Abstract
K19 is an intermediate filament protein that has been investigated in oral squamous cell carcinoma (OSCC), but that has not been correlated with the amount of keratin produced within well-differentiated OSCC grade. The aim of the present study was to objectively analyze K19 immunoexpression in OSCC and to validate the utility of K19 in differentiation among grades of oral epithelial dysplasia (OED). Formalin-fixed tissues of 36 primary OSCC (22 well, 10 moderately, 4 poorly differentiated), 43 OED (23 mild, 8 moderate, 12 severe), and 11 normal oral epithelium (NOE) were included. K19 was immunostained using HRP-DAB method. The percentage of K19-positive area was found using color deconvolution program in ImageJ® image analysis system (public domain software, National Institutes of Health, Bethesda, MD, USA) and analyzed using independent samples t tests and ANOVA test. K19 scores in NOE, mild, moderate and severe OED were: 1.8, 3.4, 21, and 50.3%, respectively, with significant association with the grade (t test P < 0.05). Well-differentiated OSCC with <30% keratin pearl formation expressed significantly higher K19 scores compared to well-differentiated OSCC with >30% keratin pearls (28.6 and 1.2%, respectively, P < 0.05). K19 scores in moderately and poorly differentiated OSCC were 60.8 and 61.3%, respectively. K19 scores significantly differentiated between two subgroups of tumors within well-differentiated OSCC grade and reflected histologic differentiation as well as probably predicting the clinical outcome. Combining K19 immunostain with the regular H&E stain may be helpful to facilitate and assure assigning a more accurate grade for OED.
Keywords: Oral squamous cell carcinoma, Epithelial dysplasia, Immunohistochemistry, K19, Image analysis
Introduction
Oral squamous cell carcinoma (OSCC) is the most common malignancy of the oral cavity [1]. Despite improvements in treatment modalities, it continues to only have a 50% 5-year survival rate [2]. OSCC may be clinically preceded by detectabl premalignant lesions. Recognition of premalignant lesions with histologic marker correlation may circumvent tumor progression if the premalignant lesions are properly diagnosed and treated [3]. These potentially malignant lesions are histologically characterized by epithelial alterations at the cellular and architectural levels, and is generally known as epithelial dysplasia [4]. Oral epithelial dysplasia is graded as mild, moderate or severe according to the extent of the dysplastic changes [4]. Unfortunately, despite attempts at standardization of oral epithelial dysplasia, there is still great inter-observer variability when determining the grade of oral epithelial dysplasia. But even with this shortcoming, the malignant transformation potential of OED is often related to the advancement of the pathologic grade [5]. Therefore, early clinical detection and accurate histological grading of epithelial dysplasia is crucially important for primary prevention of OSCC [3, 5]. However, because the diagnosis of epithelial dysplasia is essentially subjective [6–8], there is a substantial need to improve the histologic assessment of epithelial dysplasia. As a consequence, numerous attempts have been made to relate biological characteristics of OED with its malignant potential. Tumor suppressor gene products like p53, proliferative cell proteins like Ki67, and proliferating cell nuclear antigen (PCNA) along with cell cycle control proteins and cytokeratin alterations, have increased the awareness of cellular changes during carcinogenesis [9, 10]. These markers could be potential prognostic indicators of premalignant lesions. However, one or a panel of molecular markers that allow for prognostic prediction of oral pre-cancer have not yet been determined. Therefore, research in this field continues with the goal of finding more markers that could at least be considered complementary to conventional histopathologic evaluation [11].
Cytokeratins are epithelium-specific intermediate filament proteins that maintain cellular integrity and participate in cell-to-cell attachments [12]. There are 20 known cytokeratins divided into acidic and basic types [13]. The production of specific types of cytokeratins by different cell types as well as in individual cells of the oral epithelium reflects the degree of cellular differentiation and maturation [14]. For example, cells in the basal cell layer of oral epithelium produces K19 and K14 and reflects its proliferation potential, while cells in the suprabasal instead produce K1 and K10. When K19 is produced by suprabasal cells of the oral mucosa, this indicates alteration in cell behavior and probable premalignant changes [13–15].
