Abstract
Sarcomatoid carcinomas are biphasic tumors proven to be monoclonal dedifferentiated forms of conventional squamous carcinomas. This study evaluates their clinicopathologic characteristics in head and neck mucosal sites and the problems in distinguishing them from other spindle cell tumors. A total of 103 cases with a confirmed diagnosis of sarcomatoid carcinoma accessioned in the pathology department of a tertiary referral cancer centre over a period of 7 years (2004–2010) were studied. An algorithm used for their diagnosis is presented. Ages of the patients were 22–90 years (median 53 years), and male:female ratio was 3.7:1. Site distribution was oral cavity (n = 65, 63.1%), larynx (18, 17.5%), oropharynx/hypopharynx (12, 10.7%), maxilla (6, 5.8%) and metastatic nodes (2, 1.9%). A large number of patients (95%) presented with a mass lesion of less than 1 year duration. Histopathologically, epithelial differentiation was evident on morphology in 48 (46.6%) cases, only on IHC in 34 (33%) cases, and in 21 (20.4%) no epithelial differentiation was seen. Typically, tumors were polypoidal (92, 89.3%) and ulcerated (95, 92.2%) with cells arranged predominantly in fascicles (59, 57.3%) or storiform pattern (17, 16.5%) amidst collagenous (50, 48.5%) or myxoid matrix (35, 34%). Anaplasia (2+/3+) and mitosis >10 per 10 HPF were noted in 96 (93.2%) cases. IHC was done in 82 cases; 55 (66.7%) showed positivity for epithelial markers with aberrant expression of mesenchymal markers in 43 (41.7%). Diagnosis of sarcomatoid squamous carcinoma is challenging because of overlapping histopathological features with other spindle cell tumors. Understanding their clinicopathologic characteristics facilitates their diagnosis and appropriate clinical management.
Keywords: Sarcomatoid, Squamous, Carcinoma, Head and neck, Spindle cell tumors
Introduction
Sarcomatoid (spindle cell) carcinomas of the head and neck are unusual variants of squamous carcinoma commonly reported in the larynx but also described in other mucosal sites such as gingiva, tongue, hypopharynx and nasal cavity [1–17]. These tumors have now been proved to be monoclonal, evolving from conventional squamous carcinoma with dedifferentiation associated with sarcomatoid transformation [17–32]. They pose a significant diagnostic challenge to the pathologist with remarkable morphological and immunohistochemical overlap with other benign and malignant spindle cell tumors [1, 3]. An accurate diagnosis of these tumors is essential as they vary in their clinical management and outcome [3].
This study is an overview of the spectrum of a large number of sarcomatoid carcinomas arising in all head and neck mucosal sites to evaluate their clinicopathologic, detailed morphological and immunohistochemical characteristics and problems in differentiating them from other spindle cell tumors commonly occurring at these sites. An algorithm for a systematic approach to their diagnosis is also presented.
Materials and Methods
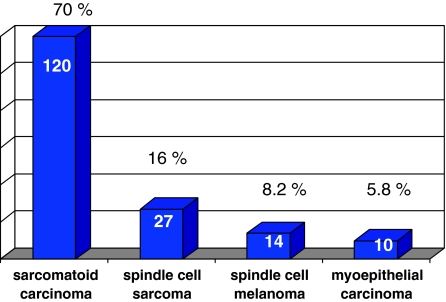
This is a retrospective review of all cases of sarcomatoid squamous carcinomas of the head and neck region accessioned in the Department of Pathology over 7 years between 2004 and 2010. The sites included were oral cavity, maxilla, oropharynx/hypopharynx, and larynx. This study was carried out in a tertiary referral cancer centre in a geographic area with a large burden of head and neck cancers constituting about 20–25% of all specimens accessioned. A total 171 cases of spindle cell tumors were reported over 7 years from 2004 to 2010, out of which 103 cases with a confirmed diagnosis of sarcomatoid squamous carcinoma were included in the study. These tumors constituted 70% of all spindle cell tumors recorded (Fig. 1). Of these, 92 cases were diagnosed and managed in the hospital while 11 cases were referral material received for pathology consultation only. There were 34 resected specimens and 69 biopsies which were evaluated where subsequent excised specimen was not available for evaluation. The 69 biopsies comprised of cases where the material was referred only for diagnosis, the patient presented with advanced disease stage and were not amenable to surgical treatment or the patient was treated subsequently with palliative intent.
Fig. 1.
Distribution of spindle cell lesions in head and neck, 2004–2010 (n = 171)
Clinical information in terms of socio-demographic profile and site of lesion was available in all the 103 patients which were taken from the hospital records. Forty-seven patients had a history of predisposing factor; while the follow-up details were available in 39 patients.
Diagnosis of Sarcomatoid Carcinoma
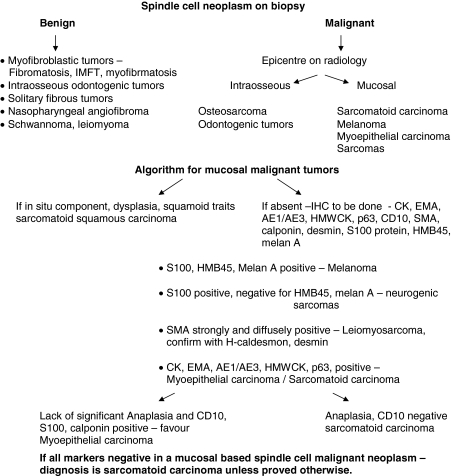
Of the total of 171 cases of mucosal spindle cell tumors of the head and neck, cases of sarcomatoid carcinoma were diagnosed and selected as per the diagnostic algorithm (Fig. 2). Immunohistochemistry (IHC) was performed on the representative paraffin blocks in 82 of the 103 cases. The remaining 21 cases had light microscopic evidence of squamous carcinoma and hence IHC was not asked for. In four cases they could not be done due to lack of availability of paraffin blocks on outside referral material. The panel of IHC done, details of the antibodies used and their dilutions are enlisted in Table 1.
