cycle of the seminiferous epithelium, although the relative roles of germ cells and Sertoli cells are unresolved. Sertoli cell gene expression continues to cycle in germ cell depleted gonads (Timmons et al., 2002; Yoshida et al., 2006), indicating an intrinsic cycle. However, germ cells continue to cycle when Sertoli cell cyclical gene expression is arrested by Sertoli-specific deletion of the RA receptor RARα (Vernet et al., 2006a), suggesting they also have an intrinsic cycle. Stronger evidence for an intrinsic germ cell cycle comes from transplantation of rat spermatogonia into mouse testes; the xenografted germ cells follow the 13 day rat cycle instead of the 8.6 day mouse cycle despite the presence of mouse Sertoli cells (Franca et al., 1998). Thus germ cell intrinsic control of the cycle may be dominant over Sertoli cell control.
Germ cell intrinsic control of the semiferous epithelial cycle likely involves modulation of RA signaling. Spermatogonia express retinoic acid receptors (Vernet et al., 2006b) and in culture exogenous RA causes them to differentiate and enter meiosis (Dann et al., 2008). In vivo, however, undifferentiated spermatogonia do not enter meiosis at stage VII like the neighboring type B spermatogonia. Instead, a subset of these cells transition to A1 differentiating spermatogonia and enter the cycle, a process that also requires RA. These distinct behaviors in the same tubule stage suggest different levels of RA responsiveness. Here we present evidence that the conserved transcription factor DMRT1 regulates RA responsiveness in undifferentiated spermatogonia by two distinct mechanisms to control the mitosis to meiosis switch.
DMRT1 is a gonad-specific transcription factor related to the invertebrate sexual regulators Doublesex and MAB-3 (Raymond et al., 1998). Human deletions removing DMRT1 cause XY sex reversal, and DMRT1 homologs are required for primary sex determination in fish, birds, and amphibians with diverse sex determination systems (Matsuda et al., 2002; Raymond et al., 1999; Smith et al., 2009; Yoshimoto et al., 2008; Zarkower, 2001). In mice, Dmrt1 is essential in Sertoli cells for differentiation and cell cycle control and in germ cells for maintenance of embryonic germ cell identity and formation of juvenile spermatogonia (Kim et al., 2007a; Krentz et al., 2009; Raymond et al., 2000).
Here we use conditional gene targeting in mice to investigate DMRT1 in adult spermatogenesis. We find that DMRT1 inhibits meiosis in undifferentiated spermatogonia by limiting RA-dependent transcription generally, and by specifically blocking robust Stra8 transcriptional induction. DMRT1 also promotes spermatogonial development by activating spermatogonial differentiation genes including Sohlh1. We suggest that DMRT1 thereby coordinates spermatogonial differentiation with the mitosis/meiosis switch.
Results
DMRT1 is expressed throughout spermatogonial development
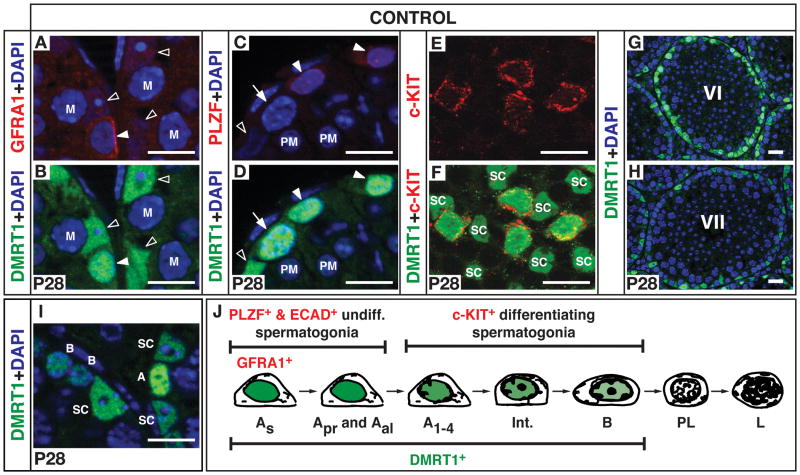
DMRT1 is expressed in germ cells and Sertoli cells, starting when the gonadal primordium (genital ridge) forms (Lei et al., 2007; Raymond et al., 1999). To evaluate its role in adult spermatogenesis we first examined the dynamics of DMRT1 expression in adult germ cells (Figure 1). DMRT1 was expressed in undifferentiated spermatogonia including GFRA1-positive cells (mainly As and Apr) and PLZF-positive cells (comprising the entire As, Apr and Aal population) (Figures 1A-1D). DMRT1 also was expressed in differentiating spermatogonia (c-KIT-positive A1-4, In, and B) (Figures 1E and 1F). At stage VI of the seminiferous epithelial cycle DMRT1 was present in B spermatogonia (Figure 1G), whereas DMRT1 was absent from premeiotic (preleptotene) cells, which form at stage VII (Figure 1H), and from meiotic and postmeiotic germ cells (Figures 1G and 1H). DMRT1 expression was strong in undifferentiated spermatogonia and lower in differentiating spermatogonia (Figure 1I, data not shown). In summary, DMRT1 is expressed in all mitotic spermatogonia but expression decreases with the onset of spermatogonial differentiation and disappears at the initiation of meiosis (Figure 1J). DMRT1 is continuously expressed in Sertoli cells (Figure 1, data not shown).
Figure 1. DMRT1 is expressed in spermatogonia and Sertoli cells, but not in meiotic and postmeiotic germ cells.
Immunofluorescence (IF) of testes from 28 day control testes. (A,B) Section IF showing DMRT1 in GFRA1-positive undifferentiated spermatogonia (filled arrowhead; primarily As and Apr, and a small proportion of Aal), and Sertoli cells (open arrowheads). (C,D) Section IF showing DMRT1 in PLZF-positive undifferentiated spermatogonia (filled arrowheads) but not postmeiotic cells (spermatids; “PM”). Arrow: differentiating spermatogonium. (E,F) Whole-mount IF of seminiferous tubules showing DMRT1 in c-KIT-positive differentiating spermatogonia. SC: Sertoli cells. (G,H) Section IF of DMRT1 at different tubule stages. DMRT1-positive germ cells in stage VI tubules (G) are mainly type B spermatogonia. In stage VII tubules (H) these have become preleptotene spermatocytes and no longer express DMRT1 (DMRT1-positive cells are Sertoli cells and A spermatogonia). (I) Section IF showing higher DMRT1 expression in type A spermatogonia relative to type B spermatogonia and Sertoli cells. Scale bars: 20 micron. (J) Schematic diagram showing progression of spermatogonial development and expression of markers used in panels A-F.
