Abstract
In sensorimotor adaptation, explicit cognitive strategies are thought to be unnecessary because the motor system implicitly corrects performance throughout training. This seemingly automatic process involves computing an error between the planned movement and actual feedback of the movement. When explicitly provided with an effective strategy to overcome an experimentally induced visual perturbation, people are immediately successful and regain good task performance. However, as training continues, their accuracy gets worse over time. This counterintuitive result has been attributed to the independence of implicit motor processes and explicit cognitive strategies. The cerebellum has been hypothesized to be critical for the computation of the motor error signals that are necessary for implicit adaptation. We explored this hypothesis by testing patients with cerebellar degeneration on a motor learning task that puts the explicit and implicit systems in conflict. Given this, we predicted that the patients would be better than controls in maintaining an effective strategy assuming strategic and adaptive processes are functionally and neurally independent. Consistent with this prediction, the patients were easily able to implement an explicit cognitive strategy and showed minimal interference from undesirable motor adaptation throughout training. These results further reveal the critical role of the cerebellum in an implicit adaptive process based on movement errors and suggest an asymmetrical interaction of implicit and explicit processes.
Keywords: Motor learning, Motor control, Cerebellum, Reaching, Cognitive, Adaptation
Introduction
When learning a new motor skill, the output of the motor system is adjusted following movement errors. A paradigmatic approach to examine this process is to induce an angular mismatch between vision and proprioception during reaching [1]. Adaptation to a visuomotor rotation is seemingly automatic and gradual [2]. Nonetheless, when the errors are large, participants are aware that the stimulus-response mapping has been perturbed, and this may lead them to explore a compensatory strategy. Indeed, when participants are made aware of task manipulations, strategies can facilitate performance [3–5], leading to the idea that strategic and adaptation processes may work in concert.
Studies of visuomotor adaptation have generally not controlled or manipulated strategic processes: The generation and use of a strategy has been left up to the prerogative of the participant and probed in post-experimental interviews. Participants find it hard to verbalize use of a strategy and their descriptions tend to be highly idiosyncratic. Taking a more direct approach, Mazzoni and Krakauer [6] provided an explicit strategy to facilitate learning of a visuomotor rotation. Participants were instructed that they could minimize the perturbing effects of the rotation by aiming at a landmark that was shifted in the opposite direction (and angular size) as the rotation. This strategy was immediately effective in counteracting the rotation. However, the participants exhibited a surprising increase in errors over time. This counterintuitive behavior was attributed to an implicit adaptation process that utilizes a movement error signal resulting from the mismatch between the strategic aiming location and visual feedback of the hand [6]. The persistent adaptation of the motor system to reduce this error resulted in learning that was counterproductive to good task performance. This profile—one in which performance actually becomes worse with practice—suggests a strong segregation of explicit and implicit learning processes [7].
Traditionally, the cerebellum has been hypothesized as a critical site for computing movement errors essential for implicit adaptation. Patients with cerebellar pathology exhibit impairments in sensorimotor adaptation tasks, with their performance marked by persistent error and reduced aftereffects [8–11]. In most conditions, the deficit is not absolute, with the patients showing some degree of adaptation. While this may reflect the operation of spared tissue within the cerebellum, it has been proposed that the patients’ spared learning may be largely driven by the adoption of a cognitive strategy, invoked to offset the impairment in error-based adaptation [8].
When strategic control processes and implicit adaptation work in tandem, it is difficult to isolate the contribution of explicit and implicit processes. In contrast, the strategic visuomotor rotation task introduced by Mazzoni and Krakauer [6] is perfectly suited to isolate the effects of explicit and implicit learning processes, given that the task design puts these processes in opposition to one another. This task has the additional feature that errors related to implicit adaptation progressively increase as training continues, a pattern opposite to that observed in typical studies of motor learning. If cerebellar pathology selectively disrupts implicit adaptive processes that utilize movement errors, then the patients would be expected to show more accurate and stable performance than control participants.
