Abstract
Background:
Viscous fibre in food has established health benefits, but few functional fibre preparations are both effective and palatable. Our objective was to determine the most effective dose, formulation and timing of consumption of a novel fibre supplement (PolyGlycopleX (PGX)) in reducing postprandial glycaemia.
Subjects/methods:
Three trials were undertaken, each with 10 subjects (8M and 8F; age 24.4±2.6 years). Granular supplement was tested at four doses (0, 2.5, 5.0 and 7.5 g) with breakfast (study 1). Granular and capsule forms of the supplement were given in a single dose (5 g for granules and 4.5 g in capsules) at −60, −45, −30, −15 and 0 before, and +15 min after a bread meal (study 2). Capsules at increasing doses (1.5, 3, 4.5 and 6 g) were consumed with the evening meal to determine effects on glucose tolerance at breakfast (study 3). Incremental area under the blood glucose curve was determined.
Results:
Granular PGX at breakfast time at doses of 2.5, 5 and 7.5 g reduced the incremental area under the curve by up to 50% in a linear dose–response fashion (P<0.001). The granular form of PGX (5 g), but not the capsules, reduced glycaemia by up to 28% when consumed from −45 to +15 min (P<0.001). Capsules containing 3, 4.5 and 6 g PGX consumed with the evening meal reduced glycaemia at breakfast by up to 28% (P<0.001).
Conclusions:
PGX has biologically important, dose-related effects on acute and delayed (second meal) postprandial glycaemia.
Keywords: Viscous polysaccharide, dietary fibre, postprandial glycaemia, PGX
Introduction
Increasing evidence from long term, prospective observational studies suggests that diets containing larger quantities of whole grains and dietary fibre are associated with reduced risk of type 2 diabetes (Schulze et al., 2004, 2005). In controlled trials, higher intake of cereal fibre produces improvements in insulin sensitivity (Pereira et al., 2002), whereas soluble fibre reduces postprandial glycaemia (Jenkins et al., 1978), as well as serum lipids (Jenkins et al., 1993; Vuksan et al., 1999). Most health authorities and diabetes associations now advise an increase in dietary fibre intake to at least 14 g per 1000 kcal (Canadian Diabetes Association 2000; American Diabetes Association 2008).
Despite community awareness of the health benefits of dietary fibre, intakes have remained at about half the recommended level over the last decade (Casagrande et al., 2007). Thus, supplementation of the diet with purified dietary fibres that are active in vivo (that is, ‘functional' fibre preparations) may be an option to increase fibre intake. Although both insoluble and soluble fibres can be used this way, soluble fibres that develop viscosity in solution appear to provide greater benefits for metabolism. Indeed, the higher the viscosity, the greater the improvement in glucose and lipid metabolism (Jenkins et al., 1978). Unfortunately, in practice both palatability and acceptability of functional fibres decline with increasing viscosity (Ellis et al., 1981).
Recent advances in food science suggest that optimal viscosity can be reached by using a combination of different viscous fibres resulting in an induced viscosity that is greater than the viscosity of the individual components (Wood et al., 1994). This may allow smaller doses to be used that may increase acceptability while maintaining efficacy (Vuksan et al., 2000).
Recently, PolyGlycopleX (PGX), a highly viscous polysaccharide complex has been developed that demonstrates a delayed onset of peak viscosity, allowing for a more palatable and easy-to-use functional fibre. As the effect of viscous fibre may vary according to the conditions in the lumen of the gastrointestinal tract, we undertook a series of studies to investigate the effectiveness of two alternate forms of the product (granules or capsules), the timing of the dose with respect to that of a carbohydrate-containing meal, and the presence or absence of a ‘second meal' effect (that is, the ability to reduce postprandial glycaemia the following morning after an evening dose of the supplement).
Materials and methods
Fibre supplement
PGX (α--glucurono-α--manno-β--manno-β-D-gluco), (α--gulurono-β- mannurono), β--gluco-β--mannan; (PGX); Inovobiologic Inc., Calgary, Canada) is a novel functional fibre complex manufactured by a proprietary process (EnviroSimplex) from three dietary fibres to form a highly viscous polysaccharide with high water holding and gel-forming properties. A proprietary process causes strong interactions to be formed between these to produce a resultant polysaccharide complex (Abdelhameed et al., 2010), with a level of viscosity that is higher than any currently known individual polysaccharide. The final product is a novel soluble, highly viscous polysaccharide (functional fibre) that has been shown to be well tolerated by rodents (Matulka et al., 2009) and humans (Carabin et al., 2009). A case study in relating pre-clinical, clinical and postmarketing surveillance data reviewed over 54 million serving of PGX over a 4-year period and found PGX to be well tolerated when used to supplement the diet (unpublished findings). Genotoxicity studies of PGX have shown no mutagenic effects using bacterial reverse mutation and mouse micronucleus assays (Marone et al., 2009). PGX is 87.4% dietary fibre, of which 81.8% is soluble. It is available in two physical forms: a granular product that is dissolved in water or sprinkled on food before consumption, and as a soft-gelatin capsule that is swallowed whole.