The aim of the present study was to objectively analyze the immunohistochemical expression of K19 in OSCC and in oral epithelial dysplasia (OED), as well as in normal oral epithelium using a color deconvolution program in ImageJ® image analysis system. For the first time, K19 scores were correlated with the amount of keratin produced within well-differentiated OSCC. In OED, the utility of K19 scores in differentiation among different grades of dysplasia was also analyzed.
Materials and Methods
Case Selection
An inclusive sample of 36 OSCC and 43 OED specimens were retrieved from the Surgical Pathology records at King Abdullah University Hospital, Jordan University of Science and Technology, Jordan, between the years 1991 and 2007. Eleven specimens of polypoid fibrous hyperplasia were selected from the same archival material to represent normal oral epithelium (NOE) controls.
Inclusion criteria for polypoid fibrous hyperplasia specimens to be considered as representative of NOE were: absence of hyperkeratosis, mucositis, ulceration or atrophic changes. Hematoxylin and eosin (H&E) stained sections of NOE, OSCC and OED were prepared and evaluated by a certified oral and maxillofacial pathologist (R.S.). Two certified oral pathologists reviewed and graded OED cases individually in three different sessions. The most consistent grade of each case was recorded for evaluation.
OSCC specimens were graded based on Broder’s classification [16] into well-differentiated (22 specimens), moderately differentiated (10 specimens), and poorly differentiated (4 specimens). OED specimens were graded using WHO criteria [4] which relies primarily on the extent of cytologic and architectural epithelial changes into mild (23 specimens), moderate (8 specimens) and severe (12 specimens).
Immunohistochemical Staining Procedure
Immunohistochemistry was performed on 5-μm sections of paraffin embedded tissues (n = 90). Tissue sections were cut using a tissue microtome and then mounted on aminosilane-coated glass microslides (3-aminopropyltriethoxysilane; Sigma, USA). After de-waxing and rehydration, microslides were immersed in an antigen retrieval solution containing 0.3% hydrogen peroxide (Reveal Decloaker 10×; Biocare Medical, USA) for 7 min in an autoclave, at a temperature of 121°C and a pressure of 1.5 Bar followed by cooling overnight in the same solution. Non-specific staining of proteins was blocked by non-specific protein block (Protein block; Biogenex, San Ramon, CA, USA) for 20 min. Immunohistochemistry was performed using the standard method of horseradish peroxidase (HRP)-diaminobenzidine (DAB) detection kits (Super sensitiveTM link-label immunohistochemical detection system, DAB substrate; Biogenex) and Dako automatic stainer (Autostainer Plus; Dako, Denmark). All incubations were carried out at room temperature. Tissue sections were defined using a wax pen (Biogenex) which improved visualization of the tissue and helped to keep the applied solutions from drying out. The primary antibody was monoclonal (RK108, clone A53-B/A2; Santa Cruz Biotechnology, CA, USA) with dilution of 1:150. It was applied for 30 min, followed by biotylinated multi-link peroxidase agent for 20 min and DAB substrate for 10 min. Counterstaining was performed with hematoxylin for 2 min, washing in water, and dehydration by passing through graded ethanol and xyline solutions was then accomplished. Microslides were coverslipped using Depex (DPX Mountant for Histology; Sigma, Germany).
In each staining session, positive and negative controls were produced for comparison and to assure consistent interpretation. Positive control tissue consisted of sections of oral squamous cell carcinoma specimens known to intensely express K19. Negative control tissue consisted of sections OED or OSCC specimens immunostained using the same procedure as for positive control except for replacing K19 antibody with non-specific mouse IgG (mouse IgG1; Santa Cruz Biotechnology). The negative control antibody was isotyped and the concentration was matched to that of the primary antibody. Titration of both antibodies was performed to select the best concentration in which the positive control tissue gave the best positive staining with mininimization background staining.
Microscopic Evaluation
All immunostained microslides were examined under the light microscope (Olympus model U-MDO10B, USA) using objective lenses magnifications of 4×, 10×, and 20×. K19 positive cells showed DAB positive brown cytoplasmic staining, while negative cells stained with the hematoxylin counterstain only.
DAB positive brown cytoplasmic staining of any intensity was considered positive for K19. Negative controls did not show any brown cytoplasmic or nuclear staining. One representative field from each section was selected and digitally photographed, using 10× objective lens magnification (Olympus; resolution 5.1 megapixels). All images were captured using the same light filter settings.