Materials and Methods
Participants
We recruited ten patients (average age = 49.7, SD = 13.7) with spinocerebellar ataxia (SCA) and ten age/gender-matched controls (57.2, SD = 7.81) with no known neurological conditions (Table 1). While all of the patients presented clinical evidence consistent with a diagnosis of cerebellar ataxia, the etiology was mixed. Three had confirmed genetic subtyping (two with SCA7 and one with SCA6). Three patients reported a family history of ataxia, but genetic testing had failed to identify a specific subtype. The other four patients had cerebellar ataxia of unknown origin, termed sporadic adult onset ataxia (SAOA), with no known family history.
Table 1.
Demographics of the ten patients with cerebellar ataxia and ten control participants
| Participant | Gender | Age | Handedness | Type | Years | ICARS | NART IQ | TRAILS | |
|---|---|---|---|---|---|---|---|---|---|
| TMTA | TMTB | ||||||||
| Ataxics | |||||||||
| CBL1 | Female | 31 | Right | SAOA | 14 | 26 | 114 | 0.12 | −0.43 |
| CBL2 | Female | 38 | Right | SAOA | 1 | 10 | 104 | 1.08 | 0.26 |
| CBL3 | Male | 37 | Right | Family history | 6 | 16 | 100 | 0.31 | 0.47 |
| CBL4 | Female | 66 | Right | Family history | 16 | 15 | 115 | −0.44 | −1.19 |
| CBL5 | Female | 52 | Right | SCA7 | 12 | 23 | 115 | −2.35 | −1.57 |
| CBL6 | Female | 54 | Right | SCA7 | 5 | 22 | 113 | −0.56 | −0.60 |
| CBL7 | Female | 47 | Right | SAOA | 1 | 26 | 108 | −1.74 | −4.02 |
| CBL8 | Male | 68 | Right | Family history | 8 | 26 | 112 | 0.57 | 0.08 |
| CBL9 | Male | 38 | Right | SAOA | 19 | 18 | 108 | 0.88 | 1.15 |
| CBL10 | Female | 66 | Right | SCA6 | 8 | 54 | 115 | 0.65 | −0.20 |
| Controls | |||||||||
| CON1 | Female | 54 | Right | ||||||
| CON2 | Male | 54 | Right | ||||||
| CON3 | Female | 55 | Right | ||||||
| CON4 | Female | 62 | Right | ||||||
| CON5 | Female | 70 | Right | ||||||
| CON6 | Male | 51 | Right | ||||||
| CON7 | Female | 46 | Right | ||||||
| CON8 | Female | 50 | Right | ||||||
| CON9 | Female | 65 | Right | ||||||
| CON10 | Male | 65 | Right | ||||||
For the patients (top), the table displays the type of ataxia (when known), the years since initial diagnosis, scores on the ICARS test of ataxia, and scores on the neuropsychological assessments of higher cognitive function
The International Cooperative Ataxia Rating Scale (ICARS) [12] was administered to assess the severity of the ataxia and was scored independently by two trained individuals. The mean ICARS score was 23.6 (SD = 11.9), with the individuals exhibiting mild to moderate levels of ataxia. The neurological exam also allowed us to exclude individuals with overt signs of extracerebellar pathology (e.g., Parkinson-like symptoms).
The patients also completed a series of neuropsychological assessments, with the tests selected to provide an assessment of dementia and frontal lobe function given that the task required the retained use of a strategy (Table 1). This abbreviated battery included the Mini-Mental State Examination (MMSE) [13] to assess dementia, the Tool for Real-time Assessment of Information Literacy Skills (TRAILS) test [14] to assess attention and working memory, and the National Adult Reading Test (NART) [15] to assess intelligence. None of the patients had any indication of dementia (all MMSE scores >28), and as a group, they exhibited above average intelligence (NART estimate: mean = 110.3, SD = 5.15). As a group, they scored within normal limits on the TRAILS test part A (mean z score, relative to published norms = −0.15, SD = 1.14) and part B (mean z = −0.61, SD = 1.44).
The protocol was approved by the university’s institutional review board, and participants provided informed consent. Participants were reimbursed for their time and travel.
