Abstract
Major histocompatibility complex (MHC) class II molecules present antigenic peptides derived from engulfed exogenous proteins to CD4+ T cells. Exogenous antigens are processed in mature endosomes and lysosomes where acidic proteases reside and peptide-binding to class II alleles is favoured. Hence, maintenance of the microenvironment within these organelles is probably central to efficient MHC class II-mediated antigen presentation. Lysosome-associated membrane proteins such as LAMP-2 reside in mature endosomes and lysosomes, yet their role in exogenous antigen presentation pathways remains untested. In this study, human B cells lacking LAMP-2 were examined for changes in MHC class II-restricted antigen presentation. MHC class II presentation of exogenous antigen and peptides to CD4+ T cells was impaired in the LAMP-2-deficient B cells. Peptide-binding to MHC class II on LAMP-2-deficient B cells was reduced at physiological pH compared with wild-type cells. However, peptide-binding and class II-restricted antigen presentation were restored by incubation of LAMP-2-negative B cells at acidic pH, suggesting that efficient loading of exogenous epitopes by MHC class II molecules is dependent upon LAMP-2 expression in B cells. Interestingly, class II presentation of an epitope derived from an endogenous transmembrane protein was detected using LAMP-2-deficient B cells. Consequently, LAMP-2 may control the repertoire of peptides displayed by MHC class II molecules on B cells and influence the balance between endogenous and exogenous antigen presentation.
Keywords: exogenous antigens, human B cells, MHC class II presentation
Introduction
Major histocompatibility complex (MHC) class II molecules present antigenic peptides derived from exogenous proteins to CD4+ T cells.1 These MHC class II proteins are constitutively expressed on the surface of a number of professional antigen-presenting cells (APC) such as dendritic cells, B cells and macrophages. The MHC class II complexes consist of α and β subunits which are first assembled in the endoplasmic reticulum with the chaperone molecule invariant chain (Ii).2,3 The cytoplasmic tail of Ii contains a motif that targets the Ii–MHC class II complexes to endosomal/lysosomal compartments. Here, acidic proteases degrade Ii to a small fragment known as class II-associated invariant chain peptide (CLIP), which remains associated with the MHC class II peptide-binding groove.4,5 Antigens delivered into the endosomal/lysosomal network via receptor-mediated or fluid-phase endocytosis are also exposed to proteases and denaturing reactions, yielding peptide ligands for class II molecules.6 CLIP removal and the capture of antigenic peptides by MHC class II proteins is catalysed by the MHC-encoded molecule HLA-DM7–9 and occurs in mature endosomes or pre-lysosomes known as MIIC.10 The resulting peptide–MHC class II complexes are ultimately trafficked to the cell surface for immune surveillance by CD4+ T cells.
Mature endosomes and lysosomes play critical roles in routine intracellular processes such as protein degradation as well as more specialized functions related to antigen presentation by MHC class II molecules.10,11 These morphologically heterogeneous organelles are distinguishable from other intracellular compartments in most cells by the presence of mature acid-dependent hydrolases and lysosome-associated membrane proteins such as LAMP-1 and LAMP-2.12 LAMP-1 and LAMP-2 are members of a family of highly glycosylated transmembrane proteins primarily located in mature endosomes and lysosomes.13 A deficiency in LAMP-2 is linked with the development of an X-linked lysosomal storage disorder known as Danon disease;14 genetic analysis of patients with this disorder demonstrated several mutations in the LAMP-2 gene causing protein truncations and an absence of protein expression in patient tissues.15 Danon disease patients display an accumulation of dense and translucent vacuoles, possibly autophagosomes, in the cells of multiple tissues.15 Additionally, studies with LAMP-2 knockout mice reveal an accumulation of autophagic vacuoles in many tissues possibly because of impaired lysosomal trafficking.16,17
The LAMP-2 gene encoded on the X-chromosome gives rise to several alternative transcripts encoding protein isoforms that differ primarily in their cytoplasmic tail domains.18 Among these isoforms, LAMP-2A and -2B proteins are ubiquitously expressed in most tissues including lymphocytes.19 LAMP-2A serves as the lysosomal receptor for chaperone-mediated autophagy, a pathway promoting the transport of specific cytosolic proteins into lysosomes via a molecular chaperone/receptor complex.20–22 Over-expression of LAMP-2A or hsc70, a chaperone protein that co-operates with LAMP-2A in chaperone-mediated autophagy, enhanced the MHC class II-restricted presentation of two cytoplasmic autoantigens in human B cells, hence establishing a role for LAMP-2 in cytoplasmic antigen presentation.19 Remarkably, a partial decrease in total LAMP-2 expression in human B cells reduced not only cytoplasmic antigen presentation but also exogenous antigen presentation by MHC class II molecules.19 Studies here address how the complete loss of LAMP-2 in human B cells modulates epitope selection and display in the context of MHC class II. In the absence of LAMP-2, human B cells displayed a reduced capacity for MHC class II-restricted presentation of exogenous antigen and peptides but maintained the presentation of epitopes from an endogenous transmembrane protein.