Fig. 2.
Approach to the diagnosis of spindle cell neoplasms from mucosal based sites in the head and neck on biopsy material
Table 1.
Immunohistochemistry (IHC), antibodies and their dilutions
| Antibody | Clone | Dilution | Vendor |
|---|---|---|---|
| Cytokeratin (CK) | MNF116 | 1:200 | DAKO |
| Epithelial membrane antigen (EMA) | E29 | 1:200 | DAKO |
| Vimentin | V9 | 1:400 | DAKO |
| Smooth muscle actin (SMA) | IA4 | 1:400 | DAKO |
| Calponin | CALP | 1:50 | DAKO |
| CD31 | JC/70A | 1:50 | DAKO |
| CD34 | QBEnd10 | 1:200 | DAKO |
| High molecular weight cytokeratin (HMWCK) | 34bE12 | 1:200 | DAKO |
| S100 protein | Polyclonal | 1:1,500 | DAKO |
| HMB45 | HMB45 | 1:200 | DAKO |
| CD10 | 56C6 | 1:40 | BIOCARE |
| Ck5/6 | D5/16B4 | 1:50 | DAKO |
Parameters Assessed Light Microscopy
Hematoxylin and eosin stained slides of all the cases were assessed for parameters like overall appearance of the tumor, status of the overlying epithelium, presence or absence of dysplasia, carcinoma—in situ, presence or absence of squamous differentiation within the tumor, pattern of arrangement of the tumor cells, presence or absence of intervening matrix such as collagen, myxoid change, presence or absence of necrosis, tumor giant cells, nucleoli, degree of nuclear anaplasia, graded as 1+ (mild), 2+ (moderate) or 3+ (severe) and number of mitotic figures calculated per ten high-power fields.
Results
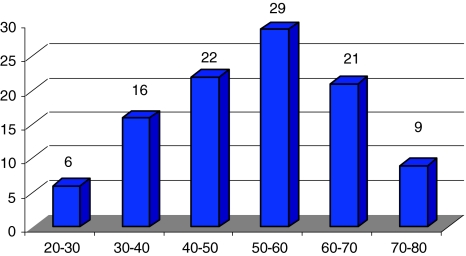
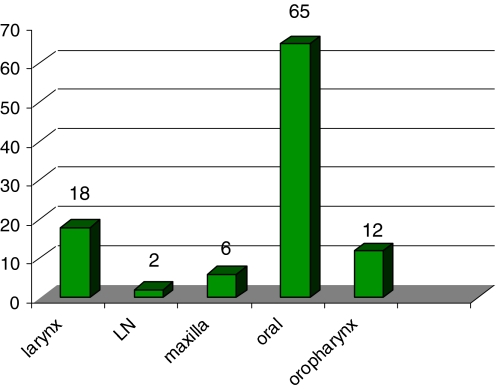
This study included a total of 103 cases of sarcomatoid carcinoma. The age at presentation ranged from 20 to 90 years, with maximum number of patients in the fifth and sixth decades (Fig. 3), and a median age of 53 years. There were 81 male patients and 22 female patients (male:female ratio was 3.7:1). The oral cavity was the most common affected site seen in 65 cases (63.1%) as shown in Fig. 4, followed by the larynx in 18 cases (17.5%), the oropharynx/hypopharynx in 12 cases (11.7%), and the maxilla in six cases (5.8%). In two cases (1.9%) only metastatic cervical lymph nodes were biopsied and this was the specimen evaluated. One of these was a large metastatic cervical mass from an unknown primary and the other was referral material where clinical information of the primary was not available.
Fig. 3.
Age range of patients
Fig. 4.
Site distribution of cases
The subsite distribution in the oral cavity showed that the most common site involved was buccal mucosa and gingivobuccal sulcus (25 cases, 38.5%) followed by upper or lower alveolus in 20 cases (30.8%), tongue (13 cases, 20%), hard palate (5 cases, 7.8%) and lip (2 cases, 3.1%). The subsite distribution in the larynx included involvement of supraglottis in six cases (33.3%), glottis in ten cases (55.6%) and subglottis in two cases (11.1%).
Predisposing Factors
History of presence of a predisposing risk factor was available in 47 patients of which 30 (63.8%) patients had a history of chewing tobacco, ten (21.3%) patients had history of smoking and six (12.8%) had a history of consuming alcohol. None of the patients had prior history of exposure to radiation.
Clinical Presentation
The oral cavity lesions manifested with polypoidal or ulcerated growth, difficulty in mouth opening and pain. Oropharyngeal/hypopharyngeal lesions presented with growth, foreign body sensation, dysphagia or odynophagia. The most common presentation in laryngeal lesions was change in voice. Tumors in the maxilla or nasal cavity presented with excessive tearing from the eye, mass lesion and bleeding. Duration of symptoms ranged from 20 days to 2 years with less than 1 year in 95% of patients.
The size of the tumor on clinical appearance ranged from 2 to 6 cm in size and the clinical appearance of the lesion was described as proliferative or ulceroproliferative tumor with a polypoidal configuration. The clinical stage was available in 56 cases which were T1 (8 cases), T2 (17 cases), T3 (9 cases), and T4 (19 cases). In three cases the primary stage was not known, one of these presented with a large nodal mass as an unknown primary and two others had had treatment elsewhere and then referred to the hospital for further management. Lymph node metastasis was seen at presentation in 21/50 (42%) patients where data was available. Lymph node metastasis were present in 14/65 (21.5%) oral lesions, 4/12 (33.3%) oro/hypo pharyngeal lesions, 1/18 (0.5%) laryngeal lesions and in two cases metastatic nodes were seen with unknown primary.