Study design
Three single-blind, randomized controlled trials were undertaken in three groups of 10 healthy subjects selected from a pool of 16 (8M and 8F; age 24.4±2.6 years (range: 20.3–29.2 years); body mass index 21.7±2.3 kg/m2 (range: 18.2–24.8 kg/m2)). Subjects were recruited through the Sydney University Glycemic Index Research Service volunteer roster. Entry criteria included body mass index < 25 kg/m2 and fasting blood glucose <5.5 mmol/l. Subjects taking medications or dietary supplements were excluded. The study was conducted at the University of Sydney and was approved by the Human Research Ethics Committee of the University of Sydney. Informed written consent was obtained from all subjects before the start of the study. Subjects received a small financial reward for their participation.
Treatments were randomized within each series with 6, 7 and 16 treatments in study 1, 2 and 3, respectively. Each subject undertook up to two tests per week with at least 2 days between tests. On each test day, subjects arrived at the metabolic kitchen in the morning after a 10–12 h overnight fast. After being weighed and having two fasting blood samples obtained by finger-prick, the subject consumed the test meal within 10 min, and further blood samples were obtained at 15, 30, 45, 60, 90 and 120 min after the start of eating.
Study 1
The aim was to investigate the dose–response relationship using four doses of PGX granules taken with a white bread meal (Tip Top, George Weston Foods, Sydney, NSW, Australia) containing 50 g available carbohydrate (defined as total carbohydrate minus dietary fibre). There were six treatments consisting of three placebo tests (0 g) and one each with 2.5, 5 and 7.5 g supplement. The active granules were dissolved in 2 × 250 ml glasses of water.
Study 2
The aim was to determine whether the timing of a single dose of PGX (5 g) was critical, and whether the ability to lower postprandial glycaemia (effectiveness) varied with the form of the supplement (capsules or granules). Of the 16 treatments, four were placebo, six were capsules and six were granules, given at times −60, −45, −30, −15, 0 and +15 min relative to the start of eating. The meal consisted of a 50 g available carbohydrate portion of white bread. The granular supplement was dissolved in 2 × 250 ml water and consumed within 5 min, whereas capsules were consumed with the same volume of water (0 time).
Study 3
The aim was to investigate whether increasing amounts of the capsule form of PGX had a ‘second meal' effect, that is, a beneficial effect on glucose tolerance at breakfast following supplementation at the previous evening meal. Subjects underwent a total of seven sessions in which the same meal (teriyaki chicken stir-fry with vegetables and Jasmine rice, with a cereal snack bar for dessert) containing 120 g available carbohydrate and 3000 kJ (approximately 717 Cal) (female meal) and 3300 kJ (approximately 789 Cal) (male meal) was consumed in the evening together with 1.5, 3, 4.5 or 6 g of PGX (2, 4, 6 and 8 capsules, respectively, given in random order) or 4, 6 or 8 placebo capsules (that is, a total of three placebo tests) and 2 × 250 ml water. The evening meal was consumed within 20 min and the capsules were taken at the start of the meal. On the following morning after a 12-h fast, subjects consumed a standard breakfast of white bread containing 50 g available carbohydrate.
Blood glucose analysis
Finger-prick blood samples (0.8 ml) from warmed hands were collected into Eppendorf tubes containing 10 U heparin, centrifuged and the plasma stored on ice until same-day analysis in duplicate using a glucose hexokinase assay (Roche Diagnostic Systems, Sydney, NSW, Australia) and an automatic centrifugal spectrophotometric analyser (Roche/Hitachi 912, Boehringer Mannheim Gmbh, Germany) with internal controls.
Statistical analysis
For each test, the incremental area under the curve (iAUC) was calculated according to the trapezoidal method. Any area under the baseline (fasting value) was ignored. In each study, the results were analyzed using a general linear model (analysis of variance) for iAUC with treatment or food and time as fixed factors and subject as a random factor. PASW Statistics 18 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Results are expressed as means±s.e.m.