Conditional deletion of Dmrt1 in undifferentiated spermatogonia disrupts spermatogenesis
Deletion of Dmrt1 in embryos disrupts germ cell development prior to formation of spermatogonia, causing complete azoospermia (Kim et al., 2007a; Krentz et al., 2009; Raymond et al., 2000). To bypass the embryonic requirement for Dmrt1, we conditionally deleted Dmrt1 with a Ngn3-cre transgene that is active within the adult testis only in undifferentiated spermatogonia (Figures S1A to S1E) (Sada et al., 2009; Yoshida et al., 2004). To confirm that Ngn3-cre was not active in Sertoli cells we used a cre-responsive Yfp reporter transgene. Fewer than 0.5% of Sertoli cells expressed YFP (3/653 Sertoli cells; Figure S1E); any phenotypes must therefore result from deletion of Dmrt1 in germ cells. Because Ngn3-cre is active in undifferentiated spermatogonia, we first examined DMRT1 expression in these cells, which express E-Cadherin (ECAD). As expected, seminiferous tubules from conditionally targeted testes (“mutant” hereafter) had ECAD-positive undifferentiated spermatogonia lacking DMRT1 (Figures 2A-2D). Ngn3-cre also is active in a subset of neonatal germ cells (Yoshida et al., 2006); the resulting mutant cells arrested during the first postnatal week and were not further analyzed in this study. Because Ngn3 is not expressed in the vast majority of steady-state spermatogonial stem cells (Nakagawa et al., 2010), the mutant testes should continuously produce undeleted spermatogonia that are mutated as they develop (Figure S1B). Indeed, analysis of testes at ages up to six months confirmed the continued production of mutant spermatogonia (below and data not shown).
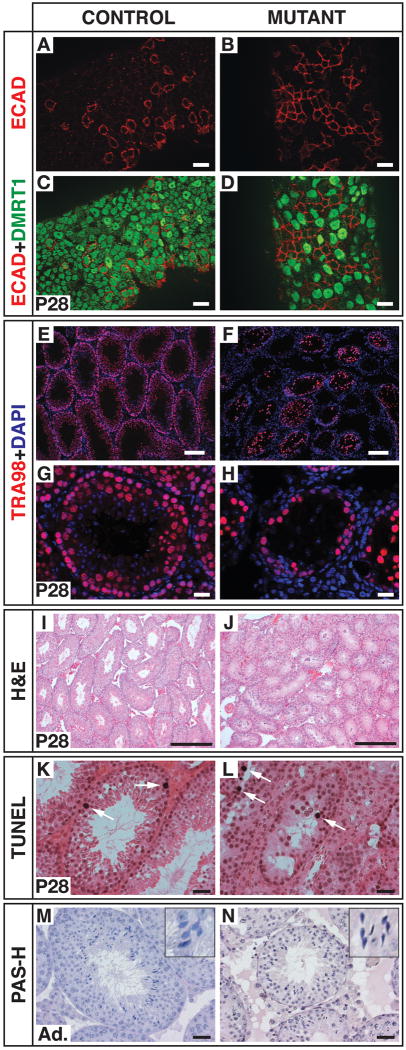
Figure 2. Loss of DMRT1 in early spermatogonia disrupts spermatogenesis.
(A-D) Whole-mount analysis of seminiferous tubules. ECAD IF alone (A,B) and merged with DMRT1 IF (C,D). DMRT1-positive Sertoli cells (with dark spots), are not affected by Ngn3-cre conditional mutation. Scale bars: 20 microns. (E-H) Section IF staining for pan-germ cell marker TRA98 and DAPI at low magnification (E,F; scale bars: 50 microns) and higher magnification (G,H; scale bars: 20 microns). (I,J) Hematoxylin and eosin staining. Scale bars: 100 microns. (K,L) TUNEL labeling. Arrows: apoptotic cells. Scale bars: 20 microns. (M,N) Periodic acid/Schiff (PAS-H) staining of adult (eight-week-old) testes. Insets: darkly staining elongating spermatids. Scale bars: 20 microns.
Mutant testes were small and had greatly reduced numbers of germ cells (Figures 2E-2J). This reduction could potentially result either from elevated apoptotic cell death or from a defect in some aspect of spermatogenesis. TUNEL labeling indicated that apoptosis in mutant testes was not elevated relative to controls at one, two and six months (Figures 2K and 2L; data not shown). We conclude that the reduced germ cell numbers in mutants do not result from inappropriate cell death. Although most tubule sections contained germ cells, some were empty, and the proportion of empty tubule sections was stable over time. Because Ngn3-cre is active in a small fraction of spermatogonial stem cells (Nakagawa et al., 2010; Yoshida et al., 2004), these empty tubule sections could have arisen from loss of spermatogonial stem cells, reduced progenitor cell expansion, or both.
Mutant germ cells undergo uncontrolled meiotic entry
To determine why germ cell numbers were reduced in mutant testes we examined spermatogenesis. Adult mutant testes had spermatogonia, spermatocytes, and spermatids (Figures 2M and 2N), indicating that mutant germ cells can complete meiosis. However, the control of meiotic initiation appeared highly abnormal in mutants. Normally STRA8 is expressed at low levels in spermatogonia and at greatly elevated levels in preleptotene spermatocytes (Vernet et al., 2006a; Zhou et al., 2008a). Strikingly, however, mutant testes had single, paired, and chained ECAD-positive spermatogonia with high STRA8 expression (Figures 3A-3D). Also, while robust STRA8 expression was present only at stage VII in control tubules, cells strongly expressing STRA8 were present in all tubule sections of mutant testes that contained germ cells (Figures 3E and 3F).
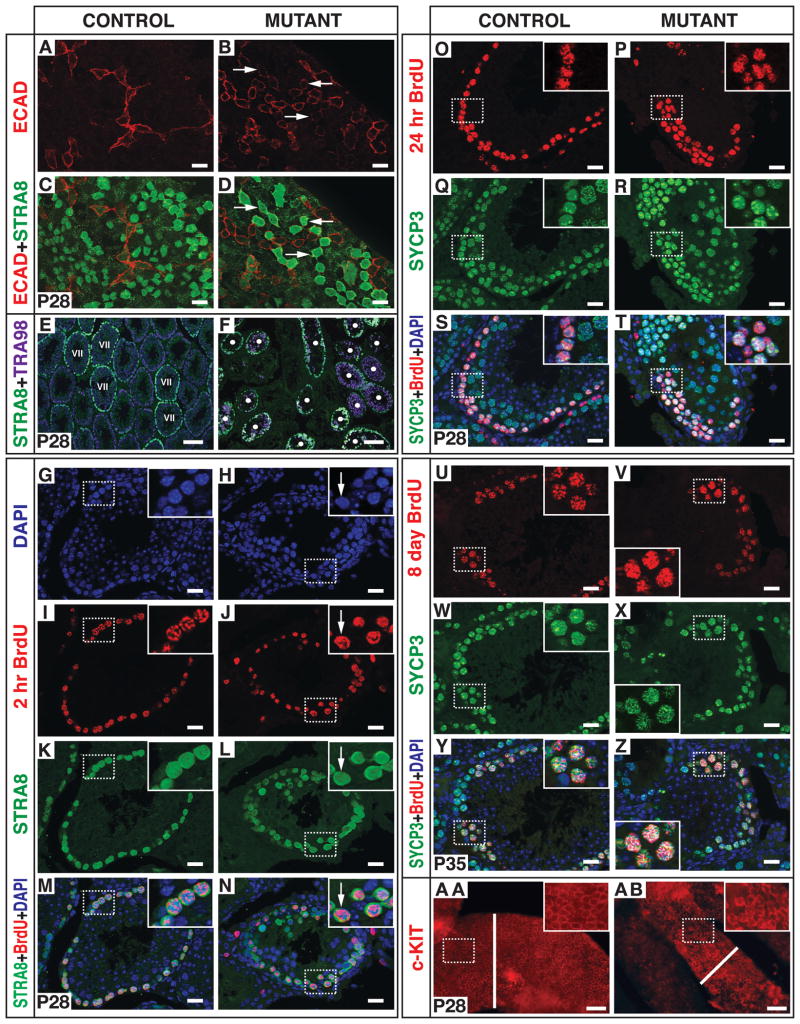
Figure 3. Precocious and uncontrolled meiotic initiation in Dmrt1 mutant germ cells.