Gross Examination
All the biopsies received were documented as polypoidal mucosa covered or ulcerated bits. The excision specimens showed polypoidal masses with surface ulceration with a firm fibrous appearance submucosally.
Microscopy
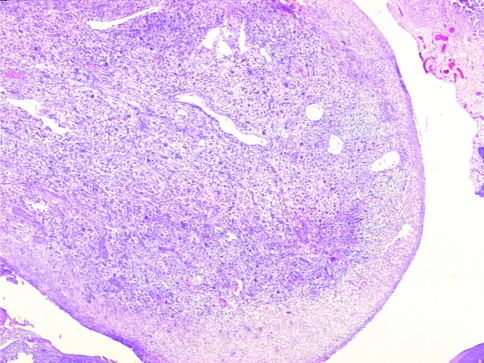
A large number of tumors (92/103 cases, 89.3%) had a polypoid configuration on the biopsy (Fig. 5). The overlying epithelium was ulcerated and covered with fibrinoid material and slough (95/103, 92.2%). As a result, in some of these cases changes in the overlying epithelium could not be assessed. The tumor was seen to invade the underlying bone in 19/34 cases (55.9%) where surgical excision was done.
Fig. 5.
Section showing typical polypoidal configuration of the tumor with ulceration of the overlying mucosa (H&E ×40)
The cases were divided into 3 groups.
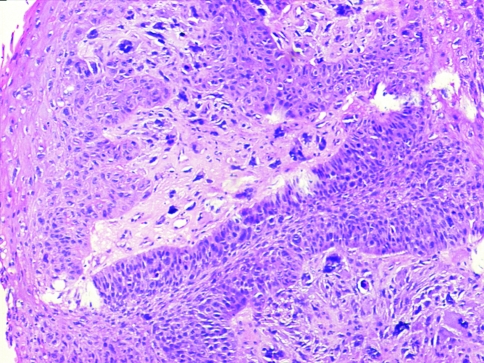
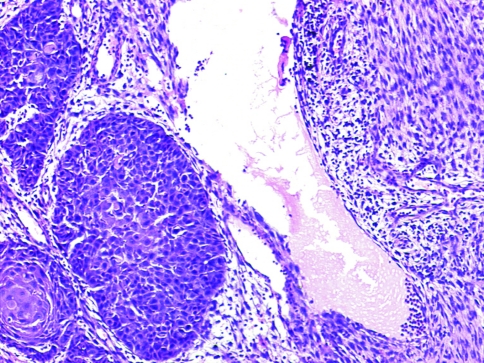
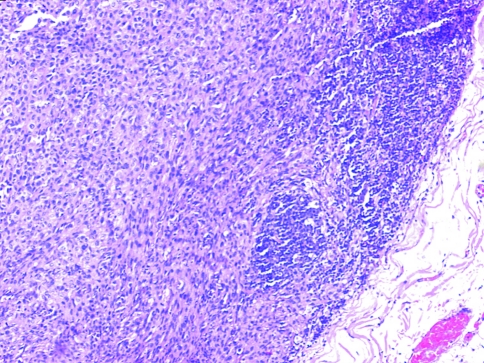
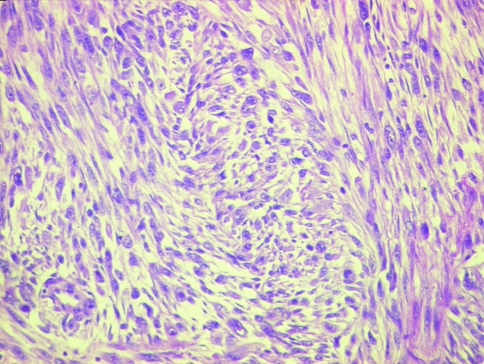
Group I (48/103 cases, 46.6%) had frank epithelial differentiation at light microscopic level (dysplasia/squamous carcinoma in situ/squamous nests/lymph node metastasis) (Figs. 6, 7, 8).
Group II (34/103 cases, 33%) had epithelial differentiation at immunohistochemistry level only (using antibody panel CK, EMA, HMWCK, p63, CK5/6, AE1/AE3) (Fig. 9a–c).
Group III (21/103 cases, 20.4%) displayed no evidence of epithelial differentiation.
Fig. 6.
Epithelial differentiation evident on light microscopy showing tumors with nests of frank squamous carcinoma intimately admixed with sarcomatoid elements (H&E ×100)
Fig. 7.
Epithelial differentiation evident on light microscopy showing overlying carcinoma in situ (H&E ×40)
Fig. 8.
Lymph node metastasis of a case of sarcomatoid carcinoma (H&E ×40)
Fig. 9.