Materials and methods
Cell lines
The human B lymphoblastoid cell lines (B-LCL) Priess [(homozygous DR4 (DRβ1*0401)] and Frev [DR1(DRβ1*0101), DR4(DRβ1*0401)] were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% heat inactivated calf serum. The human B-LCL 7C3.DR4 was retrovirally transduced to express HLA-DR423 and cultured in IMDM supplemented with 5% heat inactivated calf serum. A B-LCL from a Danon disease patient (Danon B-LCL) [DR14(DRβ1*1401), DR15(DRβ1*1502)] was cultured in IMDM supplemented with 10% heat inactivated calf serum. In these cells, a 2-base-pair deletion in exon 3 of the LAMP-2 gene in the single X-chromosome-encoded copy disrupts LAMP-2 gene expression. Priess and 7C3.DR4 cells express endogenous immunoglobulin G (IgG) κ light chain while Frev and Danon B-LCL are negative for κ light chain expression by Western blot analysis and instead, express IgG λ light chain. Danon B-LCL were transduced with DRβ1*0401 complementary DNA along with the mammalian selection marker histidinol using the retroviral cell line PA317hddw4c1 obtained from Dr William Kwok (Benaroya Research Institute at Virginia Mason, Seattle, WA). HLA-DR4+ Danon B-LCL clones (DB.DR4) were selected by their growth in IMDM supplemented with 10% heat inactivated calf serum and 8 mm histidinol (Sigma-Aldrich, St Louis, MO). HLA-DR4 expression in the DB.DR4 transfectants was evaluated by flow cytometry using the HLA-DR4-specific antibody 3.5.9-13F10. The murine B-cell CH27 was retrovirally transduced with DRα and DR4β to express HLA-DR4 and cultured in Dulbecco's modified Eagle's minimal essential medium supplemented with 10% fetal bovine serum and 0·1%β-mercaptoethanol. The T-cell hybridoma 17.9 is specific for the HSA64–76 epitope from human serum albumin (HSA).24 The T-cell hybridomas 2.18 and 1.21 are specific for the κI188–203 and κII145–159 epitopes from the human IgG κ light chain, respectively.25 The T-cell hybridoma 33.4 is specific for the HLA-A52–70 epitope from the α chain of HLA-A.26 All T-cell hybridomas were generated in the DR4(DRβ1*0401) transgenic mice27 and were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 0·1%β-mercaptoethanol, 50 U/ml penicillin, and 50 μg/ml streptomycin.
Peptides and antigens
Human GAD273–285 (IAFTSEHSHFSLK), HSA64–76 (VKLVNEVTEFAKT), human IgG immunodominant κI188–203 (KHKVYACEVTHQGLSS), biotinylated κI188–203 (biotin-KHKVYACEVTHQGLSS), human IgG subdominant κII145–159 (KVQWKVDNALQSGNS) and human HLA-A52–70 (VDDTQFVRFDSDAASQRME) peptides were synthesized, purified to > 90% purity by reverse-phase high-performance liquid chromatography, and the sequences were confirmed by mass spectral analysis in conjunction with Quality Controlled Biochemicals (QCB; Hopkinton, MA). The HSA and human IgG antigens were purchased from Sigma-Aldrich.