Procedure
The participants made horizontal reaching movements, sliding their hand along the surface of a table in an attempt to reach a visually displayed target. The target was displayed on a 15-in. LCD computer monitor (1,280 × 1,024 pixel resolution) horizontally mounted 25.4 cm above the table. With this arrangement, vision of the hand was occluded. The movements were tracked by a 3D motion tracking system (miniBIRD, Ascension Technology, Burlington, VT, USA; sampling rate = 138 Hz) with a sensor placed on the tip of the index finger. While the motion tracking system has a spatial resolution of approximately 0.05 cm, the monitor limited the actual resolution to 0.10 cm/pixel.
On each trial, eight blue circles, 10 cm from the starting location and separated by 45°, were presented on the monitor. A green circle, the target, appeared at one location (Fig. 1a). Participants were instructed to make ballistic-style movements and reach through the target [9]. Feedback was provided by a red cursor that appeared at the position where the hand crossed an invisible ring (10 cm radius). The feedback cursor served as the only source of on-line feedback. Following a feedback interval of 1,000 ms, the participants were guided back to the starting position by a white circle whose radius corresponded to the distance of the hand from the starting position. A 2-cm circular piece of felt was attached on the table to help the participants identify the start position. When the hand was within 1 cm of the start position, a red cursor appeared at the position of the hand. Once the cursor was at the starting position for 500 ms, the next target appeared. The ordering of the target locations was pseudorandom such that each target location appeared once every eight trials. The target, start region, and feedback cursor were all small circles, 0.8 cm in diameter.
Fig. 1.
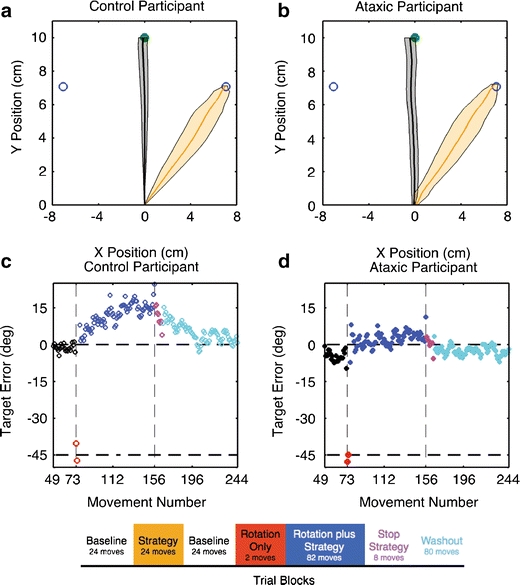
a, b Mean trajectory during the baseline (black) and strategy-only (orange) blocks for a representative control participant (a) and patient with ataxia (b). Movements are approximately straight and directed toward the cued green target in the baseline block and to the adjacent (45°CW) blue “aiming target” in the strategy-only block. Shading indicates the 95% confidence intervals of the trajectories. c, d Target errors for these two participants across the phases of the experiment: second baseline phase (black), rotation phase (blue; between vertical dashed lines), washout without feedback (magenta), and washout with feedback (cyan). The rotation was turned on without warning for two movements (red) before the participants were instructed to use a strategy to counteract the rotation. Lower Participants performed 244 movements throughout seven phases of the experiment
Participants made a total of 244 movements, divided into a series of blocks (Fig. 1—lower). For the first three blocks, no rotation was imposed. In an initial baseline block of 24 trials, participants were instructed to reach toward the green target. The participants were then taught to use a 45° clockwise (CW) strategy. For this strategy-only block of 24 trials, they were instructed to aim to the blue circle (aiming target) that was adjacent in the clockwise direction from the cued, green target. The red feedback cursor was presented at the true position at which the hand intersected the target ring (i.e., near the aiming target). This strategy-only block was included to make sure that participants understood the aiming instructions that would be required when the rotation was introduced later in the experiment. Following this block, participants completed a second baseline block of 24 trials in which they again aimed for the cued, green target.