Digital Image Analysis
Digital images were prepared for analyses using ImageJ® computer program. ImageJ® is a Java image processing program developed by the National Institute of Health [Bethesda, Maryland, USA, (http://rsb.info.nih.gov/ij/download.html)]. In each image, the epithelium to be analyzed was selected and separated from the rest of the field using image brush tool. K19 positive area (brown stain) and total epithelial area in the analyzed field were automatically measured using color deconvolution plugin. Color deconvolution involves isolation of the color information from histological red, green, and blue (RGB) images containing multiple stains [17]. This was achieved by calculating the contribution of each stain based on the stain-specific RGB absorption. In the present study, color deconvolution was used to isolate DAB stain—which represents the K19-positive area—from hematoxylin which represents the whole epithelial area. Each image was changed into 8-bit type (gray) then processed into binary (black and white) color image. Measurements icon was calibrated to calculate the area of the field (total epithelial area) and area fraction which was area of the object in the field (black color representing DAB stain).
A score for K19 expression in the analyzed field was calculated by dividing the positively stained area over the total epithelial area.
Statistical Analysis
The K19 score for each specimen was entered into a statistical computer program, Statistical Package for Social Science (SPSS) version 11, (SPSS, Chicago, IL, USA). The mean percentage of K19 scores for each diagnostic group was then calculated. K19 scores were compared among the different diagnostic groups using ANOVA test. Paired comparisons were carried out using Student’s t test (the significance level was set as <0.05).
Results
Microscopic Evaluation of Stained Sections
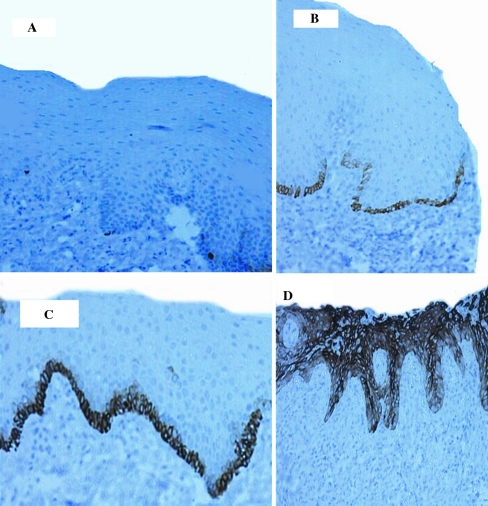
The brown cytoplasmic staining of normal oral epithelium was confined to the basal cell layer in all cases (11/11) (Fig. 1a). The positive brown staining was scant and sparse. Mild epithelial dysplasia was positive in 95.6% of the cases (22/23). The brown cytoplasmic staining was noted in the basal cell layer. The distribution pattern was continuous rather than intermittent with some occasional suprabasal extension (Fig. 1b). Moderate epithelial dysplasia was positive in 87.5% of the cases (7/8). There was supra basal extension of K19 positive cells compared to basal cell layer-limited staining of mild grade (Fig. 1c). Severe epithelial dysplasia was positive in 91.7% of the cases (11/12) with extension of K19 positive cells to involve the superficial one-third of epithelial thickness (Fig. 1d). In all grades of oral epithelial dysplasia, the extent of suprabasal K19 positivity was consistent with the cytomorphologic epithelial changes as confirmed by H&E stain.
Fig. 1.