Antibodies
The mouse monoclonal antibodies (mAb) specific for either human LAMP-1 (H4A3) or human LAMP-2 (H4B4) were purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA) for use in Western blots. The mouse mAb specific for human LAMP-1 and conjugated with AlexaFluor647 for use in immunofluorescence was purchased from eBioscience (San Diego, CA). The rat antibody 3.5.9-13F1028 was used to detect surface HLA-DR4β chains while the mouse mAb L24329 was used to detect intracellular and surface HLA-DRαβ dimers by flow cytometry. L243 conjugated with fluorescein isothiocyanate (FITC) was purchased from BD Biosciences (San Jose, CA) and used to detect HLA-DRαβ dimers in immunofluorescence. The mouse mAb W6/32 was used to detect intracellular MHC class I molecules. The mouse mAb MaP.DM1 was a gift from Dr Peter Cresswell (Yale University, New Haven, CT) and was used to detect intracellular HLA-DM molecules. A mouse mAb used to detect intracellular HLA-DO molecules by flow cytometry was purchased from BD Biosciences. The mouse mAb DA6.147 was used to detect intracellular HLA-DRαβ dimers by Western blotting.30 The mouse mAb specific for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was purchased from Chemicon (Temecula, CA). For immunoblotting, the polyclonal anti-mouse secondary antibody conjugated to horseradish peroxidase (HRP) was purchased from Jackson Laboratories (West Grove, PA). For flow cytometry, the FITC-conjugated F(ab′)2 fragment of goat anti-mouse IgG and the Cy2-conjugated F(ab′)2 fragment of donkey anti-rat IgG were purchased from Jackson Laboratories. The phycoerythrin (PE) -conjugated F(ab′)2 fragment of rabbit anti-mouse immunoglobulin was purchased from Dako (Carpinteria, CA).
Western blotting
Danon or Frev B-LCL were lysed on ice for 20 min in buffer containing 10 mm Tris–HCl, pH 7·2, 150 mm NaCl, 1% Triton X-100, and the following protease inhibitors: 4-(2-aminoethyl)benzenesulphonyl fluoride hydrochloride, pepstatin A, E-64, bestatin, leupeptin and aprotinin (Sigma-Aldrich). Total protein concentration of the cell lysates was determined using the Bio-Rad Protein Assay reagent (BioRad Laboratories, Inc., Hercules, CA). Between 50 and 100 μg of protein/sample were resolved on 8% sodium dodecyl sulphate (SDS) –polyacrylamide gel electrophoresis gels, transferred onto nitrocellulose membranes (BioRad), and immunoblotted using antibody specific for LAMP-1 or LAMP-2 followed by incubation with a polyclonal anti-mouse-HRP-conjugated secondary antibody. To detect HLA-DRαβ dimers, samples were prepared in non-reducing, non-boiled conditions. Blots were visualized with enhanced chemiluminescence (Pierce, Rockford, IL). The membranes were stripped in buffer containing Tris–HCl, SDS, and β-mercaptoethanol and reprobed for GAPDH as a control for protein loading among samples.
Quantitative real-time polymerase chain reaction analysis
Total RNA was prepared from wild-type or LAMP-2-deficient B-LCL using Tri-reagent (Molecular Research Center, Inc., Cincinnati, OH). Reverse transcription was performed using an Advantage RT-for-PCR kit (Clontech Laboratories Inc., Palo Alto, CA) according to the manufacturer's instructions. The 5′ primer for HLA-DRα chain was 5′-CAAAGAAGGAGACGGTCTGG-3′ and the 3′ primer was 5′-AGCATCAAACTCCCAGTGCT-3′. GAPDH primers were used as a control. The correct sizes of the amplification products using these primers in reverse transcription–polymerase chain reaction (RT-PCR) were confirmed by ethidium bromide staining and UV transillumination before their use in quantitative RT-PCR. For quantitative RT-PCR, SYBR® GREEN PCR Master Mix (Applied Biosystems, Foster City, CA) was used for all amplifications, which were performed in a 7500 Real-Time PCR thermal cycler (Applied Biosystems) using the following parameters: 95° for 15 seconds, then 60° for 60 seconds for 40 cycles. GAPDH was used as the endogenous reference while Priess messenger RNA (mRNA) was used as the calibrator. Quantification of gene expression was determined using the relative standard curve method developed by Applied Biosystems. Briefly, a standard curve is generated with gene-specific oligonucleotide primers and cellular mRNA from the calibrator sample (Priess), and this curve is used to determine the quantity of specific mRNA in the unknown samples. All samples are normalized to the endogenous reference mRNA (GAPDH) and are then divided by the normalized calibrator value. The normalized calibrator therefore has a value of 1, and the normalized unknown samples are expressed as an n-fold difference relative to the calibrator.