(A-D) Whole-mount IF staining of seminiferous tubules for ECAD and STRA8. Arrows indicate examples of single, paired, and aligned double-positive germ cells found only in mutant. Scale bars: 20 microns. (E,F) Section IF for STRA8 and TRA98. Control tubules at stage VII are indicated (“VII”). White dots: mutant tubules with germ cells. Scale bars: 50 microns. (G-N) Section IF two hours after BrdU labeling. Arrow: STRA8- and BrdU-positive cell with spermatogonial DAPI morphology. Scale bars: 20 microns. (O-T) Section IF 24 hr after BrdU labeling. Insets: BrdU-labeled SYCP3-positive leptotene spermatocytes in control and similar cells in mutant. Scale bars: 20 microns. (U-Z) Section IF eight days after BrdU labeling. Insets: BrdU-labeled pachytene spermatocytes with SYCP3 localized to synaptonemal complexes. Scale bars: 20 microns. (AA, AB) Whole mount IF of seminiferous tubules for c-KIT. Bars indicate diameters of seminiferous tubules. Scale bars: 50 microns.
The widespread presence of STRA8-positive germ cells in mutant testes might reflect meiotic entry independent of the seminiferous epithelial cycle or could result from arrest and accumulation of STRA8-positive cells. To distinguish between these possibilities we asked whether the mutant cells with high STRA8 expression were actively dividing, using a brief (2 hr) treatment with 5-bromo-2-deoxyurodine (BrdU) to detect DNA replication (Figures 3G to 3N). Controls had robust BrdU incorporation in STRA8-positive spermatocytes at stage VII as they entered meiosis (Figures 3G, 3I, 3K, and 3M). Mutants also had robust BrdU incorporation in STRA8-positive cells, but these were present in virtually all tubule cross-sections with germ cells, and some labeled cells had morphology typical of undifferentiated spermatogonia rather than preleptotene spermatocytes (Figures 3H, 3J, 3L, and 3N). BrdU- and STRA8-positive cells were abundant in mutant testes at all times tested, from two weeks to six months (Figure 3J and data not shown), indicating that this population arises continuously from sustained activity of Ngn-3-cre in undifferentiated spermatogonia. We conclude that loss of DMRT1 in undifferentiated spermatogonia causes uncontrolled meiotic entry, independent of the seminiferous epithelial cycle, rather than arrest of differentiation.
To confirm that unregulated meiotic entry of mutant spermatogonia leads to bona fide meiosis, we followed the fates of mutant cells. After twenty-four hours, BrdU-labeled control and mutant germ cells had entered meiosis as leptotene spermatocytes and had accumulated SYCP3 in nuclear foci (Figures 3O to 3T). Seven days later, control and mutant BrdU-labeled germ cells had reached pachynema and SYCP3 was localized to the synaptonemal complex (Figures 3U to 3Z). These data indicate that although spermatogonia initiate meiosis from precocious developmental stages and at inappropriate stages of the seminiferous epithelial cycle, they can proceed normally into the meiotic program.
Precocious meiotic entry truncates spermatogonial proliferation and differentiation
The finding that mutant spermatogonia express STRA8 while still positive for ECAD and can reach meiotic prophase within a week suggests that the program of mitotic proliferation and spermatogonial differentiation is likely to be truncated in the mutant testes. This “skipping” of developmental stages by mutant cells, either before or after the onset of spermatogonial differentiation, would be expected to severely deplete the population of differentiating spermatogonia. Indeed whole-mount analysis of mutant tubules showed that differentiating (c-KIT-positive) spermatogonia were greatly depleted (Figures 3AA and 3AB). We confirmed this depletion by counting ECAD-positive and c-KIT-positive germ cells in control versus mutant testes. ECAD-positive undifferentiated spermatogonia were relatively normal in the mutant (149+/-6 cells/mm tubule in mutant vs 116+/-7 cells/mm in control; p = 0.03), but c-KIT-positive germ cells were significantly depleted (735 +/- 156 cells/mm in mutant versus 2030 +/- 162 cells/mm in control; p = 0.015). These results can explain why germ cell numbers are severely reduced in mutants even though germ cell development is not blocked: amplifying divisions of the differentiating spermatogonial population are skipped by mutant cells when they precociously abandon the spermatogonial program to switch from mitosis to meiosis. The slight increase in ECAD positive cells in the mutant may reflect a delay in their developmental progression as they switch to the meiotic program or a compensatory amplification in response to loss of DMRT1.
Kinetics of the transition from mitotic to meiotic programs
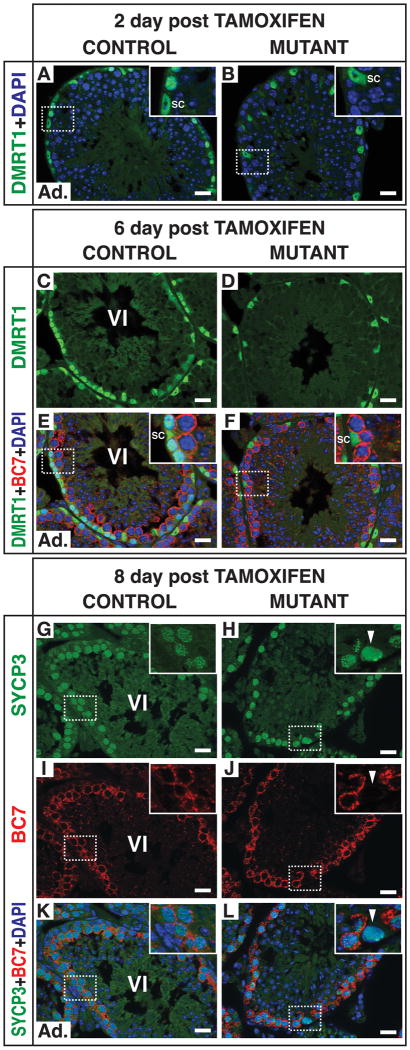
To further examine the transition of mutant spermatogonia into meiosis, we used a tamoxifen-inducible cre transgene (Ubc-cre/ERT2) to delete Dmrt1 in spermatogonia at a defined time and follow their fates. We deleted Dmrt1 in adults and examined germ cell development over the following eight days. Ubc-cre/ERT2 is ubiquitously expressed, but DMRT1 expression declined rapidly in germ cells while remaining constant in Sertoli cells over the course of the experiment (Figure 4; two animals of each genotype examined at each time point). The differential stability of DMRT1 in the two cell types allowed its role in spermatogonia to be examined specifically. Two days after tamoxifen injection most spermatogonia lacked DMRT1 expression (Figures 4A and 4B) and ectopic STRA8 was detectable in mutant spermatogonia (not shown). Six days post-tamoxifen, mutant tubule sections with abundant BC7-positive zygotene/pachytene spermatocytes (found in stage VI in controls) lacked the normal basal layer of spermatogonia (Figures 4C to 4F). Eight days after tamoxifen, cells with leptotene patterns of SYCP3 accumulation (normally found in stage VIII when BC7 is absent) were ectopically present in tubules with abundant BC7 expressing cells (Figures 4G to 4L, arrowheads; compare 4K and 4L). TUNEL labeling confirmed that apoptosis was not elevated in mutants (not shown). This experiment reveals that loss of DMRT1 in germ cells causes rapid depletion of the spermatogonial population and ectopic appearance of meiotic cells at inappropriate stages of the seminiferous epithelial cycle. We conclude that mutant spermatogonia, differentiated and undifferentiated, can enter meiosis within a week after loss of DMRT1, whereas undifferentiated Aal spermatogonia normally require 8.6 days to form preleptotene spermatocytes and a further twenty hours to reach leptonema. These results confirm the uncontrolled meiosis and truncation of the spermatogonial differentiation program observed in the Ngn3-cre deletion mutants.