Results
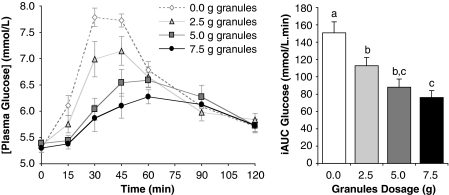
Study 1 investigated the dose–response effect of 0, 2. 5, 5 and 7.5 g of the granular form of PGX dissolved in water and taken with the meal. The highest dose reduced the iAUC by 50% from 151±5 to 76±9 m/120 min (P<0.005). Treating dose as a continuous variable with values 0, 1, 2 and 3, there was a significant linear reduction of postprandial glycaemia (iAUC 151±5, 113±9, 88±9 and 76±9 m/120 min, P<0.001, Figure 1) with each additional gram of supplement reducing iAUC by ∼11 units or 7%. In pairwise comparisons, adjacent doses were not significantly different, but all other comparisons were significant at P=0.005.
Figure 1.
Acute dose–response effects. Incremental postprandial blood glucose responses and corresponding incremental area under the curve (iAUC) of 10 healthy subjects after four meals containing 50 g available carbohydrate as white bread supplemented with 0, 2.5, 5 or 7.5 g of PGX granules dissolved in 500 ml of water. Columns with different letters are significantly different (P<0.01). P-values are for analysis of variance with pairwise comparisons.
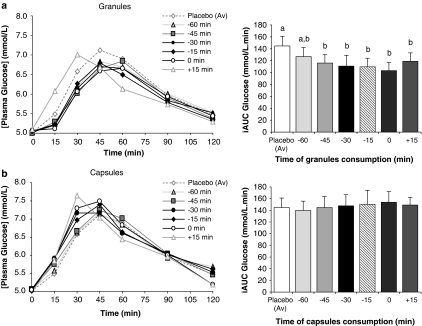
Study 2 determined whether the timing of a single dose (5 g) relative to the meal influenced postprandial glycaemia, and whether the physical form of the supplement (capsules or granules) was important. The granular form, but not the capsules, significantly reduced glycaemia relative to placebo (P<0.001) when consumed at −45, −30, −15, 0 or +15 min, but not –60 min, relative to the meal (Figure 2a). The most effective reduction in iAUC occurred when the granules were consumed at the start of the meal (0 time, 28% reduction, 145±5 vs 103±11 m/120 min, P<0.001), followed by −30 and 15 min. There was no significant effect of capsules, irrespective of timing (Figure 2b).
Figure 2.
Timing of consumption. Incremental postprandial blood glucose responses and corresponding incremental area under the curve (iAUC) of 10 healthy subjects after a meal containing 50 g available carbohydrate as white bread supplemented at −60, −45, −30, −15, 0 and +15 min with PGX granules (5 g) dissolved in 500 ml of water (a) and as PGX capsules consumed with 500 ml water (b). Bars with different letters are significantly different (P<0.001). P-values are for analysis of variance with pairwise comparisons.
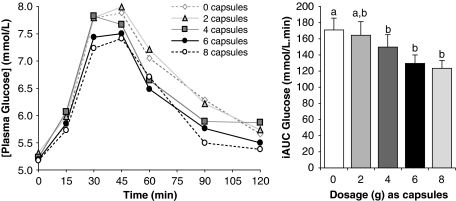
Study 3 determined whether there was a ‘second meal' effect at breakfast following supplementation with capsule form in the previous evening meal. Doses of 3, 4.5 and 6 g (but not 1.5 g) reduced blood glucose iAUC relative to placebo (171±4 (0 g), 150±8 (3 g), 130±8 (4.5 g) and 123±8 (6 g) m/120 min, respectively), with the greatest reduction seen with the largest number of capsules (Figure 3). There was a significant dose–response effect at the second meal, indicating that each extra active capsule reduced the incremental AUC by ∼6 units or ∼3–4%.
Figure 3.
Delayed ‘second meal' effects. Incremental postprandial blood glucose responses and corresponding incremental area under the curve (iAUC) of 10 healthy subjects after a standard breakfast containing 50 g available carbohydrate as white bread. On the evening before the breakfast, 0, 1.5, 3, 4.5 and 6 g PGX in the form of capsules were consumed with the evening meal. Bars with different letters are significantly different (P<0.01). P-values are for analysis of variance with pairwise comparisons.