Flow cytometric analysis
Wild-type or LAMP-2-deficient B-LCL were incubated with the rat 3.5.9-13F10 antibody or the mouse L243 mAb for 60 min on ice to detect surface HLA-DR4β or HLA-DR dimers, respectively. After washing with phosphate-buffered saline (PBS) + 1% bovine serum albumin (BSA) + 0·1% NaN3, cells were incubated with the FITC-conjugated F(ab′)2 fragment of goat anti-mouse IgG or the Cy2-conjugated F(ab′)2 fragment of donkey anti-rat IgG secondary antibody for 30 min on ice. Cells were washed again and fixed in 1% paraformaldehyde. Additionally, wild-type or LAMP-2-deficient B-LCL were fixed with 1% paraformaldehyde, permeabilized with 0·1% saponin, blocked with goat serum in PBS + 1% BSA + 0·1% NaN3, and incubated for 60 min on ice with the mouse mAb W6/32 or L243 to detect intracellular MHC class I molecules and HLA-DR dimers, respectively or with the mouse mAb MaP.DM1 or a mouse mAb for HLA-DO to detect intracellular HLA-DM or HLA-DO, respectively. After washing with PBS + 1% BSA + 0·1% NaN3, cells were incubated with the PE-conjugated F(ab′)2 fragment of rabbit anti-mouse immunoglobulin for 30 min on ice. Cells were washed again before analysis. Flow cytometry was performed on a FACScan™, and the data were analysed with cellquest™ software (BD Biosciences).
Endocytosis assay
Wild-type 7C3.DR4 and LAMP-2-deficient DB.DR4 B-LCL were washed with cold Hanks’ balanced salt solution (HBSS) + 3% BSA and incubated with 5 mg/ml FITC-albumin (Sigma-Aldrich) for 0 and 120 min at 37°. At each time-point, cells were again washed with cold HBSS + 3% BSA and fixed with 1% paraformaldehyde. Uptake of FITC-albumin was determined using flow cytometry performed on a FACScan™, and the data were analysed with cellquest™ software (BD Biosciences).
Indirect immunofluorescent microscopy
Wild-type Frev or LAMP-2-deficient DB.DR4 B-LCL were incubated with 200 nm LysoTracker Red (Invitrogen, Carlsbad, CA) for 18 hr at 37°. Cells were washed and plated on poly-L-lysine-treated (Sigma-Aldrich) coverslips. The cells were then fixed with 4% paraformaldehyde, permeabilized with 0·1% saponin, blocked with PBS + 2% BSA, and incubated for 60 min at room temperature with FITC-conjugated L243 to detect HLA-DR dimers. Additionally, unlabelled Frev or DB.DR4 cells were plated on poly-L-lysine-treated coverslips, fixed with 4% paraformaldehyde, and permeabilized with 0·1% saponin. After blocking with PBS + 2% BSA, cells were incubated for 60 min at room temperature with FITC-conjugated L243 to detect HLA-DR dimers and with AlexaFluor647-conjugated-anti-LAMP-1 antibody to detect LAMP-1. All samples were washed again before analysis. Cells were viewed using a Perkin Elmer Spinning Disk Confocal Microscope, and a single plane through the cell is depicted. Images were processed using NIH Image J software.