Figure 4. Kinetics of the switch from mitosis to meiosis in mutant spermatogonia.
Conditional deletion of DMRT1 in adult testes using the tamoxifen inducible cre transgene Ubc-cre/ERT2. (A,B) Section IF for DMRT1two days after tamoxifen injection showing DMRT1 in spermatogonia and Sertoli cells (SC) in control but only in Sertoli cells in mutant. (C-F) Section IF six days after tamoxifen injection. BC7-positive cells at stage VI are primary spermatocytes (late zygotene to mid-pachytene) with underlying layer of DMRT1-positive B spermatogonia (SC: Sertoli cell). Inset shows missing layer of spermatogonial cells in mutant; DMRT1-positive cells in mutant are Sertoli cells. (G-L) Section IF eight days after tamoxifen injection. In stage VI control tubule the germ cells underlying the layer of BC7- and SYCP3-positive primary spermatocytes are negative for SYCP3. In mutant tubules with abundant BC7- and SYCP3-positive primary spermatocytes, underlying cells have SYCP3 distribution typical of leptotene spermatocytes (arrowheads), not normally found together with BC7-positive spermatocytes. Scale bars: 20 microns.
Uncontrolled meiosis in mutant germ cells requires RA
To ask whether uncontrolled meiotic initiation by mutant spermatogonia requires RA and Stra8 induction, we examined vitamin A deficient (VAD) animals (Figure 5). Lack of RA causes wild type germ cells to arrest as undifferentiated spermatogonia (van Pelt and de Rooij, 1990). As expected, in VAD controls undifferentiated spermatogonia arrested development and all tubule sections were STRA8-negative (Figures 5A and 5C). Similarly, spermatogonia in VAD mutants lacked STRA8, indicating that RA is required to induce STRA8 expression in mutant spermatogonia (Figures 5B and 5D). Unexpectedly, and in contrast to VAD controls, mutant spermatogonia arrested with much brighter foci of SYCP3 staining than seen in arrested undifferentiated spermatogonia (Figures 5C-5H). This accumulation of SYCP3 resembled that described in Stra8 mutant germ cells (Anderson et al., 2008), suggesting that the VAD mutant spermatogonia had arrested at a more advanced developmental stage than controls. Based on SYCP3 accumulation, these cells might be premeiotic (similar to preleptotene cells) but unable to enter meiosis due to the absence of RA and STRA8. The mutant VAD cells were BrdU-positive, possibly indicating that they underwent premeiotic DNA replication (not shown).
Figure 5. Meiosis of mutant spermatogonia requires retinoic acid.
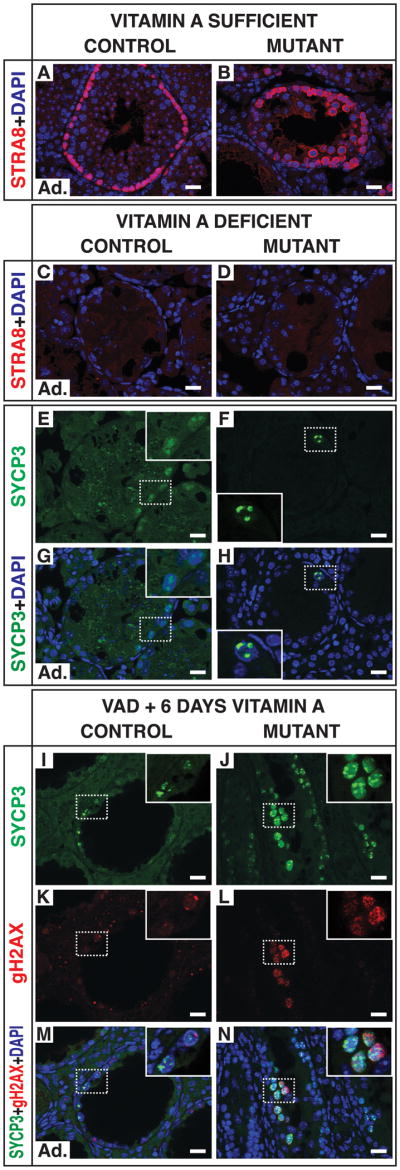
(A,B) Section IF for STRA8 in adult vitamin A sufficient (VAS) testes. (C-H) Section IF from vitamin A deficient (VAD) testes at fifteen weeks. Arrested undifferentiated A spermatogonia in VAD mice have low expression of SYCP3 (C,E,G), whereas mutant VAD tubules contain germ cells with intense foci of SYCP3 similar to those of preleptotene spermatocytes. SYCP3 channel in panels E and G is intentionally overexposed to show faint puncta in A spermatogonia of control. (I-N) Section IF six days after vitamin A replenishment. Differentiating spermatogonia of control have weak expression of γH2AX and SYCP3. Leptotene spermatocytes in mutant have intense and distributed SYCP3 localization and punctate accumulation of γH2AX at presumptive double-strand DNA breaks (for comparison, see control leptotene spermatocytes in Figure 3Q). Scale bars: 20 microns.
When vitamin A is readministered to VAD mice, the arrested undifferentiated spermatogonia require nine to ten days to complete differentiation (van Pelt and de Rooij, 1990). Six days after vitamin A resupplementation control spermatogonia were still differentiating and had not yet reached meiosis (Figures 5I-5N). By contrast, mutants had cells with nuclear distribution of SYCP3 and foci of γH2AX characteristic of leptotene spermatocytes (Figures 5I-N). These cells were not abundant (1.6 per tubule, n = 80 tubules; 2 mutants and 2 controls analyzed), likely because they arose from early and unamplified spermatogonia. In summary, the uncontrolled meiosis of Dmrt1 mutant spermatogonia requires RA and Stra8, like normal meiosis, but VAD mutant germ cells arrest at a stage closer to meiotic initiation than do VAD control germ cells.