K19 immunostain in normal epithelium and OED groups. Objective lens magnification of ×10, and resolution of 1.10 μm. a Normal epithelium showing intermittent cytoplasmic staining of the basal cell layer. b Mild epithelial dysplasia, showing continuous intense cytoplasmic staining of the basal cell layer. c Moderate epithelial dysplasia showing continuous intense cytoplasmic staining of the basal and suprabasal cell layers with occasional positive cells in the middle third of the surface epithelium. d Severe epithelial dysplasia showing intense cytoplasmic staining of the entire epithelial thickness
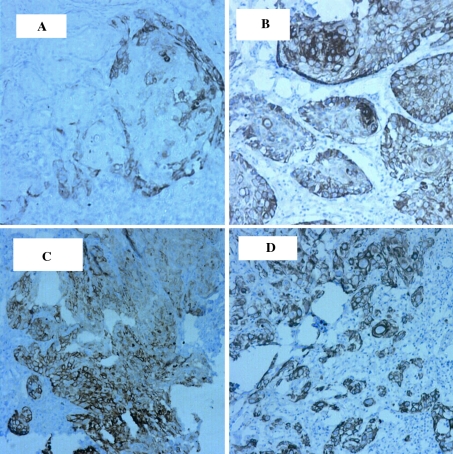
Well-differentiated OSCC was positive in 63.4% of the cases (14/22). The specimens showed relatively mature tumor cells with few nuclear aberrations, keratin pearl formation and/or individual cell keratinization. Well-differentiated OSCC grade exhibited two staining patterns: OSCC with abundant keratin pearl formation (30–100% of invasive tumor islands) and OSCC with infrequent keratin pearl formation (<30% of invasive tumor islands). About 57.1% of the specimens of OSCC with abundant keratin pearls were K19 positive (4/7). K19 positive cells were at the periphery of keratin pearl-producing tumor islands. There was no staining of the keratinized cells or keratin pearls (Fig. 2a). In well-differentiated OSCC with infrequent keratin pearl formation, 73% of the specimens were positive (11/15). Positive K19 staining was diffusely evident within tumor cells of the invasive tumor islands as well as in the peripheral layer of invasive islands where keratin pearls were present (Fig. 2b).
Fig. 2.
K19 immunostain in OSCC groups. Objective lens magnification of ×10, and resolution of 1.10 μm. a Well-differentiated OSCC with abundant keratin pearl formation. Note the pale staining of the outer layer of the invasive epithelial islands. b Well-differentiated OSCC with rare keratin pearl formation. Note the diffuse staining of invasive islands. c Moderately differentiated OSCC showing diffuse staining of invasive islands. d Poorly differentiated OSCC with diffuse positivity
Moderately differentiated OSCC was positive in 70% of specimens (7/10). In this grade, tumor cells exhibited a wide range of differentiation. Keratinization was occasionally present and nuclear aberrations were moderately abundant. There was diffuse positive staining of invasive tumor islands (Fig. 2c).
Poorly differentiated OSCC was positive in 75% of the specimens (3/4). In this grade, the tumor exhibited small invasive islands, strands, files and individual malignant cells which were disorderly and poorly differentiated. There was no tendency for keratinization and nuclear aberrations were abundant. The positive cases exhibited diffuse pattern of K19 staining (Fig. 2d).
Image Analyses of Stained Sections
Table 1 displays the mean percentages of K19-positive areas (K19 scores) in NOE, mild, moderate and severe OED and in OSCC groups. The K19 score in NOE was 1.8%. In mild, moderate and severe OED, the K19 score significantly increased from 3.4 to 21.0%, and to 50.5%, respectively (ANOVA test F = 227.4, P value = 0.0005). In OSCC, the K19 score significantly increased from well-differentiated (20.8%) to moderately and poorly differentiated grades (60.8 and 61.3%, respectively, ANOVA test F = 6.3, P < 0.001). In well-differentiated OSCC with abundant keratin pearls, the K19 score was 1.2% compared to 28.6% in well-differentiated OSCC with infrequent keratin pearls (<30%).
Table 1.
The mean, standard deviation, minimum, and maximum values of K19 scores in normal oral epithelium, mild, moderate, severe oral epithelial dysplasia and OSCC grades
| Diagnostic group | n | Mean K19 score | SD | Min. K19 score | Max K19 score |
|---|---|---|---|---|---|
| Normal oral epithelium | 11 | 1.80 | 2.00 | 0.14 | 6.07 |
| Mild epithelial dysplasia | 22 | 3.40 | 2.93 | 0.51 | 12.42 |
| Moderate epithelial dysplasia | 8 | 21.00 | 11.72 | 10.27 | 40.61 |
| Severe epithelial dysplasia | 12 | 50.30 | 31.24 | 0 | 96.83 |
| Well-differentiated OSCC | 22 | 20.80 | 15.58 | 0 | 70.00 |
| Moderately differentiated OSCC | 10 | 60.80 | 36.81 | 0 | 96.50 |
| Poorly differentiated OSCC | 4 | 61.30 | 42.86 | 0 | 97.30 |
Paired comparisons of K19 scores of NOE with that of each grade of OED are presented in Table 2. The K19 score of mild OED was higher than that of NOE without a statistically significant difference (P = 0.09). However, the K19 scores of moderate and severe OED grades were significantly higher than NOE (P < 0.001 for both). Paired comparisons of K19 scores between mild and moderate and between moderate and severe OED grades revealed significant increase. (P = 0.01 for both, Student’s t test).