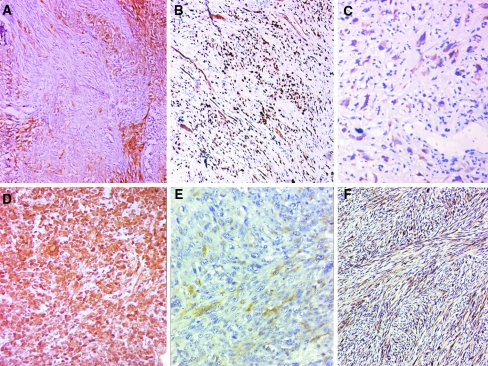
a–c Epithelial differentiation in sarcomatoid carcinomas identified at the immunohistochemistry (IHC) level with varying proportion and intensity of positive staining. a Focal strong positivity for cytokeratins (CK). b Diffuse strong positivity for p63. c Diffuse weak positivity for high molecular weight cytokeratins (HMWCK). d, e Aberrant mesenchymal marker expression on IHC. d Strong and diffuse positivity for vimentin. e Focal weak positivity for smooth muscle actin (SMA). e Strong diffuse positivity for calponin
A squamous component in the form of carcinoma in situ or dysplasia of the overlying epithelium was identified in 26 (25.2%) cases (Figs. 6, 7). Nests of conventional squamous carcinoma or squamoid differentiation within the tumor was seen in 32 (31.1%) cases. Both these components were identified in 13 (12.6%) cases. The sarcomatoid and the squamous component were intricately mixed. At times the area of elongation and spindling seemed to arise from the basal epithelial cells making indistinct any demarcation between the surface epithelial origin and the underlying tumor.
The invasive squamous component was seen in 35 cases; of these in 60% of cases the squamous element was poorly differentiated while in 40% of cases it was moderately differentiated. The sarcomatoid component was divided into three grades based on the degree of anaplasia, seven cases were grade 1 (mild anaplasia), 60 cases were grade 2 (moderate anaplasia) and 36 cases were grade 3 (severe anaplasia). Lymph node metastasis morphologically showed either a frank squamous carcinoma or admixture of both epithelial as well as spindle cell component or purely spindle cell component.
In 55 cases squamous differentiation was absent on morphology. This may partly be due to lack of availability of the overlying epithelium due to extensive ulceration. In 34 (33%) the epithelial differentiation was identified only on IHC (Fig. 9a–c).
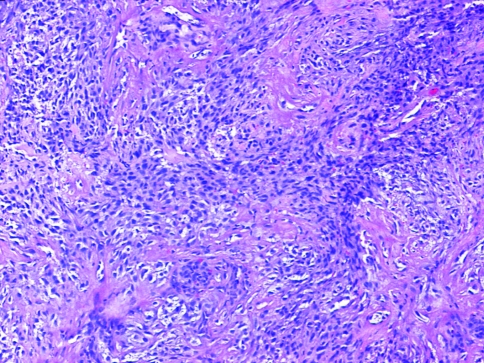
The sarcomatoid component of the tumor was arranged in various patterns mimicking mesenchymal malignancies both by light microscopy as well as on IHC (Fig. 9d–f). Tumor cells were seen arranged in fascicles (59–57.3%), in a storiform pattern (17–16.5%), or admixed with extensive inflammation and granulation tissue simulating inflammatory myofibroblastic tumors (16, 15.5%). Marked epithelioid appearance of the tumor cells was identified in 20 (19.4%) cases and only single cell infiltration admixed with abundant collagenous or myxoid matrix was seen in two cases (1.2%).
Another characteristic of mesenchymal malignancies seen in these tumors was presence of variable amount of intervening matrix (Figs. 10, 11, 12) such as collagen in 50 (48.5%) cases and prominent myxoid stroma in 35 (34%) cases. The matrix material in varying proportions admixed with the tumor cells gave rise to various patterns within the tumor. When the biopsy was sampled from an extensively ulcerated area, we observed scanty spindloid malignant tumor cells amidst granulation tissue making the diagnosis difficult.
Fig. 10.
Fasciculated arrangement of tumor cells mimicking a mesenchymal malignancy (H&E ×40)
Fig. 11.
Spindled tumor cells separated by abundant myxoid matrix
Fig. 12.
Tumor cells separated by collagenous matrix
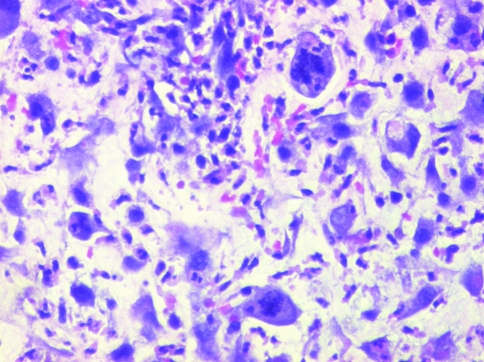
Diagnosis of malignancy was not difficult in all 103 cases, as anaplasia was graded as at least 2+ in 96 cases (93.2%), of which in 36 cases (35%) the grading was 3+ or severe (Fig. 13a–d). Mitotic count of more than 10/10 high-power fields was noted in 96 (93.2%) cases, of these 60 cases (58.2%) showed a very high mitotic count of 3–8 per high power field. Bizzare atypical mitotic were easily identified in most cases (Fig. 13). Prominent eosinophilic nucleoli were observed in 31 (30.1%) cases. Cytoplasm was abundant eosinophilic and dense giving the clue towards epithelial differentiation. Spotty necrosis was present in 23 (22.4%) cases within the tumor, this did not include ulcerated areas. None of the case in our study showed metaplasic mesenchymal elements such as bone or cartilage formation within the tumor.
Fig. 13.