The rotation phase began immediately at the end of the second baseline block. For these trials, the position of the feedback cursor was shifted by −45° counterclockwise (CCW rotation) from the actual hand position. The rotation was introduced without warning and was highly salient given that the red feedback circle now appeared close to the blue circle located CCW from the cued, green target. After two rotation-only trials, the participants were informed that they could cancel out the rotation by using the strategy that they had learned in the strategy-only block. That is, to offset the rotation, they should reach to the blue target 45°CW to the green target. The rotation+strategy block consisted of 80 trials, ten to each of the eight targets.
The final phase of the experiment was used to assess aftereffects. Participants were told that the rotation was no longer going to be applied and that they should again aim to the green target. For the first eight trials, the red feedback dot was not displayed. This allowed us to assess the magnitude of adaptation in the absence of a strategy and without further trial-by-trial learning. Following this, the participants performed 80 more trials in which the red feedback was visible, allowing for deadaptation.
Data Analysis
Kinematic information was analyzed with Matlab (MathWorks, Natick, MA, USA). Endpoint error was computed as the angle of the hand at 10 cm, relative to a straight line connecting the starting position and the target (green circle except for the strategy-only block). To quantify peak drift on an individual basis, we averaged the error over each set of eight movements (one movement per target). Peak drift was defined as the bin with the maximum angular error during the rotation block. These bins were then averaged across participants. Movement onset was defined by identifying the maximum velocity and scanning the kinematic record backward to identify the last sign reversal in the velocity record. Movement time was defined as the interval between movement onset and when the hand crossed the virtual target ring. As a measure of movement curvature, we computed the absolute area between the actual hand path and a straight-line path from the start position to the target. Our estimates of the variance are reported as the 95% confidence interval of the mean.
Results
As a group, the ataxics tended to move slightly slower than controls (312 ± 57.6 vs. 273 ± 45.3 ms), but this difference was not significant (F 1, 18 = 1.8, p = 0.20). Movements were approximately straight for both groups, with a slight bias in the CCW direction. In the baseline block, in which no rotation was present and endpoint feedback was veridical, there was no difference in endpoint accuracy between the patients and controls (F 1, 18 = 1.10, p = 0.31), and the degree of curvature was only marginally larger in the ataxic group compared to the controls (F 1, 18 = 3.17, p = 0.09). Participants had little difficulty in adopting the 45°CW strategy, indicating that they were able to understand and implement the instructions. Averaging over the last eight movements of the strategy-only block, the mean headings for the ataxic patients and controls were 43.3 ± 1.5° and 44.5 ± 1.4°, respectively (F 1, 18 = 1.47, p = 0.24). Figure 1a, b shows the mean trajectories during the baseline (black) and strategy-only (orange) blocks for a representative control participant and individual with ataxia.
After the strategy-only block, participants completed a second round of baseline movements (Fig. 1c, d—black). The visuomotor rotation was then introduced without warning. As expected, this resulted in an error of approximately −45° (in cursor space) for both groups (Fig. 1c, d—red). After two movements with this rotation, we instructed the participants that they could counteract the rotation-induced displacement and thus minimize their error, by adopting an explicit “corrective” strategy of aiming to the blue circle located 45°CW from the target. Initial use of the strategy was very effective for the ataxics and controls. The mean endpoint error over the first eight reaches was 2.5 ± 1.7° and 1.9 ± 2.0° for the patients and controls, respectively.
As training continued, the control participants became less accurate (see Fig. 1c for representative participant, group averages in Fig. 2a). The increase in endpoint error was manifested as a drift in the direction of the strategy reaching a maximum of 11.3 ± 2.2° in the CW direction (t 9 = 9.80, p < 0.001). While the ataxic patients also exhibited drift, with the error rising to 5.9 ± 1.9° in the CW direction (t 9 = 6.13, p < 0.001), the magnitude of this effect was approximately half that observed for the controls (F 1, 18 = 12.9, p = 0.002). The patients had no difficulty maintaining the strategy during the entire rotation block. Thus, given their reduced drift, their overall performance during the rotation + strategy block, as measured by endpoint error, was less for the patients with ataxia compared to the controls (F 1, 18 = 6.02, p = 0.02).