Table 2.
Comparison of K19 scores between normal oral epithelium and all grades of OE and OSCC using independent samples t tests
| Type of lesion | n | Mean K19 score | T value | Mean difference | P value |
|---|---|---|---|---|---|
| Normal oral epithelium | 11 | 1.84 | −1.76 | −1.52 | 0.09 |
| Mild epithelial dysplasia | 22 | 3.37 | |||
| Normal oral epithelium | 11 | 1.84 | −5.36 | −19.13 | <0.001 |
| Moderate epithelial dysplasia | 8 | 21.00 | |||
| Normal oral epithelium | 11 | 1.84 | −5.18 | −48.42 | <0.001 |
| Severe epithelial dysplasia | 12 | 50.26 | |||
| Normal oral epithelium | 11 | 1.80 | −5.01 | −19.01 | <0.001 |
| Well-differentiated OSCC | 22 | 20.80 | |||
| Normal oral epithelium | 11 | 1.84 | 5.31 | 58.92 | <0.001 |
| Moderately differentiated OSCC | 10 | 60.80 | |||
| Normal oral epithelium | 11 | 1.84 | 4.12 | 47.20 | <0.001 |
| Poorly differentiated OSCC | 4 | 61.30 | |||
| Normal oral epithelium | 11 | 1.84 | 3.28 | 26.72 | <.001 |
| Well-differentiated OSCC with <30% keratin pearls | 15 | 28.56 | |||
| Normal oral epithelium | 11 | 1.84 | 0.82 | 0.66 | <0.4 |
| Well-differentiated OSCC with >30% keratin pearls | 7 | 1.18 |
Comparisons of K19 scores between NOE and each grade of OSCC are also presented in Table 2. The K19 score in NOE was significantly lower than that of well, moderately and poorly differentiated OSCC (P < 0.001). Comparing K19 scores of NOE with that of well differentiated OSCC producing abundant keratin pearls revealed no significant difference (P = 0.4). On the other hand, the K19 score of NOE was significantly lower than that of well differentiated OSCC producing infrequent keratin pearls (P < 0.001).
Table 3 presents paired comparisons of K19 scores in well-differentiated OSCC with moderately and poorly differentiated OSCC scores. Well-differentiated OSCC showed significantly lower K19 scores than moderately and poorly differentiated grades (P < 0.001). Interestingly, comparing K19 scores between well-differentiated OSCC with abundant keratin pearls and that of well-differentiated OSCC with infrequent keratin pearls revealed a significant difference (P < 0.001).
Table 3.
Comparison of K19 scores between different groups of OSCC using independent samples t tests
| Type of lesion | n | K19 score | T value | Mean difference | P value |
|---|---|---|---|---|---|
| Well-differentiated OSCC | 22 | 20.80 | −4.24 | −59.58 | <0.001 |
| Moderately differentiated OSCC | 10 | 60.80 | |||
| Well-differentiated OSCC | 22 | 20.80 | −3.23 | −47.86 | <0.001 |
| Poorly differentiated OSCC | 4 | 61.30 | |||
| Well-differentiated OSCC with <30% keratin pearl formation | 15 | 28.56 | −3.95 | −27.38 | <0.001 |
| Well-differentiated OSCC with >30% keratin pearl formation | 7 | 1.18 |
Discussion
The present study is an immunohistochemical investigation of keratin 19 expression in oral squamous cell carcinoma and oral epithelial dysplasia. K19 is an intermediate protein normally present in glandular epithelium and in undifferentiated stem cells as well as in the basal cell layer of oral mucosa.
K19 positive cytoplasmic areas were automatically measured under the same magnifications and light settings, providing an objective, reproducible and quantitative analysis of K19 expression. Using the color deconvolution program as part of a computer-based image analysis, we were able to detect even scant immunoreactivity and obtain accurate calculations of positive cytoplasmic area. Using true negative controls in the present study gave us more confidence to consider any cytoplasmic brown staining detected by color deconvolution as truly positive staining [18].