Adverse events
Although no formal assessment was made, all test meals were well tolerated and no adverse events were reported.
Discussion
This series of studies shows that PGX has clinically important, dose-related effects on acute and delayed postprandial glycaemia. The highest dose of the granular form (7.5 g) dissolved in water and consumed with a carbohydrate meal at breakfast time, reduced the blood glucose response in healthy individuals by 50%. Effectiveness of PGX granules was also related to timing: consumption within 15 min of the start of the meal, but not 45 or 60 min, reduced glycaemia just as effectively as when taken with the meal (0 time). In contrast, PGX (4.5 g) consumed as capsules did not produce acute lowering of glycaemia, yet had important ‘second meal' effects, improving glucose tolerance at breakfast time when consumed with the previous evening meal. These findings indicate that the effectiveness of PGX is dependent on dose, timing of consumption and physical form.
The magnitude of the reduction in glycaemia achieved with PGX appears to be comparable with or superior to other commercially available functional fibre preparations (Jenkins et al., 1978). High viscosity guar gum (∼5 g) also reduces glycaemia by up to 50% when intimately mixed with a meal, but it is not effective when viscosity is low (Leclere et al., 1994). In contrast, pysllium fibre (5 g) produced only a 14% reduction in postprandial glycaemia when consumed with a breakfast meal (Pastors et al., 1991). β-glucans (5 g) derived from oats, but not barley, reduced glycaemia by <20% when consumed with a bread meal (Biorklund et al., 2005).
The beneficial effects of functional fibre preparations are highly dependent on the food matrix. PGX has also been found to be just as effective when sprinkled on food as dissolved in water (unpublished data). This ability provides a significant advantage in terms of palatability because it delays the onset of viscosity. Nonetheless, PGX was not effective when consumed encased in gelatin capsules, even when a 15, 30, 45 or 60-min time gap was permitted between consumption of the capsules and the start of the meal to allow development of viscosity in vivo.
The effectiveness of various fibre preparations has been directly related to their ability to create viscosity (Jenkins et al., 1978). Nonetheless, extreme viscosity is not desirable. Guar gum is so highly viscous that its applications are limited because of the difficulties in incorporating the product into normal food processing operations. To be effective, guar gum must be mixed with a blender to prevent clumps, and allowed to reach its full viscosity before incorporation, often resulting in an undesirable food texture or mouth-feel. In contrast, the present studies show that PGX reduces glycaemia very effectively when consumed in water before significant gelling has taken place. Guar is also noted for its capacity to produce excessive gastrointestinal discomfort (Ellis et al., 1981). In a double blind, randomized controlled trial (n=54), gastrointestinal symptoms after PGX supplementation were rated as mild to moderate, and generally well tolerated (Carabin et al., 2009).
In the third study in this series, we showed that PGX in capsule form had dose-related ‘second meal' effects. This phenomenon describes the ability of a food or supplement to improve glucose tolerance at the following meal, for example, from dinner in the evening to breakfast the next day or from breakfast to lunch or lunch to dinner on the same day. In the case of PGX, 6 g consumed as capsules in the evening reduced glycaemia at breakfast by almost 30%. This is a useful attribute because it implies that not all meals must be consumed with the supplement in order to see benefits on postprandial glycaemia. Many fibres, both soluble and insoluble, as well as low glycaemic index foods, have been shown to produce second meal effects (Brighenti et al., 2006). The effect of wheat fibre is thought to be directly related to an acute improvement (within 24 h) in whole-body insulin sensitivity caused by the absorption of the products of fermentation of the fibre in the large bowel into the portal bloodstream (Weickert et al., 2005). In the case of low glycaemic index foods, the mechanism may be related to prolonged carbohydrate absorption and suppression of free fatty acid release (Jenkins et al., 1990). It is, therefore, possible that the second meal effects of PGX could be even greater in the granular form.