Marked anaplasia with atypical bizarre mitotic figures (H&E ×100)
Immunohistochemistry was performed in 82 of the 103 cases. The remaining 21 cases had the presence of an epithelial component and hence IHC was not considered essential for diagnosis. At least one of the epithelial markers (CK, EMA, HMWCK, p63, CK5/6, AE1/AE3) was positive in 55 (53.4%) cases (details in Table 2). This helped in establishing the diagnosis of an epithelial malignancy in 34 (33%) cases where other morphological features of squamous epithelial differentiation such as carcinoma in situ or invasive components were absent. CK was positive in 40 of 80 cases in which it was done (50%); EMA was positive in 23 of 80 cases done (28.8%). CK was positive in 26 cases where EMA was negative and EMA was positive in nine cases where CK was negative. Either CK or EMA were positive and hence useful in 49 of the 80 cases (61.3%) in which they were done. HMWCK was positive in seven of 30 (23.3%) cases done, all of which were also positive for either CK or EMA. p63 was positive in nine out of 30 cases in which it was done. In the two other cases epithelial nature was determined by positivity for CK5/6 and AE1/AE3. Percentage and intensity of staining in tumor cells which were positive for epithelial markers varied widely; in some cases the cells showed diffuse and intense positivity, and in some cases the staining was weak and focal. Vimentin was positive in all the 29 cases where it was done. Occasional aberrant expression of other mesenchymal markers gave rise to diagnostic confusion. Calponin was positive in all 8/8 cases (100%) where it was asked for. SMA and S100 were positive in 4/25 (16%) and 2/30 (6.7%) cases, respectively. None of the cases were positive for CD31 (0/9 cases), CD34 (0/11 cases), or HMB45 (0/13 cases) in which they were done.
Table 2.
A detailed comparison of the key features in our study compared to that of Thompson et al. [3]
| Parameters | Thompson et al. [3] | Our study |
|---|---|---|
| General parameters | ||
| Number of cases | 187 | 103 |
| Site | Larynx only | All head neck mucosal sites |
| Median age (years) | 66 | 53 |
| M:F | 13:1 | 3.7:1 |
| Pathological features | ||
| Squamoid differentiation | 149 (79.6%) | 48 (46.6%) |
| Polypoid configuration | 185 (98.9%) | 92 (89.3%) |
| Ulcerated | 144 (77%) | 95 (92.2%) |
| Pattern—fasciculated/storiform | 132 (70.5%) | 69 (71.2%) |
| Tumor giant cells | 106 (56.7%) | 46 (44.7%) |
| Stroma—collagen | 93 (49.7%) | 50 (48.5%) |
| Stroma—myxoid | Not mentioned | 35 (34%) |
| Necrosis | 26 (13.9%) | 23 (22.4%) |
| Anaplasia (2+/3+) | 114 (70%) | 96 (93.2%) |
| Atypical mitosis | 171 (91.4%) mean 11.9/10 hpf | 96 (93.2%) (>10/10 hpf) |
| Nucleoli | Not mentioned | 31 (30.1%) |
| Metaplastic component | 13 (7%) | Absent |
| Immunohistochemistry | ||
| Performed in cases | 123 (65.8%) | 82 (79.6%) |
| Epithelial marker positivity | 84 (68.3%) | 55 (53.4%) |
| Pan CK—Intermediate and LMWCK (CK 5, 6, 8, 17, 19) | Not mentioned as a cocktail | 40/80 (50%) |
| Epithelial membrane antigen | 21/117 (17.9%) | 23/80 (28.8%) |
| P63 | Not mentioned | 9/30 (30%) |
| HMWCK (CK1, 5, 10, 14) | Not mentioned as a cocktail | 7/30 (23.3%) |
| Vimentin | 108/108 (100%) | 29/29 (100%) |
| Calponin | Not mentioned | 8/8 (100%) |
| Smooth muscle actin | 40/123 (32.5%) | 4/25 (16%) |
| S100 protein | 6/123 (4.9%) | 2/30 (6.7%) |
| Desmin | 4/123 (3.3%) | 2/11 (18.2%) |
Cocktail of low molecular weight cytokeratins (LMWCK) and high molecular weight cytokeratins (HMWCK)
A total lack of epithelial differentiation as well as absence of epithelial immunomarker positivity was seen in 21 cases including four cases where IHC could not be done due to non-availability of paraffin blocks. Diagnosis of sarcomatoid carcinoma in these cases was hence by exclusion (algorithm as shown in Fig. 2).
No significant difference was found on comparison of clinical and pathological features of sarcomatoid carcinoma arising from different sites. However, oral cavity lesions predominated while the numbers for the other subsites were few. Epithelial differentiation was seen in 28/65 (43.1%) oral, 7/12 (58.3%) oropharyngeal/hypopharyngeal, 10/18 (55.6%) laryngeal and 1/5 (20%) maxillary lesions. Immunopositivity for epithelial markers were identified in 36/53 (67.9%) oral lesions, 3/4 (75%) maxillary lesions, 8/15 (53.3%) laryngeal lesions, 6/8 (75%) oropharyngeal/hypopharyngeal lesions and 2/2 (100%) lymph nodal metastasis.
Follow-Up
Treatment details and follow-up was not known in the 11 cases sent only for pathology consultation. Hospital records could be obtained in 65 of the remaining 92 cases managed within the institution. Among these, 39 patients were treated with curative intent, five were treated with palliative intent while the remaining 21 patients were lost to follow-up. Hence, follow-up data was available in 39 patients and the duration of follow-up ranged from 1 to 39 months with an average follow-up of 8 months. Of these, 20 patients were alive without evidence of disease and six patients were alive with disease at last follow-up. Local recurrence of the tumor was seen in eight patients (20.5%), one at presentation and in the rest of the seven patients at 2, 4, 5, 7, 24 and 27 months after treatment. Distant metastasis was seen in two patients to the lung and soft tissues of the shoulder region at 2 and 9 months respectively while three patients (5.8%) died of disease at 1, 2 and 6 months after treatment.
Discussion
Sarcomatoid (spindle cell) carcinomas are unusual variants of squamous carcinoma reported to account for 3% of all squamous carcinomas in the head and neck region [1–3]. Both the sarcomatoid as well as conventional squamous carcinoma components have now been proven to arise monoclonally from a single stem cell, and there is further evidence to prove that the sarcomatoid component represents a dedifferentiation and suggests molecular progression of the conventional component [18–32]. The clinical behavior of sarcomatoid carcinoma is however reported to parallel that of conventional squamous carcinoma with the outcome being determined by the location and clinical stage [2, 3, 19].