The expression of K19 in NOE was scant, intermittent and confined to the basal cell layer of oral epithelium. This finding was consistent with previous reports [10, 14, 19]. This localization and pattern of K19 positivity reflects the presence of undifferentiated progenitor stem cells that are normally present in the basal cell layer. The extension of K19 positive cells suprabasally is, therefore, considered abnormal and has been correlated with disturbed stem cell distribution [19, 20].
Oral epithelial dysplasia showed altered pattern of K19 expression compared to NOE. Mild OED expressed K19 continuously in the basal cell layer with only occasional suprabasal extension reflecting the dysplastic epithelial cells that extend into, but not beyond, the lower third of oral epithelium. In moderate and severe OED, dysplastic cells extended up into the middle and upper thirds of oral epithelium, respectively. The pattern of K19 extension reflects the extension of dysplastic epithelial cells when compared with H&E stain alone. Interestingly, some occult dysplastic changes were overlooked on H&E stain but were readily obvious with the K19 immunostain. The immunostain therefore facilitates the determination and extension of dysplastic changes and therefore helps assigning a dysplastic grade. The conventional criteria of grading epithelial dysplasia published in 1978 by the World Health Organization (WHO) collaborating centre for oral precancerous lesions were challenged for consistency among reviewers [6–8]. Researchers in this field called for the need of more objective and uniform criteria and/or markers for improving grading and decreasing the variability in the diagnosis of oral epithelial dysplasia. Classification systems for OED include the conventional oral epithelial dysplasia scoring system and squamous intraepithelial dysplasia. The former system has been recommended for routine use [21, 22].
In addition to grading OED as mild, moderate and severe grades, a new binary system proposed by Kujan et al. [23] has been suggested to reduce the choices to “low-risk” and “high-risk”. However, the utility of this new system needs to be further evaluated in future studies [22]. Regardless of the classification system used, the finding that the K19 score and pattern were significantly associated with the presence and extension of dysplastic cells should undoubtedly improve any H&E system used to grade OED. As the clinical appearance and the histological grading of the dysplasia are still the most important prognostic factors, the clinical implication of the use of K19 expression in this context is that combining the evaluation of K19-stained sections with H&E stain will provide a diagnostic aid to the pathologist in grading OED. Studies on this implication are encouraged especially in evaluating the impact of K19 immunostain on inter- and intra-examiner variability.
K19 expression in OSCC has been studied using different methods such as PCR and immunohistochemistry, emphasizing the potential significance of K19 use in clinical diagnosis. However, some studies considered only the number of positive cases without considering the extent and intensity of K19 positivity [24]. One study reported K19 scores as 0 for negative, 1 for weak (1–25% of reactive cells), 2 for moderate (26–50% of reactive cells), and 3 for severe (more than 50% of reactive cells) [15]. Another study gave only 2 scores; either zero or one [19]. Categorization of K19 scores in this way will reduce the value of K19 scores as some cases may be considered in either of the categories at the cut-off points. The present study attempted to avoid the above shortcoming by analyzing the percentage of positively stained area of cells out of the total epithelial area in the analyzed sections in addition to the percentage of positive cases.
In the present study, an overall percentage of K19 positive OSCC specimens was 66.7%. Previous studies reported a range for the percentage of K19 positive OSCC specimens from 29 to 100% [15, 20, 24, 25]. In this study, an increase in the percentage of K19 positive OSCC cases by advancement of the grade of OSCC, from well-differentiated to moderately differentiated to poorly differentiated (63.6 to 70 to 75%) was noted. This trend is in line with other studies concerning this topic [15, 20, 24]. It is more likely that a higher percentage of moderately and poorly differentiated OSCC specimens will express K19 as less-differentiated cells express more K19. However, the percentage of positive cases per se is of little clinical significance. As a result, K19 mean percentage of positive area was calculated in the present study. Advancement of OSCC grade was also reflected on the percentage of K19 positive area. There was progressive increase in K19 percentage of positive area as the pathologic grade of OSCC increased from well-, to moderately, to poorly differentiated (22.8, 60.8 to 61.3%, respectively). Poorer grade indicates less-differentiated tumors with increased expression of progenitor-cell keratins, like K19. The increased expression of this type of keratin has been associated with higher tumor proliferation rate and poorer prognosis [15, 25, 26]. Although 63.6% of well-differentiated OSCC cases were K19 positive, the percentage of positive area was only 22.8%. This percentage was significantly different from normal oral epithelium score.