Spermatogonia-specific binding of DMRT1 to Stra8 regulatory sequences in vivo
By what RA-dependent mechanism does DMRT1 block meiosis? A likely candidate for regulation by DMRT1 is the RA-responsive meiotic inducer Stra8, which is expressed at abnormally high levels in Dmrt1 mutant spermatogonia. DMRT1 and STRA8 are reciprocally expressed in B spermatogonia and preleptotene spermatocytes: B spermatogonia express DMRT1 but not STRA8 whereas preleptotene spermatocytes lack DMRT1 and accumulate high levels of STRA8 as they enter meiosis (Figures 6A and 6B; Figure S2). We asked, using chromatin immunoprecipitation (ChIP), whether this reciprocal expression might involve DMRT1 directly regulating Stra8 transcription. Using ChIP-chip (Murphy et al., 2010) and quantitative ChIP (qChIP) in P9 and P28 testes, we found that DMRT1 associates with a region of the Stra8 proximal promoter (Figures 6C and 6D). The interval bound by DMRT1 contains a close match to the preferred DMRT1 in vivo DNA binding consensus (Figure 6C) (Murphy et al., 2010), located between two retinoic acid response elements (RAREs) that mediate RA transcriptional response in F9 embryonal carcinoma cells (Wang and Tilly, 2010).
Figure 6. Regulation of Stra8 transcription and RA activity by DMRT1.
(A,B) Section IF for DMRT1 and STRA8. At stage VII DMRT1 is expressed in Sertoli cells but not preleptotene spermatocytes (A) and STRA8 is robustly expressed in preleptotene spermatocytes (B). Scale bars: 20 microns. (C) ChIP-Chip of DMRT1 in P9 testis (Murphy et al., 2010) detects binding of DMRT1 to the Stra8 promoter. Red dots: positions of RAREs. Black dot: DMRT1 consensus DNA binding site, sequence of which is at top of panel, aligned to in vivo-derived DNA binding consensus. More distal peak of DMRT1 binding lacks a clear consensus element and is presumably bound either indirectly or via a non-canonical binding site. (D) qChIP of DMRT1 at P28 detects DMRT1 bound to DNA near Stra8 transcriptional start site (0) but not 3 kb upstream or downstream. B2m is negative control promoter. (E) qChIP of DMRT1 on Stra8 promoter in testes with germ cell specific deletion of Dmrt1 (GCKO) or Sertoli cell-specific deletion of Dmrt1 (SCKO). (F-I) Expression of RA-dependent RAREhsplacZ reporter transgene in control and mutant testes at P28. (F,G) Beta-galactosidase (BGAL) IF. Expression in control is higher in meiotic germ cells relative to surrounding mitotic spermatogonia (arrowheads). Reporter expression in mutant is higher in the spermatogonia that ring the seminiferous tubule (arrowheads). (H,I) DMRT1 IF showing that control spermatogonia with high DMRT1 expression in spermatogonia have low reporter expression and germ cells with elevated reporter expression in mutant testes lack DMRT1. Scale bars: 20 microns. (J,K) Expression of mRNAs involved in RA-dependent transcription. qRT-PCR reveals decreased Cyp26a1 and Tbx1 transcript levels at P28 and increased Crabp2 levels in mutant testes (J). Similar expression changes are detected by qRT-PCR in c-KIT sorted mutant germ cells (K). Error bars: SD.
Because DMRT1 is expressed both in spermatogonia and in Sertoli cells, it was important to determine in which cell type(s) binding to the Stra8 promoter occurs. We combined qChIP with conditional deletion of Dmrt1 in germ cells (Ngn3-cre) or Sertoli cells (Dhh-cre; (Lindeboom et al., 2003)). Deletion of Dmrt1 in spermatogonia reduced DMRT1 binding to Stra8, whereas deletion in Sertoli cells had no significant effect (Figure 6E). This shows that DMRT1 binds the Stra8 promoter in spermatogonia, and we conclude that DMRT1 likely regulates Stra8 expression by direct transcriptional repression, preventing its activation by the retinoic acid receptor transcriptional regulatory complex. This model predicts that ablation of the DMRT1 binding site will cause ectopic expression of Stra8 in spermatogonia.
DMRT1 suppresses RA-dependent transcription in spermatogonia
Blocking Stra8 transcription likely contributes to the inhibition of meiosis by DMRT1, but is unlikely to be sufficient because Stra8 mutant germ cells can retain the ability to initiate meiosis (Mark et al., 2008). Thus DMRT1 is likely to repress other meiosis-promoting factors.
Since RA is required for male meiosis, an effective way for DMRT1 to prevent precocious meiosis would be to inhibit RA-dependent transcription in spermatogonia. To test this idea we first assessed RA-dependent transcription using a reporter transgene (RAREhsplacZ) with three RAREs controlling beta-galactosidase expression (Rossant et al., 1991). In controls, DMRT1-positive spermatogonia had low reporter expression, whereas DMRT1-negative meiotic and postmeiotic cells had higher expression (Figures 6F and 6H). By contrast, mutant spermatogonia had elevated reporter expression, indicating abnormally high RA-dependent transcriptional activity (Figures 6G and 6I). As a control, we tested binding of DMRT1 to the reporter transgene by qChIP. We detected no binding, either in the vicinity of the RAREs or elsewhere. We conclude that regulation of the reporter by DMRT1 is likely indirect and that DMRT1 functions to inhibit RA-dependent transcriptional activity.
To further assess how DMRT1 affects RA-dependent transcription we examined mRNA expression in P9 testes (Murphy et al., 2010). This identified many known RA-induced mRNAs with elevated expression in the mutant testes (Table S1). Sixteen of 83 RA-inducible mRNAs identified from the literature (19.3%) were significantly overexpressed in mutant testes, compared with 910 of 23,850 mRNAs overall (p < 0.0001). Among the upregulated mRNAs was Crabp2, whose protein CRABPII can directly load RA onto its nuclear receptor and stimulate RA-dependent transcription (Budhu and Noy, 2002; Dong et al., 1999; Noy, 2000) (qRT-PCR data are in Figures 6J and 6K). We also detected lower expression at P9 and P28 of the retinoid response inhibitor Tbx1, a transcription factor whose expression is inhibited by RA (p=0.0001; Figures 6J and 6K) (Roberts et al., 2005). The altered expression of Crabp2 and Tbx1 are expected to reinforce DMRT1 control of RA signaling activity. One way TBX1 inhibits RA response is by inducing expression of the three CYP26 RA oxidizing enzymes (Guris et al., 2006; Roberts et al., 2006). Mutant testes and purified c-KIT positive germ cells from mutants had reduced expression of the RA inactivating enzyme Cyp26a1 (Figure 6J and 6K), suggesting that RA accumulation might be affected in mutant spermatogonia. Expression of Cyp26b1 and Cyp26c1 was normal (data not shown). Another notable RA-responsive gene with elevated expression (Table S1) was the mitotic inhibitor Cdkn1a (encoding p21Cip1); this suggests that RA signaling may jointly promote meiosis and inhibit mitosis. Stra8 expression was not elevated in P9 mutant testes; Ngn3-cre is not expressed in first wave spermagonia and the lack of elevated Stra8 at P9 may indicate that the mechanism activating Stra8 transcription is not yet active in second wave spermatogonia at this stage.
We used ChIP-chip to ask whether DMRT1 binds genes that modify RA signaling. We did not detect binding to Cyp26a1, Tbx1 or Crabp2 promoter regions, suggesting their regulation may be indirect. ChIP-chip and qChIP did detect DMRT1 binding to 5′ regulatory regions of the dehydrogenases Adh4 and Aldh1a1 (which convert retinol to RA) in Sertoli cells and spermatogonia (data not shown). Expression of these genes was not altered in mutant testes, however, so further analysis will be needed to determine whether they are regulated by DMRT1 in Sertoli cells or in the later differentiating spermatogonial populations that are severely depleted in mutants.