In the past, the ability of fibre preparations to improve lipid metabolism was the focus of most research (Brown et al., 1999). But increasingly, reductions in postprandial glycaemia (glycemic ‘spikes') have been encouraged as part of the management and prevention of impaired glucose tolerance (pre-diabetes) and type 2 diabetes (Ceriello, 2004; Dickinson and Brand-Miller, 2005). In the STOP-NIDDM trial, treatment with the α-glucosidase inhibitor, acarbose, a compound that specifically reduces postprandial hyperglycaemia, reduced the risk of type 2 diabetes (Chiasson et al., 2002), weight gain over time (Chiasson et al., 2002, 2003), and cardiovascular disease (Chiasson et al., 2003). The repeated challenge to β-cell function induced by low-fibre, high-carbohydrate meals is of concern in insulin-resistant individuals who must increase insulin secretion to re-establish glucose homeostasis. In this phenotype, adherence to a conventional low fat diet produces both higher postprandial glycaemic and insulinaemic excursions and greater demands on β-cell function, which could eventually promote β-cell dysfunction and type 2 diabetes. PGX's ability to reduce postprandial glycaemia also suggests a potential role in the treatment and prevention of obesity. Low glycaemic index carbohydrates have been associated with lower fat mass accretion in animal models (Pawlak et al., 2004; Isken et al., 2009) and greater fat loss in some weight loss trials (McMillan-Price et al., 2006; Ebbeling et al., 2007). In adolescents, PGX in solution (5 g) was shown to reduce energy intake from a pizza meal given 90 minutes later more effectively than less viscous fibres, such as glucomannan and cellulose (Vuksan et al., 2009).
In conclusion, PGX in granular form has biologically important, dose-related effects on acute postprandial glycaemia. As little as 7.5 g of the granules reduces blood glucose responses over 120 min by 50%. Precise timing was not critical and consumption within 15 min either side of the start of the meal was effective. PGX in capsule form did not reduce acute glycaemia with the meal it was taken with but had a biologically important effect when consumed with the evening meal whereby it improved glucose tolerance at breakfast (that is, a ‘second meal effect'). Further research is needed to evaluate the long-term health benefits of this promising viscous polysaccharide.
Acknowledgments
InovoBiologic Inc., Calgary, AB, Canada supported the study and supplied PGX.
JCBM received financial remuneration for the preparation of the paper. FSA was employed by the University of Sydney to undertake the studies. RJG owns the Factors Group of Companies, which retains an interest in PGX. VK is an employee of the Canadian Centre for Functional Medicine. MRL receives consulting fees from the Factors Group of Companies. SW receives consulting fees from InovoBiologic Inc.
PGX and Envirosimplex are trademarks of InovoBiologic Inc. All other marks are the property of their respective owners.
References
- Abdelhameed AS, Ang S, Morris GA, Smith I, Lawson C, Gahler R, et al. An analytical ultracentrifuge study on ternary mixtures of konjac Glucomannan supplemented with sodium alginate and xanthan gum. Carbohydr Polym. 2010;81:145–148. [Google Scholar]
- American Diabetes Association Nutrition recommendations and interventions for diabetes. A position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- Biorklund M, van Rees A, Mensink RP, Onning G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with [beta]-glucans from oats or barley: a randomised dose-controlled trial. Eur J Clin Nutr. 2005;59:1272–1281. doi: 10.1038/sj.ejcn.1602240. [DOI] [PubMed] [Google Scholar]
- Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, et al. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83:817–822. doi: 10.1093/ajcn/83.4.817. [DOI] [PubMed] [Google Scholar]
- Brown L, Rosner B, Willett W, Sacks F. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- Canadian Diabetes Association Guidelines for the nutritional management of diabetes mellitus in the new millennium. A position statement by the Canadian Diabetes Association. Can J Diabetes Care. 2000;23:56–69. [Google Scholar]
- Carabin I, Lyon M, Wood S, Pelletier X, Donazzolo Y, Burdock G. Supplementation of the diet with the functional fiber PolyGlycoplex® is well tolerated by healthy subjects in a clinical trial. Nutr J. 2009;8:9. doi: 10.1186/1475-2891-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande S, Wang Y, Anderson C, Gary T. Have Americans increased their fruit and vegetable intake? The trends between 1988 and 2002. Am J Prev Med. 2007;32:257–263. doi: 10.1016/j.amepre.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164:2090–2095. doi: 10.1001/archinte.164.19.2090. [DOI] [PubMed] [Google Scholar]