Previous studies on this topic exhibited differences in their results. For example, Toyoshioma et al. [25] showed no significant difference in K19 expression rate between normal oral epithelium and OSCC, while Youshida [27] reported that K19 expression of OSCC was significantly different from NOE. Differences between studies can be attributed to the differences in the amounts of keratin pearl formation produced by tumors within this grade. The problem in level of obtaining statistical significance may have also been related to their use of non-parametric data ranking rather than the quantitative method utilized in this study. In the present study, we found that classifying well-differentiated OSCC tumors according to Bryne’s criteria [28] of producing <30% keratin pearls and those producing >30% provided two categories with significantly different K19 scores (Student’s t test, P < 0.001). Comparing K19 scores of well-differentiated OSCC with <30% keratin pearl formation with NOE gave a significant difference (P < 0.001) while comparing K19 scores of well-differentiated OSCC producing >30% keratin pearls gave no significant difference (Student’s t test, P = 0.40).
Furthermore, the pattern of K19 expression in well-differentiated OSCC with <30% keratin pearls was similar to that of moderately differentiated OSCC implying that the amount of extracellular keratin formation rather than intracellular keratinization is the determinant of the pattern of K19 expression and scores. K19 expression has been associated with poor prognosis of tumors and higher proliferation rates [15, 25, 26]. The significant difference in K19 scores and pattern within well-differentiated OSCC grade in the present study indicate a prognostic difference between these two subgroups despite the fact that both are graded as well differentiated. We suggest grading well-differentiated OSCCs producing keratin pearls in less than 30% as moderately well differentiated or at least to be given a diagnostic comment.
In conclusion, the amount and distribution of K19 positive areas significantly differentiated among OSCC grades and within the well-differentiated OSCC grade in specific cases. The K19 score significantly differentiated among two subgroups of tumors within the well-differentiated OSCC grade which reflects different histologic differentiation and may predict different clinical outcomes of tumors in this grade. The amount and distribution of K19 positive areas and pattern of expression may be a promising method of separating the different OED grades. We recommend the use of K19 immunostain regularly as a complementary stain to the conventional H&E stain to assign a more accurate grade of OED.
Contributor Information
Rima A. Safadi, Phone: +962-7-95644353, FAX: +962-2-7201080, Email: rsafadi@just.edu.jo
Atika S. Musleh, Email: layla3223@yahoo.com
Taiseer H. Al-Khateeb, Email: khateeb@just.edu.jo
Abed Al-Hadi Hamasha, Email: hamasha@just.edu.jo.
References:
- 1.Vicente J, Recio O, Pendás S, et al. Oral squamous cell carcinoma of the mandibular region A survival study. Head Neck. 2001;23(7):536–543. doi: 10.1002/hed.1075. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Silverman SJ, Sugerman P. Oral premalignancies and squamous cell carcinoma. Clin Dermatol. 2000;18(5):563–568. doi: 10.1016/S0738-081X(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 4.Kramer I, Lucas R, Pindborg J, et al. Definition of leukoplakia and related lesions: An aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46(4):518–539. doi: 10.1016/0030-4220(78)90382-1. [DOI] [PubMed] [Google Scholar]
- 5.Silverman SJ, Gorsky M, Kaugars G. Leukoplakia, dysplasia, and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(2):117. doi: 10.1016/S1079-2104(96)80209-5. [DOI] [PubMed] [Google Scholar]
- 6.Abbey L, Kaugars G, Gunsolley J, et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Pathol Oral Radiol Endod. 1995;80(2):188–191. doi: 10.1016/S1079-2104(05)80201-X. [DOI] [PubMed] [Google Scholar]
- 7.Brothwell DJ, Lewis DW, Bradley G, et al. Observer agreement in the grading of oral epithelial dysplasia. Community Dent Oral Epidemiol. 2003;31(4):300–305. doi: 10.1034/j.1600-0528.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 8.Fischer DJ, Epstein JB, Morton TH, et al. Interobserver reliability in the histopathologic diagnosis of oral pre-malignant and malignant lesions. J Oral Pathol Med. 2004;33(2):65–70. doi: 10.1111/j.1600-0714.2004.0037n.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu L, Crowe DL, Rheinwald JG, et al. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51(15):3972–3981. [PubMed] [Google Scholar]