Based on the activity of RAREhsplacZ and the misexpression of RA-dependent endogenous genes, we conclude that DMRT1 restricts RA-dependent transcriptional activity. By acting to generally inhibit RA activity and also to specifically block RA-dependent activation of Stra8 transcription, DMRT1 can prevent meiosis during spermatogonial proliferation and differentiation.
DMRT1 promotes spermatogonial differentiation
Dmrt1 mutant spermatogonia precociously abandon spermatogonial development in favor of meiosis. Clearly, therefore, DMRT1 inhibits meiosis in spermatogonia, but we reasoned that it might also promote spermatogonial development and differentiation. To investigate this possibility we exploited the VAD testes. In VAD testes spermatogonial differentiation is blocked but the fate of spermatogonia depends on DMRT1 expression: wild type VAD germ cells arrest as undifferentiated spermatogonia, while mutant VAD germ cells arrest as apparent premeiotic cells (Figure 5). Mutant VAD testes had reduced expression of many genes normally expressed in spermatogonia (Table S2), including Dazl, Piwil2, and Taf7l (Ballow et al., 2006; Lee et al., 2006; Pointud et al., 2003; Reynolds et al., 2005), consistent with their abandonment of the spermatogonial program. Of 59 mRNAs significantly downregulated in adult VAD mutant testes (0.22% of mRNAs tested), sixteen (27%) had known roles in spermatogonial differentiation (p < 0.0001). We also examined Sohlh1, which was detected in DMRT1 ChIP-chip analysis (Murphy et al., 2010) and also in the VAD mutant mRNA expression analysis, but below the significance threshold. SOHLH1 is a transcription factor expressed in undifferentiated spermatogonia and essential for spermatogonial development (Ballow et al., 2006). Antibody staining confirmed that SOHLH1 is coexpressed with DMRT1 in control spermatogonia and absent from mutant spermatogonia (Figures 7A to 7D). Regulation of Sohlh1 by DMRT1 appears to be direct: ChIP-chip detected binding of DMRT1 to the promoter region of Sohlh1 and qChIP confirmed that DMRT1 the binding in spermatogonia (Figures 7E and 7F). The bound region contained a close match to the DMRT1 preferred in vivo DNA binding consensus (Figure 7E). Reduced expression of Sohlh1 and other germ cell differentiation factors in the mutant spermatogonia helps explain why these cells exit from spermatogonial development.
Figure 7. Regulation of spermatogonial development and meiosis by DMRT1.
(A-D) IF of DMRT1 and SOHLH1 in adult testis sections. Control spermatogonia are double-positive (A,C), and mutant spermatogonia are double-negative (B,D). Cells positive for DMRT1 alone are Sertoli cells. Scale bars: 20 microns. (E,F) Binding of DMRT1 to Sohlh1 promoter in vivo. (E) ChIP-chip at P9 (Murphy et al., 2010) detects DMRT1 bound at the Sohlh1 promoter. Black dot: DMRT1 consensus DNA binding site, sequence shown at top of panel aligned to in vivo-derived DNA binding consensus. (F) DMRT1 binds Sohlh1 in both germ cells and Sertoli cells. GCKO: Deletion of Dmrt1 in spermatogonia. SCKO: deletion of Dmrt1 in Sertoli cells. B2m is negative control promoter. Error bars: SD. (G) Model for control by DMRT1 of spermatogonial development and meiosis. Solid lines indicate direct transcriptional control and dashed lines indicate undefined regulatory interactions.
Loss of DMRT1 in germ cells disrupts cyclical gene expression in Sertoli cells
Loss of Dmrt1 in spermatogonia causes them to undergo meiotic entry independent of the seminiferous epithelial cycle, despite the presence of wild type Sertoli cells. We asked whether this disruption of germ cell development affects cyclical gene expression in Sertoli cells. Indeed, Sertoli cells in all tubule sections of mutant testes expressed levels of GATA1 and Androgen Receptor (AR) typical of stages VII-VIII, regardless of germ cell abundance (Figure S3). Thus loss of DMRT1 activity in spermatogonia disrupts cyclical protein expression in Sertoli cells. RA would be a likely candidate to signal from the mutant germ cells to the Sertoli cells. However, RAREhsplacZ reporter expression was not elevated in the Sertoli cells (Figures 6G and 6I, and data not shown), suggesting that a different signal may be responsible.
The disrupted Sertoli cell gene expression in testes with germ cell specific loss of Dmrt1 raises the possibility that the disrupted Sertoli cells could, in return, affect germ cell development and contribute to uncontrolled meiosis. To test this idea, we examined tamoxifen-induced mutant testes. Six days post-tamoxifen, when cycle-independent meiosis was present (Figure 4), Sertoli cell cyclical gene expression was normal (data not shown). We conclude, therefore, that the primary defect leading to uncontrolled meiosis is in the mutant spermatogonia.
Discussion
We have examined the role of DMRT1 in spermatogonial development and differentiation by conditional gene targeting. Loss of DMRT1 in undifferentiated spermatogonia causes them to precociously exit the normal program of mitotic proliferation and differentiation, instead switching to the meiotic program and eventually completing germ cell differentiation to form spermatids. DMRT1 represses Stra8 activation by both direct transcriptional regulation and by a general inhibition of RA-dependent transcriptional activity. Repressing both Stra8 transcription and RA signaling may provide a “fail-safe” mechanism for DMRT1 to block meiosis and ensure the completion of spermatogonial proliferation and differentiation. A model for the control of spermatogonial development by DMRT1 is shown in Figure 7G.
How does DMRT1 limit RA-dependent transcription? In Stra8 DMRT1 binds near two promoter-proximal RA response elements and prevents expression, likely by blocking activation of Stra8 by the retinoic acid receptor transcriptional regulatory complex. DMRT1 may regulate other RA-responsive genes by this mechanism. However, we also detected changes in expression of genes involved in RA-dependent transcription that did not have promoter-proximal binding of DMRT1, including Crabp2, Tbx1, and Cyp26a1, and DMRT1 bound the promoters of the synthetic enzymes Adh1 and Aldh1a1. These observations and the misregulation of the RAREhsplacZ reporter suggest that DMRT1 also controls the accumulation and/or transcriptional potency of RA in germ cells. RA signaling involves feedback regulation, so further work will be needed to determine precisely how DMRT1 controls this signaling network.
DMRT1 levels vary in spermatogonia as they develop: DMRT1 is highest in undifferentiated spermatogonia, lower in differentiating spermatogonia, and disappears as B spermatogonia make the transition to preleptotene cells and initiate meiosis. The two decrements in DMRT1 expression correspond to steps of spermatogonial differentiation known to require RA. Since DMRT1 inhibits RA signaling, we suggest that DMRT1 may act as a transcriptional “rheostat” to modulate RA signaling during spermatogonial progression. Our previous finding that Dmrt1 tumor-suppressive activity in fetal germ cells is highly sensitive to gene dosage (Krentz et al., 2009) is consistent with this model. A key remaining question is how DMRT1 expression is reduced to allow the switch to meiosis. Tamoxifen-induced deletion of Dmrt1 caused rapid depletion of DMRT1 in germ cells but not in Sertoli cells; this differential stability may be an important part of the regulatory mechanism.