Our study comprised a total of 103 cases of sarcomatoid carcinoma involving all mucosal sites in the head and neck region. Of these, 34 were excision specimens while 69 specimens were biopsies where the excision specimen was not available.
This study, to the best of our knowledge, is among the largest series of sarcomatoid carcinomas reported which includes the exhaustive and detailed review of 187 cases in the larynx by Thompson et al. [3]. A detailed comparison of key features between the two studies are presented in Table 2. In our study, the incidence of sarcomatoid carcinoma was approximately 70% of all the malignant spindle cell tumors of the head and neck region accessioned in the pathology department over a period of 7 years.
Clinical Parameters
The age at presentation ranged from 20 to 90 years, with predominance in the fifth and sixth decades. This finding is similar to other reports in literature which suggest maximum occurrence between the fifth and seventh decades of life [3, 10–14]. The male to female ratio in our study was 3.7:1, similar to the male predominance reported by all studies [3, 10–14, 33, 34]. It has been noted that tobacco use (both chewed and smoked forms) and alcohol consumption are the strong causative agents for the development of squamous carcinoma in general as well as for sarcomatoid carcinoma [3, 10, 13–15, 33, 34]. All these factors were seen in our study. Additionally, tobacco chewing (63.8%) was more commonly observed than smoking (21.3%) in our study, similar to the reported figures of 77% and 42% from this part of the world [33, 34]; hence oral cavity was the predominant site affected. This is however in contrast to Western studies [3, 10–15, 17, 24] where smoking of tobacco is reported to be the predominant habit and maximum number of cases are reported in the larynx. None of the patients had prior history of exposure to radiation which has been reported as one of the etiological agents for sarcomatoid carcinoma [3, 17]. This may possibly be due to the oral cavity being the most common site involved in this study where surgery is the mainstay of treatment, in contrast to laryngeal cancers where radiation alone or in combination with chemotherapy are the chief therapeutic modalities.
The most commonly involved subsites of buccal mucosa in the oral cavity and glottis in laryngeal tumors are similar to other reports [2, 3, 10–15, 17, 33, 34]. The type of symptoms observed and their short duration of onset of less than 1 year have also been reported [3]. A large number of patients presented with advanced disease stage, again a common occurrence in this part of the world [33, 34]. The clinical and gross appearance of a polypoid configuration have been widely reported and characterize these tumors [2, 3, 10–17, 23–25].
Histomorphology
Sarcomatoid carcinoma contains both a conventional epithelial squamous component and a sarcomatous spindle cell component [2, 3, 17–32]. As also noted in our study, epithelial differentiation evident within the tumor is reported to be highly variable with either changes in the overlying epithelium in the form of dysplasia or carcinoma in situ or as part of the infiltrating tumor [1–3]. The latter may be demonstrable at light microscopy or by the expression of epithelial markers at immunohistochemistry, electron microscopy or at the molecular level [2, 3, 17–32]. The degree and intensity of epithelial marker expression is also reported to vary [3] as was observed in our study as well. Immunopositivity with any of the epithelial markers was observed in 53.4% of cases in our study, comparable to reported figures of between 48 and 75% [3, 17, 19, 23, 25–27]. This may be due to a number of technical and histological factors as detailed by Thompson et al. [3] which include subjectivity in interpretation, sampling error, nonhomogeneous tumors, poor preservation or fixation, inappropriate antibody or technique, or epithelial features less than the threshold of detection. One of the other reasons for low immunopositivity for epithelial markers in this study may be due to the large number of biopsies studied where the entire tumor is not available for assessment. However, this also represents the diagnostic difficulty encountered with sarcomatoid carcinomas in practice while reporting on limited biopsy material prior to definitive surgical excision or in advanced diseases which are not amenable to surgical treatment. The immunohistochemical results are reported to improve by including a larger panel of antibodies [3]. However, in daily practice, depending on the type of pathology centre, cost constraints limit the number of antibodies that can be asked in each case. In our study pan CK (including intermediate and LMWCK) and EMA were found to be most useful practically and were positive in 61.3% of cases. In sarcomatoid carcinomas it has been reported that epithelial marker expression decreases as the degree of epithelial differentiation decreases and may be lost entirely [3]. Hence, although an immunopositivity can help, a negative result does not rule out the diagnosis of sarcomatoid carcinoma. This was observed in 21 (20. 4%) of our cases.
It has been documented that the epithelial cells go through a spectrum of progressive phenotypic changes, acquiring a mesenchymal pathway of differentiation metamorphosing to a spindle shape, undergoing a loss of cellular polarity, producing mesenchymal matrix components, and gaining vimentin while losing keratin expression. It has been demonstrated that the phenotypic plasticity of interconversion of epithelium to mesenchyme cells (as seen during embryogenesis) is expressed by a loss of intercellular cohesion, elongation of the cells, loss of basement membrane, production of connective tissue (collagen), and invasion into the stroma [3, 20, 28–30].
This divergent mesenchymal differentiation of the tumor cells seems to be supported by the carcinoma cells acquiring the potential to express a mesenchymal phenotype at the light microscopic, immunohistochemical, and ultrastructural levels [2–32]. The degree of mesenchymal metaplasia within the tumor may vary widely and may be seen as bony or cartilagenous elements within the tumor or as aberrant immunohistochemical marker expression [2–8, 17–32]. Although no aberrant metaplastic elements were observed in any of our cases, we did see aberrant expression of vimentin, calponin, SMA, desmin and S100 protein, which is also well reported in literature [3]. Although diffuse and strong positivity for vimentin and calponin was seen in all cases, SMA, desmin and S100 protein were weak, faint and patchy helping in ruling out leiomyosarcoma (strong SMA/desmin positivity) or melanoma (strong S100 positivity).
