Abstract
The regulation of the collagen II gene was investigated by transfecting plasmids containing potential regulatory sequences of this gene coupled to the gene for chloramphenicol acetyltransferase (CAT) into various cells. The 5' flanking region of this gene functioned as a weak promoter when transfected into chicken chondrocytes or fibroblasts. Inclusion of an 800-base fragment from the first intron, however, increased transcription of CAT approximately 18-fold in chicken chondrocytes and in differentiating limb bud cells but not in fibroblasts, myoblasts, muscle-derived fibroblasts, or teratocarcinoma cells. Furthermore, this fragment was active when placed either upstream or downstream of the CAT gene and when present in either orientation. The activity of this enhancer was examined in relation to differentiation by using "micromass" culture of limb bud mesenchyme cells undergoing chondrogenesis. In this system, no response to the enhancer was observed in undifferentiated limb bud mesenchyme cells. Following differentiation into chondrocytes, a 13-fold enhancer effect was observed in these cells. Finally, all-trans-retinoic acid, a known teratogen, dramatically suppressed enhancer activity in chondrocytes and differentiating limb bud mesenchyme cells. These results suggest that the collagen II gene contains an enhancer element in the first intron that is involved in cell-specific expression.
Full text
PDF
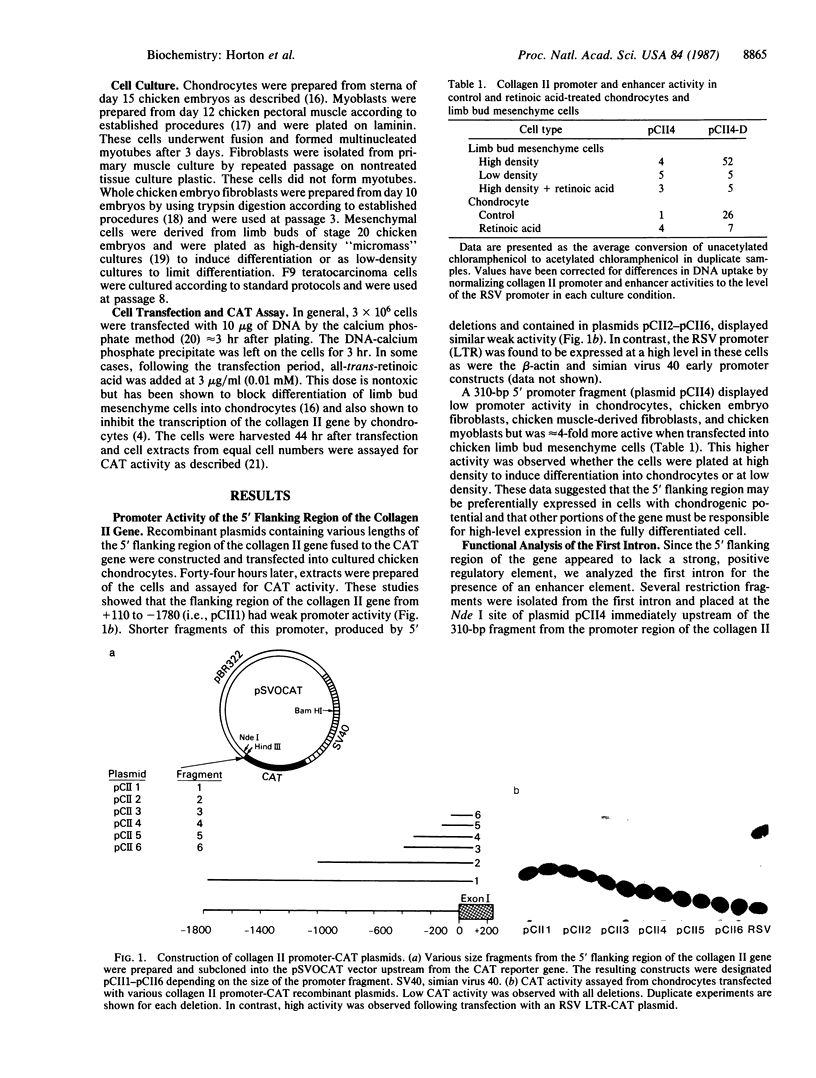
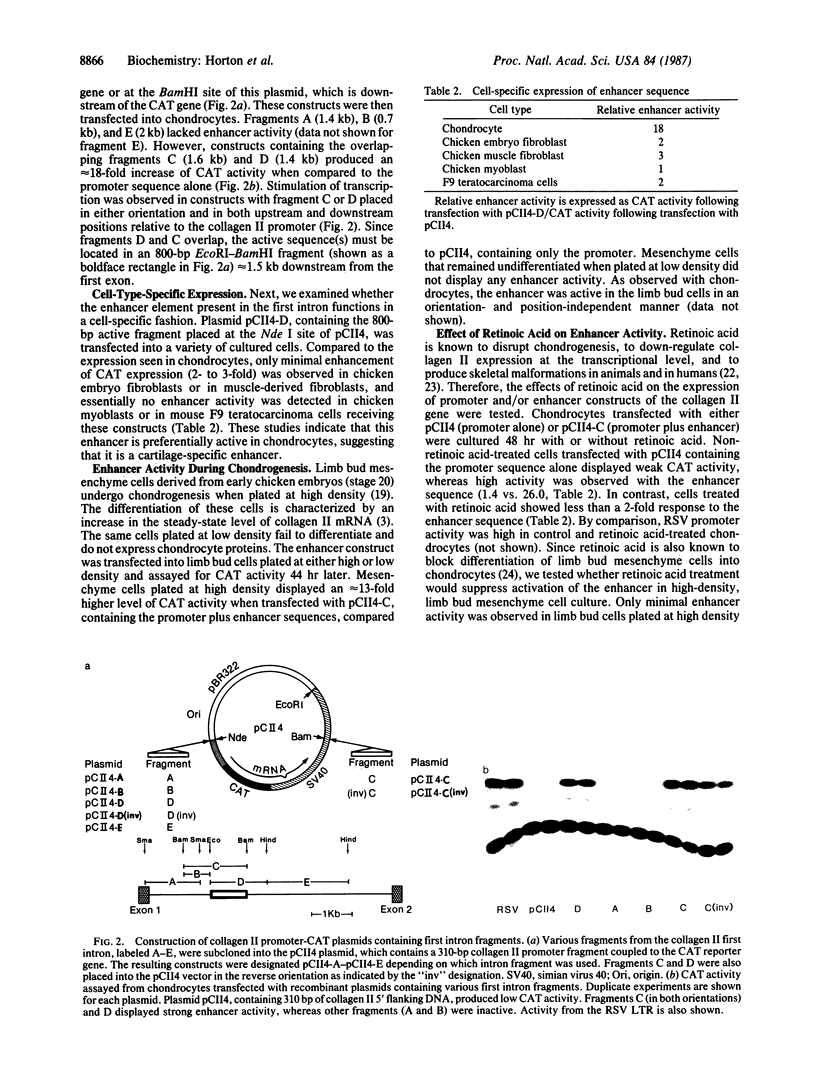


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P., McKay J., Morishima J. K., Devarayalu S., Gelinas R. E. Regulatory elements in the first intron contribute to transcriptional control of the human alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8869–8873. doi: 10.1073/pnas.84.24.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Pennypacker J. P., Yamada K. M., Pratt R. M. Changes in cell surface proteins during normal and vitamin A-inhibited chondrogenesis in vitro. Ann N Y Acad Sci. 1978 Jun 20;312:406–409. doi: 10.1111/j.1749-6632.1978.tb16820.x. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Yamada Y., Hassell J. R. Retinoic acid rapidly reduces cartilage matrix synthesis by altering gene transcription in chondrocytes. Dev Biol. 1987 Oct;123(2):508–516. doi: 10.1016/0012-1606(87)90409-x. [DOI] [PubMed] [Google Scholar]