Fig. 2.
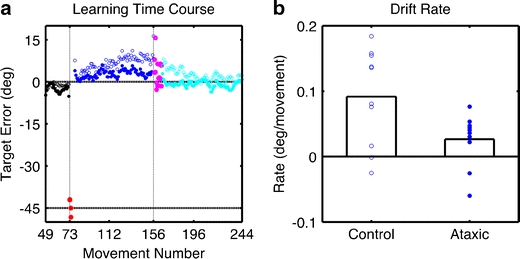
a Mean target error across the experimental session for each group (filled = ataxic; open = controls). The color coding is the same as in Fig. 1. b Drift rate as estimated from regression analysis over the rotation + strategy phase. The individual data are shown as empty circles for the control participants (left) and filled circles for the individuals with ataxia (right)
Given that drift occurred in a gradual and relatively continuous manner during the 80 trials of the rotation+strategy block (see also Mazzoni and Krakauer [6]), we fit the data from this block with a linear function to quantify the rate of drift as well as the initial bias (i.e., the intercept of the regression). Figure 2b displays the individual values for drift rate, as well as the group means. The drift rate for the control group was significantly greater than zero (t 9 = 3.92, p = 0.004); the rate was only marginally greater than zero for the ataxic group (t 9 = 2.09, p = 0.066). A comparison of the two groups revealed a reliable increase in drift rate for the controls (t 18 = 2.45, p = 0.025). There was no difference between the groups in terms of the initial bias (t 18 = 0.83, p = 0.42).
After the rotation+strategy block, the participants were told that the rotation had been turned off and that they should now move directly to the green target. To assess the degree of implicit adaptation in the absence of further learning, we did not provide feedback for the first eight movements (Fig. 2a—magenta). The movement heading of the ataxia patients was 0.3° ± 1.6°. This aftereffect is significant when compared to the baseline heading (t 9 = 3.23, p = 0.01), but not when compared to the target (t 9 = 0.38, p = 0.71). The aftereffect for the control group was larger, averaging 6.2° ± 2.4°, a significant shift when compared to the baseline (t 9 = 7.21, p < 0.001) or target (t 9 = 5.18, p < 0.001). A comparison between the two groups confirmed that the aftereffect was significantly greater for the controls compared to the patients (F 1, 18 = 16.7, p < 0.001).
Discussion
Learning curves on a range of motor tasks exhibit two stereotypical phases, an initial rapid decrease in movement errors followed by a gradual phase in which performance continues to improve until reaching asymptote [16]. Here, we bypassed the rapid phase of this process by providing an explicit strategy that allowed participants to immediately counteract the visuomotor rotation. This strategy allowed participants to immediately succeed in meeting the task goal. Nonetheless, the processes underlying learning in the gradual phase continued to operate, at least for the control participants. This obligatory motor adaptation led to a reverse learning curve in which task performance degraded with continued training, similar to that observed in young control participants [6].
Motor adaptation is most likely a combination of explicit and implicit processes when participants are aware of movement errors. Our participants were all aware of the rotation and successfully able to employ a strategy that allowed them to compensate for the rotation. The drift, reflecting a gradual deterioration in performance during extended training with the strategy, arises from the operation of implicit processes. Less clear is participants’ awareness of this drift and any adjustments they may have made to compensate for the drift. By the end of training, the drift was substantial in most of the participants in the control group (over 10°). The participants may have become aware of their poor performance and modified their strategy in an attempt to offset the drift. Indeed, as the errors increased, some of the participants verbalized their frustration with missing the target and one reported modifying his strategy to reach to an implicit location that was slightly less than 45° in the CW direction.
The patients with cerebellar degeneration were also able to employ the explicit strategy to counteract the visuomotor rotation. In contrast to the controls, the performance of these individuals remained relatively stable over the course of training. They showed attenuated drift and a minimal aftereffect. Both of these observations are consistent with the hypothesis that the contribution of the cerebellum in this task is limited to those processes associated with implicit adaptation based on movement errors. In the absence of implicit adaptation, the patients were able to maintain accurate performance throughout training by using a stable strategy.