The source(s) of the intratubular RA that promotes meiotic initiation is unknown, because genes involved in RA metabolism and storage are expressed in Sertoli cells and germ cells (meiotic and postmeiotic) (Vernet et al., 2006b) and RA readily crosses cell membranes (Kamp et al., 1993; Noy, 1992). Thus control of spermatogonial development and meiotic initiation by RA might involve both the germ cell intrinsic regulation of RA signaling that we describe here and fluctuating levels of RA produced by other cell types within the tubule.
The RNA binding protein NANOS2 is a meiotic repressor in fetal male germ cells: Nanos2 mutant germ cells express STRA8 and enter meiosis around E17 (Suzuki and Saga, 2008). The similarity with postnatal DMRT1 function raises the possibility of a mechanistic link between the two proteins. Postnatally NANOS2 is expressed only in As spermatogonia, and fetal loss of DMRT1 does not cause inappropriate meiosis, so these proteins must have at least partially separate functions. NANOS2 does not bind Stra8 mRNA in vitro, but can bind Taf7l, whose expression was affected by loss of DMRT1 (Barrios et al., 2010). Thus the two proteins may control some shared target genes, albeit by different mechanisms and in incompletely overlapping germ cell types. Genome-wide identification of downstream targets for these proteins will help resolve this question.
When DMRT1 is deleted from undifferentiated spermatogonia, the mutant cells rapidly enter meiosis and produce elongated spermatids. Is the spermatogonial differentiation program therefore dispensible for male gametogenesis? In the Ngn3-cre mutant testes most germ cells lost DMRT1 as ECAD-positive undifferentiated spermatogonia. However mutant spermatogonia expressing the differentiation marker c-KIT also were present (Figure 3AB and data not shown), suggesting that although mutant cells bypass mitotic proliferation, they do undergo at least some spermatogonial differentiation prior to meiosis. Thus this program of gene expression may be a necessary part of male gametogenesis.
Does DMRT1 regulate female meiosis? Although DMRT1 is expressed in female germ cells during the transition from mitosis to meiosis, Dmrt1 null mutant females are fertile (Raymond et al., 2000). Nevertheless, it will be important to ask whether meiotic initiation occurs normally in the fetal ovaries of Dmrt1 mutants.
Loss of DMRT1 in germ cells uncouples meiotic initiation from the seminiferous epithelial cycle and disrupts cyclical gene expression in Sertoli cells. The effect on Sertoli cell gene expression might reflect inappropriate signaling between germ cells and Sertoli cells or a deficit in a germ cell type necessary for cyclical gene expression in Sertoli cells. Either way, our results are consistent with the idea that a germ cell-intrinsic program can influence Sertoli cells to achieve coordinated progression of the seminiferous epithelial cycle. A useful test of this idea is to ask whether transplantation of rat spermatogonia into the mouse testis causes recipient Sertoli cells to adopt the 13 day rat cycle.
DMRT1 is a DM domain transcription factor (Raymond et al., 1998). These proteins control sexual differentiation and/or primary sex determination in varied phyla and occur throughout metazoans. It therefore will be important to ask whether DM domain proteins control the mitosis to meiosis transition outside mammals. The rise of the metazoans created a need for mitotic and meiotic cells to coexist in the same individual. DMRT1 controls the mitosis to meiosis switch (this work) and also prevents germ cells from adopting somatic cell fates (Krentz et al., 2009). Based on these functions, we speculate that DM domain genes may have evolved in early metazoans to allow meiotic germ cells to coexist with somatic cells, and later assumed control of other reproductive functions.
The results presented here establish DMRT1 as a key regulator of spermatogonial development and differentiation that controls the mitosis versus meiosis switch in male germ cells of mammals. These findings expand the mechanistic understanding meiotic control in male mammals and suggest that in adults as in embryos the regulation of RA signaling is critical. Our results provide an entry point for further elucidation of meiotic regulation during the cycle of the seminiferous epithelium. In addition, understanding how DMRT1 functions to promote spermatogonial development and prevents meiosis may aid in the artificial manipulation of spermatogenesis for a variety of applications.
Experimental Procedures
Mouse Breeding
Dmrt1flox/flox mice were used as controls, and Dmrt1flox/flox mice (Kim et al., 2007a) with the relevant cre transgene were used as mutants. Details of mouse breeding are in Supplemental Experimental Procedures. Experimental protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Histological Analysis
Hemotoxylin and eosin (H&E) staining was performed as described (Kim et al., 2007b) and Periodic Acid-Schiff (PAS-H) staining was performed according to the manufacturer's protocol (Sigma-Aldrich Cat. No. 395B-1KT). More details are in Supplemental Experimental Procedures.
Immunofluorescence and Immunohistochemistry
Immunofluorescence (IF) was as described (Kim et al., 2007b) except testes were fixed by perfusion with 4% paraformaldehyde (PFA; more detail in Supplemental Experimental Procedures). Immunohistochemistry (IHC) and whole-mount IF were as described (Buaas et al., 2004; Krentz et al., 2009).
Staging of Mouse Seminiferous Tubules
Staging of seminiferous tubules in testis sections was as described (Ahmed and de Rooij, 2009). To confirm staging, DMRT1/GATA1 and STRA8/GATA1 IF were performed on testis sections. In adults Sertoli cell GATA1 expression is stage-specific (Yomogida et al., 1994). In tamoxifen-induced Dmrt1 mutants and controls, BC7 IF was used to identify tubule stages XII-VI (Koshimizu et al., 1993).
BrdU Incorporation and TUNEL Assays
5-bromo-2-deoxyuridine (BrdU) incorporation and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were as described (Mark et al., 2008) except that the 2-hour and 8-day time points were from separate animals. More details are in Supplemental Experimental Procedures. TUNEL-positive aptoptotic cells were detected with the In Situ Cell Death Detection Kit (Roche). Testis sections were counterstained with eosin.
Tamoxifen-induced deletion of Dmrt1
Adult experimental and control animals were IP injected twice in 24 hours with a total of 4 mg tamoxifen/mouse. Tamoxifen (Sigma) was prepared by dissolving 100 mg of tamoxifen in 1.5 ml of 100% ethanol and diluted in 8.5 ml of sesame oil (Sigma) to 10 mg/ml. Testes were harvested 24 hr, 48 hr, six days, and eight days after tamoxifen treatment.
Vitamin A Depletion and Vitamin A Replacement
Vitamin A depletion and replacement was performed as described ((van Pelt and de Rooij, 1990) and Supplemental Exerimental Procedures).
Isolation of c-KIT-positive Germ Cells
c-KIT-positive germ cells were isolated as previously described ((Takubo et al., 2008) and Supplemental Experimental Procedures), except CD117 (c-KIT) microbeads were used instead of anti-rat microbeads.