- Horton W., Hassell J. R. Independence of cell shape and loss of cartilage matrix production during retinoic acid treatment of cultured chondrocytes. Dev Biol. 1986 Jun;115(2):392–397. doi: 10.1016/0012-1606(86)90258-7. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M., Penner J. D., Tellone C. I. Comparative teratogenic activities of two retinoids: effects on palate and limb development. Teratog Carcinog Mutagen. 1984;4(4):377–387. doi: 10.1002/tcm.1770040407. [DOI] [PubMed] [Google Scholar]
- Kohno K., Sullivan M., Yamada Y. Structure of the promoter of the rat type II procollagen gene. J Biol Chem. 1985 Apr 10;260(7):4441–4447. [PubMed] [Google Scholar]
- Kosher R. A., Kulyk W. M., Gay S. W. Collagen gene expression during limb cartilage differentiation. J Cell Biol. 1986 Apr;102(4):1151–1156. doi: 10.1083/jcb.102.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Chen W. T. Masking of extracellular collagen and the co-distribution of collagen and fibronectin during matrix formation by cultured embryonic fibroblasts. J Cell Sci. 1982 Jun;55:35–50. doi: 10.1242/jcs.55.1.35. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow T. G., Jones S. D., Bond B., Yamamoto K. R. The immunoglobulin octanucleotide: independent activity and selective interaction with enhancers. Science. 1987 Mar 20;235(4795):1498–1501. doi: 10.1126/science.3029871. [DOI] [PubMed] [Google Scholar]
- Rogers B. L., Sobnosky M. G., Saunders G. F. Transcriptional enhancer within the human placental lactogen and growth hormone multigene cluster. Nucleic Acids Res. 1986 Oct 10;14(19):7647–7659. doi: 10.1093/nar/14.19.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F. W., Wilk A. L., Kelsey F. O. Teratogen update: vitamin A congeners. Teratology. 1986 Jun;33(3):355–364. doi: 10.1002/tera.1420330315. [DOI] [PubMed] [Google Scholar]
- Rossi P., de Crombrugghe B. Identification of a cell-specific transcriptional enhancer in the first intron of the mouse alpha 2 (type I) collagen gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5590–5594. doi: 10.1073/pnas.84.16.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandra A., Leon M. A., Przybylski R. J. Suppression of myoblast fusion by concanavalin A: possible involvement of membrane fluidity. J Cell Sci. 1977 Dec;28:251–272. doi: 10.1242/jcs.28.1.251. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Rossi P., de Crombrugghe B. Transcriptional control of the mouse alpha 2(I) collagen gene: functional deletion analysis of the promoter and evidence for cell-specific expression. Mol Cell Biol. 1986 Feb;6(2):347–354. doi: 10.1128/mcb.6.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Swalla B. J., Solursh M. Inhibition of limb chondrogenesis by fibronectin. Differentiation. 1984;26(1):42–48. doi: 10.1111/j.1432-0436.1984.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]