Antigen presentation assays
To measure exogenous antigen presentation, DB.DR4, Frev, Priess, or 7C3.DR4 cells (APC) were incubated with various concentrations of purified antigen for 16 hr at 37° or synthetic peptides for 4 or 16 hr at 37°. Samples were washed and then fixed with 0·5% paraformaldehyde for 10 min at room temperature. Then, 4 × 104 APC were incubated with 2 × 104 epitope-specific T cells for 24 hr at 37°. For endogenous antigen presentation, variable numbers of APC were incubated with 2 × 104 epitope-specific T cells for 24 hr at 37°. To measure the effect of pH on exogenous peptide presentation, APC were incubated with peptide in either cell culture medium (pH 7) or 150 mm Na2HPO4 buffer adjusted to pH 5·5 with citric acid for 4 hr at 37°. To strip surface MHC class II, APC were first treated with 160 mm NaCl adjusted to pH 4 with citric acid, three treatments for 30 min each on ice. Cells were washed and fixed as described above before incubation with exogenous peptide and co-culture with epitope-specific T cells. An interleukin-2-dependent cell line, HT-2, was used to measure interleukin-2 production following T-cell activation, and HT-2 proliferation was quantified using [3H]thymidine incorporation. Data are expressed as the average counts per minute (c.p.m.) of triplicate samples for each assay.
Capture enzyme-linked immunosorbent assay (ELISA)
DB.DR4 or 7C3.DR4 cells were first fixed with paraformaldehyde and then incubated overnight at 37° with 100 μm biotinylated κI188–203 peptide. Lysates were prepared and added to plates coated with an anti-HLA-DR4 antibody to capture HLA-DR4 molecules in the lysates. The binding of biotinylated κI188–203 peptide to the captured HLA-DR4 was measured using europium-strepavidin.25
Results
Defects in exogenous antigen presentation in Danon B-LCL
A hallmark characteristic of Danon disease in humans is the absence of LAMP-2 protein expression in multiple tissues, particularly cardiac and skeletal muscle, because of mutations in the LAMP-2 gene.15 We evaluated the expression of the LAMP-2 protein in the B-LCL derived from a patient with Danon disease (Danon B-LCL) by Western blotting. As observed in fibroblasts from patients with Danon disease (data not shown), Danon B-LCL did not express any detectable LAMP-2 protein (Fig. 1a). Interestingly, the levels of another lysosomal transmembrane protein LAMP-1 were equivalent in both Danon and wild-type Frev B-LCL (Fig. 1a).
Figure 1.
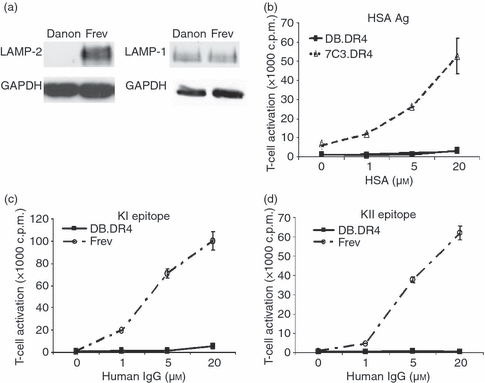
Exogenous antigen presentation is defective in LAMP-2-deficient Danon B-LCL. (a) Cell lysates were prepared from Danon or wild-type Frev B-LCL, the proteins were resolved by gel electrophoresis, and immunoblotted with an antibody to LAMP-2 or LAMP-1. The blot was stripped and reprobed with an antibody to GAPDH. Data are representative of at least two independent experiments. (b) LAMP-2-deficient DB.DR4 or wild-type 7C3.DR4 cells were incubated with various concentrations of human serum albumin antigen overnight and then cultured with HSA64–76-specific T cells to measure MHC class II presentation. (c, d) DB.DR4 or wild-type Frev cells were incubated with various concentrations of human IgG antigen overnight and then cultured with either κI188–203-specific T cells (c) or κII145–159-specific T cells (d) to measure MHC class II presentation. Neither DB.DR4 nor Frev expressed endogenous IgG κ light chain. Data are representative of three independent experiments, and the error bars indicate the mean T-cell activation ± the standard deviation. Note that the size of the symbols at some points masks the error bars.