- Chiasson J, Josse RG, R G, Hanefeld M, Karasik M, Laakso M, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Chiasson J, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose treatment and the risk kof cardiovascular disease and hypertension in patients with impaired glucose tolerance. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- Dickinson S, Brand-Miller J. Glycemic index, postprandial glycemia and cardiovascular disease. Curr Opin Lipidol. 2005;16:69–75. doi: 10.1097/00041433-200502000-00012. [DOI] [PubMed] [Google Scholar]
- Ebbeling C, Leidig M, Feldman H, Loveskym M, Ludwig D. Effects of a low–glycemic load vs low-fat siet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- Ellis P, Apling E, Leeds A, Bolster N. Guar bread: acceptability and efficacy combined. Studies on blood glucose, serum insulin and satiety in normal subjects. Br J Nutr. 1981;46:267–276. doi: 10.1079/bjn19810032. [DOI] [PubMed] [Google Scholar]
- Isken F, Klaus S, Petzke K, Loddenkemper C, Pfeiffer A, Weikert M. Impairment of fat oxidation under high-vs low-glycemic index diet occurs before the development of obese phenotype. Am J Physiol Endocrinol Metab. 2009;298:E287–E295. doi: 10.1152/ajpendo.00515.2009. [DOI] [PubMed] [Google Scholar]
- Jenkins D, Wolever T, Leeds A, Gassull M, Haisman P, Dilawari J, et al. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br Med J. 1978;1:1392–1394. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D, Wolever T, Rao A, Hegele R, Mitchell S, Ransom T, et al. Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol. N Engl J Med. 1993;329:21–26. doi: 10.1056/NEJM199307013290104. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Ocana AM, Vuksan V, Cunnane SC, Jenkins M, et al. Metabolic effects of reducing rate of glucose ingestion by single bolus versus continuous sipping. Diabetes. 1990;39:775–781. doi: 10.2337/diab.39.7.775. [DOI] [PubMed] [Google Scholar]
- Leclere C, Champ M, Boillot J, Guille G, Lecannu G, Molis C, et al. Role of viscous guar gums in lowering the glycemic response after a solid meal. Am J Clin Nutr. 1994;59:914–921. doi: 10.1093/ajcn/59.4.914. [DOI] [PubMed] [Google Scholar]
- Marone PA, Lyon M, Gahler R, Donath C, Hofman-Hutcher H, Wood S. Genotoxicity studies of PolyGlycopleX (PGX) A novel dietary fiber. Int J Toxicol. 2009;28:318–331. doi: 10.1177/1091581809338955. [DOI] [PubMed] [Google Scholar]
- Matulka R, Lyon M, Wood S, Marone P, Merkel D, Burdock G. The safety of PolyGlycopleX® (PGX®) as shown in a 90-day rodent feeding study. Nutr J. 2009;8:1. doi: 10.1186/1475-2891-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan-Price J, Petocz P, Atkinson F, O′Neill K, Samman S, Steinbeck K, et al. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomised controlled trial. Arch Intern Med. 2006;166:1466–1475. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- Pastors J, Blaisdell P, Balm T, Asplin C, Pohl S. Psyllium fiber reduces rise in postprandial glucose and insulin concentrations in patients with non-insulin-dependent diabetes. Am J Clin Nutr. 1991;53:1431–1435. doi: 10.1093/ajcn/53.6.1431. [DOI] [PubMed] [Google Scholar]
- Pawlak DB, Kushner J, Ludwig D. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. The Lancet. 2004;364:778–785. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- Pereira M, Jacobs D, Pins J, Raatz S, Gross M, Slavin J, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848–855. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- Schulze M, Liu S, Rimm E, Manson J, Willett W, Hu F. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- Schulze M, Hoffmann K, Manson J, Willett W, Meigs J, Weikert C, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–682. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuksan V, Jenkins D, Spadafora P, Sievenpiper J, Owen R, Vidgen E, et al. Konjac-mannan (glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial. Diabetes Care. 1999;22:913–919. doi: 10.2337/diacare.22.6.913. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper J, Owen R, Swilley J, Spadafora P, Jenkins D, et al. Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: results of a controlled metabolic trial. Diabetes Care. 2000;23:9–14. doi: 10.2337/diacare.23.1.9. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Panahi S, Lyon M, Rogovik A, Jenkins A, Leiter L. Viscosity of fiber preloads affects food intake in adolescents. Nutr, Metab Cardiovasc Dis. 2009;19:498–503. doi: 10.1016/j.numecd.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Weickert M, Mohlig M, Koebnick C, Holst J, Namsolleck P, Ristow M, et al. Impact of cereal fibre on glucose-regulating factors. Diabetologia. 2005;48:2343–2353. doi: 10.1007/s00125-005-1941-x. [DOI] [PubMed] [Google Scholar]
- Wood P, Braaten J, Scott F, Riedel K, Wolynetz M, Collins M. Effect of dose and modification of viscous properties of oat gum on plasma glucose and insulin following an oral glucose load. Br J Nutr. 1994;72:731–743. doi: 10.1079/bjn19940075. [DOI] [PubMed] [Google Scholar]