Differential diagnosis of sarcomatoid carcinoma includes other malignant mesenchymal tumors [1–3] such as mucosal spindle cell melanoma, leiomyosarcoma, myoepithelial carcinoma and sometimes it is not uncommon to get bony lesions such as osteosarcoma extending to the mucosa leading to a diagnostic confusion with sarcomatoid carcinoma if the radiological picture is not taken into consideration.
Clinical Outcome and Follow-Up
Disease progression in sarcomatoid carcinoma is reported to be characterized by recurrences and metastases [2, 3, 9, 14–17]. This was seen in 13 of the 39 patients (33.3%) where follow-up data could be obtained. Local recurrence was seen in eight patients (20.5%), similar to the reported figures of between 16 and 32% [10, 12]. Lymph nodal metastasis at presentation was seen in 21 (42%) patients which was the most common site of metastasis in contrast to the findings of Thompson et al. [3] who reported lung to be the most frequent site for metastasis.
In addition, the incidence of cervical nodal metastasis reported also varied widely, between 7.5 and 26% [3, 10, 13]. Distant metastasis to the lung and soft tissue was seen in two patients (5.1%) which was similar to reported figures of 5% by Lambert et al. [13]. Incidentally, lung was the most frequent site for metastasis reported by Thompson et al. who did not have soft tissue as the site for metastasis in their large number of patients [3]. Death due to disease could be documented in 3 (7.7%) patients. However, no definite conclusions on survival could be made due to limited availability of follow-up. The reported overall mortality due to the tumor is 14–32% [3, 10, 12, 17].
Thus, to summarise, diagnosis of sarcomatoid variants of squamous carcinomas in the head and neck mucosal site is challenging because of overlapping histopathological features with other spindle cell tumors. Understanding their clinical, morphological and immunohistochemical features with a systematic approach is critical for their accurate diagnosis which aids in correct patient management.
References
- 1.Anderson CE, Al-Nafussi A. Spindle cell lesions of the head and neck: an overview and diagnostic approach. Diagn Histopathol. 2009;15(5):264–272. doi: 10.1016/j.mpdhp.2009.02.009. [DOI] [Google Scholar]
- 2.Thompson LDR. Squamous cell carcinoma variants of the head and neck. Curr Diagn Pathol. 2003;9:384–396. doi: 10.1016/S0968-6053(03)00069-3. [DOI] [Google Scholar]
- 3.Thompson LD, Wieneke JA, Miettinen M, Heffner DK. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol. 2002;26:153–170. doi: 10.1097/00000478-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Katase N, Tamamura R, Gunduz M, Murakami J, Asaumi J, Tsukamoto G, Sasaki A, Nagatsuka H. A spindle cell carcinoma presenting with osseous metaplasia in the gingiva: a case report with immunohistochemical analysis. Head Face Med. 2008;4:28. doi: 10.1186/1746-160X-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munakata R, Cheng J, Nakajima T, Saku T. Spindle cell carcinoma of the gingiva: report of an autopsy case. J Oral Pathol Med. 1998;27(4):180–184. doi: 10.1111/j.1600-0714.1998.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen YK, Lin CC, Chen CH, Yan YH, Lin LM. Spindle cell carcinoma of the tongue: case report and immunohistochemistry study. Oral Med Pathol. 1998;3:51–54. doi: 10.3353/omp.3.51. [DOI] [Google Scholar]
- 7.Batsakis JG, Suarez P. Sarcomatoid carcinomas of upper aerodigestive tracts. Adv Anat Pathol. 2000;7:282–293. doi: 10.1097/00125480-200007050-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ellis GL, Langloss JM, Heffner DK, Hyams VJ. Spindle-cell carcinoma in the upper aerodigestive tract. An immunohistochemical analysis of 21 cases. Am J Surg Pathol. 1987;11:335–342. doi: 10.1097/00000478-198705000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Su HH, Chu ST, Hou YY, Chang KP, Chen CJ. Spindle cell carcinoma of the oral cavity and oropharynx: factors affecting outcome. J Chin Med Assoc. 2006;69:478–483. doi: 10.1016/S1726-4901(09)70312-0. [DOI] [PubMed] [Google Scholar]
- 10.Goellner JR, Devine KD, Weiland LH. Pseudosarcoma of the larynx. Am J Clin Pathol. 1973;59:312–326. doi: 10.1093/ajcp/59.3.312. [DOI] [PubMed] [Google Scholar]
- 11.Hellquist H, Olofsson J. Spindle cell carcinoma of the larynx. APMIS. 1989;97:1103–1113. doi: 10.1111/j.1699-0463.1989.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 12.Hyams VJ. Spindle cell carcinoma of the larynx. Can J Otolaryngol. 1975;4:307–313. [PubMed] [Google Scholar]
- 13.Lambert PR, Ward PH, Berci G. Pseudosarcoma of the larynx: a comprehensive analysis. Arch Otolaryngol. 1980;106:700–708. doi: 10.1001/archotol.1980.00790350042012. [DOI] [PubMed] [Google Scholar]
- 14.Olsen KD, Lewis JE, Suman VJ. Spindle cell carcinoma of the larynx and hypopharynx. Otolaryngol Head Neck Surg. 1997;116:47–52. doi: 10.1016/S0194-5998(97)70351-6. [DOI] [PubMed] [Google Scholar]