The importance of lysosomal proteases and thiol reductases in MHC class II-mediated antigen presentation was established using pharmacological inhibitors and gene-deficient APC.6,31–33 Yet far less is known about the role of lysosomal transmembrane proteins in modulating MHC class II function and antigen recognition. Hence, studies were conducted to address whether the absence of LAMP-2 expression observed in Danon B-LCL altered exogenous antigen presentation. Wild-type 7C3.DR4 and LAMP-2-deficient DB.DR4 were incubated with various concentrations of exogenous HSA antigen and then co-cultured with an HLA-DR4-restricted T-cell hybridoma specific for the HSA64–76 epitope.24 Even at high concentrations of HSA (20 μm) after an overnight incubation, the LAMP-2-deficient DB.DR4 were unable to activate HSA-specific T cells (Fig. 1b). The ability of DB.DR4 to present a second exogenous antigen, human IgG κ light chain, was also evaluated. 7C3.DR4 cells express endogenous IgG κ while DB.DR4 and the wild-type Frev B-LCL are negative for endogenous IgG κ by Western blotting and instead, express IgG λ light chain (data not shown). DB.DR4 or Frev cells were incubated with IgG and then co-cultured with HLA-DR4-restricted T-cell hybridomas specific for either of two epitopes from IgG, κI188–203 or κII145–159.25 Again, even at high concentrations of human IgG (20 μm), the LAMP-2-deficient DB.DR4 cells were unable to present either κI188–203 or κII145–159 epitopes to activate the κI- or κII-specific T cells (Fig. 1c,d). Together these results suggest that the absence of LAMP-2 expression in human B cells disrupts exogenous MHC class II-mediated antigen presentation.
Comparable expression of surface and intracellular MHC class II in Danon and wild-type B-LCL
We next examined whether the absence of LAMP-2 in Danon B-LCL influenced the expression of MHC class II molecules as a potential explanation for the observed defects in exogenous antigen presentation. First, the levels of HLA-DRα chain mRNA in a panel of wild-type and Danon B-LCL were determined using quantitative RT-PCR. Both wild-type and Danon B-LCL express very similar amounts of HLA-DRα mRNA (Fig. 2a). In addition, the levels of surface and intracellular HLA-DRαβ dimers were also determined for these cells using flow cytometry. Although surface expression of HLA-DRαβ was slightly increased in LAMP-2-deficient DB.DR4 compared with wild-type Frev B-LCL (Fig. 2b) as detected using an antibody that recognizes MHC class II αβ dimers, we were able to detect similar levels of HLA-DRαβ dimers upon Western blotting cell lysates of DB.DR4 and Frev (Fig. 2c). No significant difference in the total levels of cell surface and intracellular expression of HLA-DR or MHC class I proteins was observed in Danon versus wild-type B-LCL after permeabilization (Fig. 2d). Retroviral transduction of Danon or a wild-type B-LCL (7C3) with a vector encoding the complementary DNA for the DRβ1*0401 β chain resulted in efficient pairing with endogenous HLA-DRα and similar surface expression of this DR4β chain (Fig. 2e). No staining for surface HLA-DR4 was observed in untransduced Danon B cells (data not shown). The similar HLA-DR4 surface expression on DB.DR4 and 7C3.DR4 cells was by comparison approximately twofold lower than that detected on B-LCL expressing endogenous HLA-DR4. Yet as demonstrated in Fig. 1, only DB.DR4 cells displayed a deficiency in exogenous antigen presentation. Lastly, we examined whether the expression of two other MHC-encoded gene products, HLA-DM and HLA-DO, was altered in the LAMP-2-deficient Danon B-LCL. HLA-DM facilitates the removal of CLIP and the capture of antigenic peptides by MHC class II proteins7–9 whereas HLA-DO associates with HLA-DM and serves as a negative regulator of this complex.34 The levels of intracellular HLA-DM and HLA-DO were determined in a panel of wild-type and Danon B-LCL after permeabilization using flow cytometry. Both LAMP-2-deficient cell lines DB and DB.DR4 express equivalent levels of HLA-DM as compared with Frev (Fig. 2f, top) even though human B cells have been shown to express varying levels of HLA-DM.35,36 Variation in the intracellular levels of HLA-DO was also evident in the panel of wild-type and Danon B-LCL although the expression of HLA-DO in the LAMP-2-deficient and wild-type cells was almost equivalent (Fig. 2f, bottom). Taken together, these results suggest that the absence of LAMP-2 in the Danon B-LCL did not substantially alter the levels of intracellular MHC class II HLA-DR dimers, HLA-DM, and HLA-DO nor the steady-state levels of MHC class II complexes that ultimately reach the cell surface.
Figure 2.