As can be seen in Fig. 2b, there was considerable individual variability in drift rate for both the controls and patients. Exploratory analyses failed to identify factors that might account for this variance. We did not observe an effect of either age or gender on drift rate for either the controls or ataxics. Within the patient group, drift rate did not correlate with number of years since ataxia onset (r = 0.08, p = 0.40) and showed only a weak trend to be positively related to the severity of ataxia as assessed by the ICARS score (r = −0.21, p = 0.18). While various cognitive factors [17–19] such as spatial working memory capacity [20] have been shown to account for individual differences in adaptation tasks that do not entail explicit strategies, we failed to observe any correlations between drift rate and the various neuropsychological measures in our patient group.
Previous research has demonstrated that patients with damage to the cerebellum are impaired across a range of tasks involving sensorimotor adaptation, including reaching in force fields [10, 21], prism adaptation [8], and visuomotor rotations [10, 11]. Our results are in accord with these findings, providing a novel and, in some ways, a cleaner demonstration of a cerebellar-related deficit in adaptation given that drift provides an uncontaminated signature of implicit learning. In previous studies, an impairment in motor adaptation was associated with poor task performance and, correspondingly, persistent error signals. This negative feedback might lead to volitional changes in performance, making it difficult to discern the locus of impairment. In the current study, the learning deficit is revealed by task performance that is actually better than that exhibited by the control group. As such, a deficit in error-based learning is inferred by error-free performance.
Moreover, the current work provides compelling evidence of a strong dissociation between processes involved in strategic learning and those involved in implicit adaptation, linking the cerebellar contribution solely to the latter component. Previous work had suggested these two learning systems may work in tandem [5, 22, 23], perhaps with explicit strategies providing a means to bootstrap adaptive processes. When these processes are pitted against one another as in the current task, the control data show that the implicit system continues to operate, even when it is maladaptive. Indeed, the control participants were puzzled to observe their performance deteriorate over time.
When given the explicit strategy, the patients immediately performed with minimal task error. In contrast, if patients with cerebellar degeneration are not provided with an explicit strategy, they show poor adaptation [8, 10, 11]. This raises a puzzling question: If patients with cerebellar degeneration can effectively use an explicit strategy when instructed, why do they generally fail to spontaneously develop compensatory strategies [24]?
This paradox suggests that, while an explicit strategy does not influence implicit adaptation, the converse may not be true: Implicit processes may influence explicit strategies. Typically the induced visual errors in visuomotor rotation studies are quite large, ranging from 30° to 60° [10, 11], and the patients are aware of their poor performance. However, given the complex rotational pattern of the errors, generating a successful strategy may not be obvious. The error pattern would need to be maintained in working memory to decipher the appropriate strategy.
Sequence-learning deficits associated with cerebellar degeneration have been attributed to an impairment in working memory processes for maintaining stimulus-response representations [25]. A similar involvement of working memory may be present in visuomotor rotation [20] and force field adaptation [26] tasks when there are large errors. Thus, damage to the cerebellum may not only disrupt implicit adaptation but may also disrupt the generation of cognitive strategies [27], assuming that this error information is a prerequisite for the self-generation of a compensatory strategy. This hypothesis offers one account of why patients with cerebellar degeneration show a greater impairment in learning to compensate for an abrupt and large force field perturbation, compared to when the perturbation is introduced gradually [26]. Importantly, when an appropriate strategy is explicitly provided, this interaction between implicit and explicit systems is no longer required. In sum, the current results suggest that implicit and explicit processes may not be independent, but rather that implicit mechanisms can inform processes involved in explicit control.
Acknowledgments
We would like to thank Sue Hagan of the National Ataxia Foundation for her assistance with this study. Jordan Taylor was supported by National Research Service Award F32NS064749 from the National Institute of Neurological Disorders and Stroke (NINDS). Richard Ivry was supported by R01HD060306 from the National Institutes of Child Health and Human Development and P01NS040813 from NINDS.