- 15.Leventon GS, Evans HL. Sarcomatoid squamous cell carcinoma of the mucous membranes of the head and neck: a clinicopathologic study of 20 cases. Cancer. 1981;48:994–1003. doi: 10.1002/1097-0142(19810815)48:4<994::AID-CNCR2820480424>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Sherwin RP, Strong MS, Vaughn CW., Jr Polypoid and junctional squamous cell carcinoma of the tongue and larynx with spindle cell carcinoma (‘pseudosarcoma’) Cancer. 1963;16:51–60. doi: 10.1002/1097-0142(196301)16:1<51::AID-CNCR2820160108>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JE, Olsen KD, Sebo TJ. Spindle cell carcinoma of the larynx: review of 26 cases including DNA content and immunohistochemistry. Hum Pathol. 1997;28:664–673. doi: 10.1016/S0046-8177(97)90175-1. [DOI] [PubMed] [Google Scholar]
- 18.Choi HR, Sturgis EM, Rosenthal DI, Luna MA, Batsakis JG, El-Naggar AK. Sarcomatoid carcinoma of the head and neck: molecular evidence for evolution and progression from conventional squamous cell carcinomas. Am J Surg Pathol. 2003;27:1216–1220. doi: 10.1097/00000478-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Takata T, Ito H, Ogawa I, Miyauchi M, Ijuhin N, Nikai H. Spindle cell squamous carcinoma of the oral region. An immunohistochemical and ultrastructural study on the histogenesis and differential diagnosis with a clinicopathological analysis of six cases. Virchows Arch. 1991;419:177–182. doi: 10.1007/BF01626345. [DOI] [PubMed] [Google Scholar]
- 20.Guarino M, Tricomi P, Giordano F, Cristofori E. Sarcomatoid carcinomas: pathological and histopathogenetic considerations. Pathology. 1996;28:298–305. doi: 10.1080/00313029600169224. [DOI] [PubMed] [Google Scholar]
- 21.Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol. 1996;20:277–285. doi: 10.1097/00000478-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Torenbeek R, Hermsen MA, Meijer GA, Baak JP, Meijer CJ. Analysis by comparative genomic hybridization of epithelial and spindle cell components in sarcomatoid carcinoma and carcinosarcoma: histogenetic aspects. J Pathol. 1999;189:338–348. doi: 10.1002/(SICI)1096-9896(199911)189:3<338::AID-PATH429>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Zarbo RJ, Crissman JD, Venkat H, et al. Spindle-cell carcinoma of the upper aerodigestive tract mucosa: an immunohistologic and ultrastructural study of 18 biphasic tumors and comparison with seven monophasic spindle-cell tumors. Am J Surg Pathol. 1986;10:741–753. doi: 10.1097/00000478-198611000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Brodsky G. Carcino (pseudo) sarcoma of the larynx: the controversy continues. Otolaryngol Clin North Am. 1984;17:185–197. [PubMed] [Google Scholar]
- 25.Barnes L. Spindle cell carcinoma, sarcomatoid carcinoma, carcinosarcoma. In: Barnes L, editor. Surgical pathology of the head and neck, vol. 1, 3rd ed. New York: Marcel Dekker; 2001. p. 174–81.
- 26.Ellis GL, Langloss JM, Heffner DK, et al. Spindle-cell carcinoma of the aerodigestive tract: an immunohistochemical analysis of 21 cases. AmJ Surg Pathol. 1987;11:335–342. doi: 10.1097/00000478-198705000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Meijer JW, Ramaekers FC, Mannij J, et al. Intermediate filament proteins in spindle cell carcinoma of the larynx and tongue. Acta Otolaryngol (Stockh) 1988;106:306–313. doi: 10.3109/00016488809106441. [DOI] [PubMed] [Google Scholar]
- 28.Nakhleh RE, Zarbo RJ, Ewing S, et al. Myogenic differentiation in spindle cell (sarcomatoid) carcinomas of the upper aerodigestive tract. Appl Immunohistochem. 1993;1:58–68. [Google Scholar]
- 29.Battifora H. Spindle cell carcinoma: ultrastructural evidence of squamous origin and collagen production by the tumor cells. Cancer. 1976;37:2275–2282. doi: 10.1002/1097-0142(197605)37:5<2275::AID-CNCR2820370518>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Guarino M. Epithelial-to-mesenchymal change of differentiation: from embryogenetic mechanism to pathologic patterns. Histol Histopathol. 1995;10:171–184. [PubMed] [Google Scholar]
- 31.Lichtiger B, Mackay B, Tessmer CF. Spindle-cell variant of squamous carcinoma: a light and electron microscopic study of 13 cases. Cancer. 1970;26:1311–1320. doi: 10.1002/1097-0142(197012)26:6<1311::AID-CNCR2820260618>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Cassidy M, Maher M, Keogh P, et al. Pseudosarcoma of the larynx: the value of ploidy analysis. J Laryngol Otol. 1994;108:525–528. doi: 10.1017/s002221510012732x. [DOI] [PubMed] [Google Scholar]
- 33.Mohanti BK, Nachiappan P, Pandey RM, Sharma A, Bahadur S, Thakar A. Analysis of 2167 head and neck cancer patients’ management, treatment compliance and outcomes from a regional cancer centre, Delhi, India. J Laryngol Otol. 2007;121:49–56. doi: 10.1017/S0022215106002751. [DOI] [PubMed] [Google Scholar]
- 34.Walvekar RR, Chaukar DA, Deshpande MS, Pai PS, Chaturvedi P, Kakade A, Kane SV, D’Cruz AK. Verrucous carcinoma of the oral cavity. A clinical and pathological study of 101 cases. Oral Oncol. 2009;45:47–51. doi: 10.1016/j.oraloncology.2008.03.014. [DOI] [PubMed] [Google Scholar]