Quantification of c-KIT-positive and ECAD-positive germ cells
Whole mount tubule preparations were stained by IF with antibodies specific to c-KIT or ECAD. Positive cells/mm of tubule were counted in four 0.5 mm lengths of tubule from each of two animals. Cells were counted on one side of the flattened tubule. In mutants, sections of tubules that lacked germ cells were not counted.
qRT-PCR
RNA from 4-week old whole testes or c-KIT-positive isolated germ cells was extracted using TRIzol reagent according to the manufacturer's protocol (Invitrogen). cDNA was generated from 1 ug of RNA by using M-MLV reverse transcriptase according to the manufacturer's protocol (Invitrogen). cDNA was amplified with FastStart SYBR green (Roche). qRT-PCR and qChIP primers are listed in Supplemental Materials. All samples were normalized to Hprt and control values were set to 1.
ChIP
ChIP-chip and qChIP were performed as described elsewhere (Murphy et al., 2010).
mRNA expression profiling
mRNA expression profiling and data analysis were performed as previously described (Krentz et al., 2009).
Supplementary Material
Acknowledgments
We thank Pierre Chambon (IGBMC Strasbourg, France) for STRA8 antibody, Hiromitsu Tanaka and Yoshitake Nishimune (Osaka University, Japan) for BC7 antibody, Aleksander Rajkovic (University of Pennsylvania) for SOHLH1 antibody, and Aaron Sarver for bioinformatics assistance. We are grateful to David Greenstein, Anne Marie Weber-Main, and Judith Kimble for critical reading of the manuscript, Alex Bortvin for helpful discussion, and Chris Small, Keiyo Takubo, and Eric Rahrmann for reagents and technical assistance. This work was supported by grants from the NIH (GM59152) and Minnesota Medical Foundation and an NSF pre-doctoral fellowship to CKM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed EA, de Rooij DG. Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol. 2009;558:263–277. doi: 10.1007/978-1-60761-103-5_16. [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006;294:161–167. doi: 10.1016/j.ydbio.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, Rossi P, De Felici M, Jannini EA, Dolci S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–880. doi: 10.1242/jcs.057968. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134:3401–3411. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Budhu AS, Noy N. Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest. Mol Cell Biol. 2002;22:2632–2641. doi: 10.1128/MCB.22.8.2632-2641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- de Rooij DG. Stem cells in the testis. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- Franca LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- Guris DL, Duester G, Papaioannou VE, Imamoto A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev Cell. 2006;10:81–92. doi: 10.1016/j.devcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- Kamp F, Hamilton JA, Westerhoff HV. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 1993;32:11074–11086. doi: 10.1021/bi00092a017. [DOI] [PubMed] [Google Scholar]
- Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007a;307:314–327. doi: 10.1016/j.ydbio.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Namekawa SH, Niswander LM, Ward JO, Lee JT, Bardwell VJ, Zarkower D. A mammal-specific Doublesex homolog associates with male sex chromatin and is required for male meiosis. PLoS Genet. 2007b;3:e62. doi: 10.1371/journal.pgen.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu U, Watanabe D, Sawada K, Nishimune Y. A novel stage-specific differentiation antigen is expressed on mouse testicular germ cells during early meiotic prophase. Biol Reprod. 1993;49:875–884. doi: 10.1095/biolreprod49.5.875. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Kim S, Cook MS, Capel B, Zhu R, Matin A, Sarver AL, Parker KL, Griswold MD, et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci U S A. 2009;106:22323–22328. doi: 10.1073/pnas.0905431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Engel W, Nayernia K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev. 2006;73:173–179. doi: 10.1002/mrd.20391. [DOI] [PubMed] [Google Scholar]
- Lei N, Hornbaker KI, Rice DA, Karpova T, Agbor VA, Heckert LL. Sex-specific differences in mouse DMRT1 expression are both cell type- and stage-dependent during gonad development. Biol Reprod. 2007;77:466–475. doi: 10.1095/biolreprod.106.058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom F, Gillemans N, Karis A, Jaegle M, Meijer D, Grosveld F, Philipsen S. A tissue-specific knockout reveals that Gata1 is not essential for Sertoli cell function in the mouse. Nucleic Acids Res. 2003;31:5405–5412. doi: 10.1093/nar/gkg723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Feret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121:3233–3242. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- McCarthy PT, Cerecedo LR. Vitamin A deficiency in the mouse. Journal of Nutrition. 1952;46:361–376. doi: 10.1093/jn/46.3.361. [DOI] [PubMed] [Google Scholar]
- Murphy MW, Sarver AL, Rice D, Hatzi K, Ye K, Melnick A, Heckert LL, Zarkower D, Bardwell VJ. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1006243107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy N. The ionization behavior of retinoic acid in lipid bilayers and in membranes. Biochim Biophys Acta. 1992;1106:159–164. doi: 10.1016/0005-2736(92)90234-d. [DOI] [PubMed] [Google Scholar]
- Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem J. 2000;348(Pt 3):481–495. [PMC free article] [PubMed] [Google Scholar]
- Oakberg EF. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956;99:391–413. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointud JC, Mengus G, Brancorsini S, Monaco L, Parvinen M, Sassone-Corsi P, Davidson I. The intracellular localisation of TAF7L, a paralogue of transcription factor TFIID subunit TAF7, is developmentally regulated during male germ-cell differentiation. J Cell Sci. 2003;116:1847–1858. doi: 10.1242/jcs.00391. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215:208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- Reynolds N, Collier B, Maratou K, Bingham V, Speed RM, Taggart M, Semple CA, Gray NK, Cooke HJ. Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum Mol Genet. 2005;14:3899–3909. doi: 10.1093/hmg/ddi414. [DOI] [PubMed] [Google Scholar]
- Roberts C, Ivins S, Cook AC, Baldini A, Scambler PJ. Cyp26 genes a1, b1 and c1 are down-regulated in Tbx1 null mice and inhibition of Cyp26 enzyme function produces a phenocopy of DiGeorge Syndrome in the chick. Hum Mol Genet. 2006;15:3394–3410. doi: 10.1093/hmg/ddl416. [DOI] [PubMed] [Google Scholar]
- Roberts C, Ivins SM, James CT, Scambler PJ. Retinoic acid down-regulates Tbx1 expression in vivo and in vitro. Dev Dyn. 2005;232:928–938. doi: 10.1002/dvdy.20268. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinhahikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–435. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo K, Ohmura M, Azuma M, Nagamatsu G, Yamada W, Arai F, Hirao A, Suda T. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Thompson JN, Howell JM, Pitt GA. Vitamin a and Reproduction in Rats. Proc R Soc Lond B Biol Sci. 1964;159:510–535. doi: 10.1098/rspb.1964.0017. [DOI] [PubMed] [Google Scholar]
- Timmons PM, Rigby PW, Poirier F. The murine seminiferous epithelial cycle is pre-figured in the Sertoli cells of the embryonic testis. Development. 2002;129:635–647. doi: 10.1242/dev.129.3.635. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod. 1990;43:363–367. doi: 10.1095/biolreprod43.3.363. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006a;25:5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006b;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–349. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel JD, Yamamoto M. Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse Sertoli cells. Development. 1994;120:1759–1766. doi: 10.1242/dev.120.7.1759. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, Matsuda Y, Takamatsu N, Shiba T, Ito M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci U S A. 2008;105:2469–2474. doi: 10.1073/pnas.0712244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkower D. Establishing sexual dimorphism: conservation amidst diversity? Nat Rev Genet. 2001;2:175–185. doi: 10.1038/35056032. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod. 2008a;78:537–545. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008b;79:35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.