Conflicts of interest
The authors of this manuscript do not have any conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Jordan A. Taylor, Phone: +1-510-6420135, Email: jordan.a.taylor@berkeley.edu
Nola M. Klemfuss, Email: nklemfuss@berkeley.edu
Richard B. Ivry, Email: ivry@berkeley.edu
References
- 1.Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Advances in experimental medicine and biology. Progress in Motor Control. 2008;629:405–21. doi: 10.1007/978-0-387-77064-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham H. Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform. 1989;15(3):493–506. doi: 10.1037/0096-1523.15.3.493. [DOI] [PubMed] [Google Scholar]
- 3.Curran T, Keele SW. Attentional and nonattentional forms of sequence learning. J Exp Psychol Learn Mem Cogn. 1993;19:189–202. doi: 10.1037/0278-7393.19.1.189. [DOI] [Google Scholar]
- 4.Hwang EJ, Smith MA, Shadmehr R. Dissociable effects of the implicit and explicit memory systems on learning control of reaching. Exp Brain Res. 2006;173(3):425–37. doi: 10.1007/s00221-006-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner S, Bock O. Effects of variable practice and declarative knowledge on sensorimotor adaptation to rotated visual feedback. Exp Brain Res. 2007;178(4):554–9. doi: 10.1007/s00221-007-0925-0. [DOI] [PubMed] [Google Scholar]
- 6.Mazzoni P, Krakauer J. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26(14):3642. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sülzenbrück S, Heuer H. Functional independence of explicit and implicit motor adjustments. Conscious Cogn. 2009;18(1):145–59. doi: 10.1016/j.concog.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms: I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(4):1183. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- 9.Tseng Y, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98(1):54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 10.Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, et al. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol. 2009;101(4):1961–71. doi: 10.1152/jn.91069.2008. [DOI] [PubMed] [Google Scholar]
- 11.Werner S, Bock O, Gizewski ER, Schoch B, Timmann D. Visuomotor adaptive improvement and aftereffects are impaired differentially following cerebellar lesions in SCA and PICA territory. Exp Brain Res. 2009;201(3):429–39. doi: 10.1007/s00221-009-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trouillas P. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11. doi: 10.1016/S0022-510X(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Reitan R. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–4. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 15.Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8(6):847–54. doi: 10.1017/S1355617702860131. [DOI] [PubMed] [Google Scholar]
- 16.Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4(6):e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor JA, Thoroughman KA. Divided attention impairs human motor adaptation but not feedback control. J Neurophysiol. 2007;98(1):317–26. doi: 10.1152/jn.01070.2006. [DOI] [PubMed] [Google Scholar]
- 18.Taylor JA, Thoroughman KA. Motor adaptation scaled by the difficulty of a secondary cognitive task. PLoS ONE. 2008;3(6):e2485. doi: 10.1371/journal.pone.0002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galea JM, Sami SA, Albert NB, Miall RC. Secondary tasks impair adaptation to step- and gradual-visual displacements [Internet] Exp Brain Res. 2010;202:473–84. doi: 10.1007/s00221-010-2158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning [Internet] J Cogn Neurosci. 2009;22(9):1917–30. doi: 10.1162/jocn.2009.21351. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93(5):2809–21. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 22.Willingham DB. A neuropsychological theory of motor skill learning. Psychol Rev. 1998;105:558–84. doi: 10.1037/0033-295X.105.3.558. [DOI] [PubMed] [Google Scholar]
- 23.Heuer H, Hegele M. Adaptation to visuomotor rotations in younger and older adults. Psychol Aging. 2008;23(1):190. doi: 10.1037/0882-7974.23.1.190. [DOI] [PubMed] [Google Scholar]
- 24.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms: II. Specificity and storage of multiple gaze–throw calibrations. Brain. 1996;119(4):1199. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 25.Spencer RM, Ivry RB. Sequence learning is preserved in individuals with cerebellar degeneration when the movements are directly cued. J Cogn Neurosci. 2009;21(7):1302–10. doi: 10.1162/jocn.2009.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Criscimagna-Hemminger S, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–84. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304–13. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]