- 10.Takeda T, Sugihara K, Hirayama Y, et al. Immunohistological evaluation of Ki-67, p63, CK19 and p53 expression in oral epithelial dysplasias. J Oral Pathol Med. 2006;35(6):369–375. doi: 10.1111/j.1600-0714.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 11.Warnakulasuriya S. Lack of molecular markers to predict malignant potential of oral precancer. J Pathol. 2000;190(4):407–409. doi: 10.1002/(SICI)1096-9896(200003)190:4<407::AID-PATH546>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Fradette J, Germain L, Seshaiah P, et al. The type I keratin 19 possesses distinct and context-dependent assembly properties. J Biol Chem. 1998;273(52):35176–35184. doi: 10.1074/jbc.273.52.35176. [DOI] [PubMed] [Google Scholar]
- 13.Michel M, Torok N, Godbout MJ, et al. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: Keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109(Pt5):1017–1070. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg K, Rheinwald JG. Suprabasal 40kd keratin (K19) expression as an immunohistologic marker of premalignancy in oral epithelium. Am J Pathol. 1989;134(1):89–98. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong LP, Chen WT, Zhang CP, et al. Increased CK19 expression correlated with pathologic differentiation grade and prognosis in oral squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(3):377–384. doi: 10.1016/j.tripleo.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Anneroth G, Hansen LS. A methodologic study of histologic classification and grading of malignancy in oral squamous cell carcinoma. Scand J Dent Res. 1984;92(5):448–468. doi: 10.1111/j.1600-0722.1984.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 17.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23(4):291–299. [PubMed] [Google Scholar]
- 18.Elias JM, Gown AM, Nakamura RM, et al. Quality control in immunohistochemistry. Report of a workshop sponsored by the Biological Stain Commission. Am J Clin Pathol. 1989;92(6):836–843. doi: 10.1093/ajcp/92.6.836. [DOI] [PubMed] [Google Scholar]
- 19.Fillies T, Jogschies M, Kleinheinz J, et al. Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol Rep. 2007;18(3):639–643. [PubMed] [Google Scholar]
- 20.Nie M, Zhong L, Zeng G, et al. The changes of cytokeratin 19 during oral carcinogenesis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2002;37(3):187–190. [PubMed] [Google Scholar]
- 21.Küffer R, Lombardi T. Premalignant lesions of the oral mucosa. A discussion about the place of oral intraepithelial neoplasia (OIN) Oral Oncol. 2002;38(2):125–130. doi: 10.1016/S1368-8375(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 22.Warnakulasuriya S, Reibel J, Bouquot J, et al. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37(3):127–133. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 23.Kujan O, Oliver RJ, Khattab A, et al. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(10):987–993. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Su L, Morgan PR, Thomas JA, et al. Expression of keratin 14 and 19 mRNA and protein in normal oral epithelia, hairy leukoplakia, tongue biting and white sponge nevus. J Oral Pathol Med. 1993;22(4):183–189. doi: 10.1111/j.1600-0714.1993.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 25.Toyoshima T, Vairaktaris E, Nkenke E, et al. Cytokeratin 17 mRNA expression has potential for diagnostic marker of oral squamous cell carcinoma. J Cancer Res Clin Oncol Erratum in: J Cancer Res Clin Oncol. 2008;34(4):523-24,134(4):515-21. [DOI] [PubMed]
- 26.Fillies T, Werkmeister R, Packeisen J, et al. Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer. 2006;13(6):10. doi: 10.1186/1471-2407-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida Y. [Effective biological marker to detect oral squamous cancer cells–expression patterns of CK 10, 17, 19 and SCCA mRNA–]. Kokubyo, Gakkai Zasshi. 2007;73 74 (3,1):37-42. [DOI] [PubMed]
- 28.Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral Dis. 1998;4(2):70–77. doi: 10.1111/j.1601-0825.1998.tb00260.x. [DOI] [PubMed] [Google Scholar]