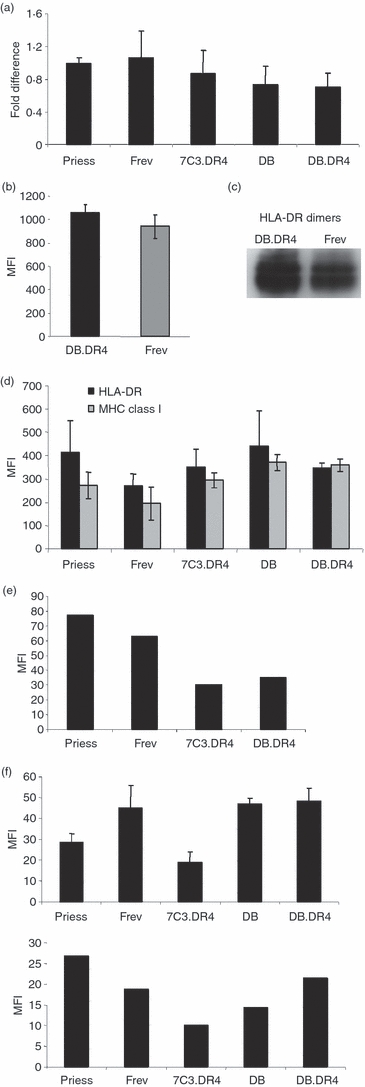
Comparable expression of MHC class II messenger RNA and protein in Danon and wild-type B-LCL. (a) Total RNA was extracted from various wild-type or Danon B-LCL, complementary DNA was synthesized, and quantitative reverse transcription–polymerase chain reaction analysis performed using primers specific for HLA-DRα chain or for GAPDH as a control. Data are representative of the fold difference in messenger RNA expression observed in three independent experiments. (b) Wild-type Frev or LAMP-2-deficient DB.DR4 cells were incubated with L243 antibody to detect total surface HLA-DR and then stained with a fluorescein isothiocyanate-conjugated F(ab′)2 fragment of goat anti-mouse IgG secondary antibody. The mean fluorescence intensity (MFI) as measured by flow cytometry indicates the level of surface HLA-DR, and data are the average MFI of three independent experiments. (c) Cell lysates were prepared from LAMP-2-deficient DB.DR4 or wild-type Frev B-LCL, the proteins resolved by gel electrophoresis under non-reducing conditions to preserve MHC class II dimers, and immunoblotted with an antibody to HLA-DRα chain. Data are representative of at least five independent experiments. The ratios of HLA-DRαβ dimers to GAPDH as a loading control were 1·5 and 1·3 for DB.DR4 and Frev, respectively. (d) Various wild-type or Danon B-LCL were first permeabilized and then incubated with L243 or W6/32 antibody to detect total intracellular HLA-DR or MHC class I molecules, respectively. Cells were then stained with a phycoerythrin-conjugated F(ab′)2 fragment of rabbit anti-mouse immunoglobulin secondary antibody. The MFI as measured by flow cytometry indicates the levels of total surface or intracellular MHC class I or class II molecules. Data are the average MFI of three independent experiments. (e) Wild-type Priess and Frev expressing endogenous HLA-DR4 and wild-type 7C3.DR4 or LAMP-2-deficient DB.DR4 transfectants were incubated with 3.5.9-13F10 antibody to detect surface HLA-DR4β chains and then stained with a Cy2-conjugated F(ab′)2 fragment of donkey anti-rat IgG secondary antibody. The MFI as measured by flow cytometry indicates the level of surface HLA-DR4, and data are representative of more than three independent experiments. (f) Various wild-type or Danon B-LCL were first permeabilized and then incubated with MaP.DM1 (top panel) or a monoclonal antibody to HLA-DO (bottom panel) to detect total intracellular HLA-DM or HLA-DO molecules, respectively. Cells were then stained with a phycoerythrin-conjugated F(ab′)2 fragment of rabbit anti-mouse immunoglobulin secondary antibody. The MFI as measured by flow cytometry indicates the levels of total intracellular HLA-DM or HLA-DO molecules. Data for HLA-DM staining are the average MFI of three independent experiments while the data for HLA-DO staining are a representative experiment.
Endocytosis and distribution of MHC class II in Danon and wild-type B-LCL
While LAMP-2 deficiency in the Danon B-LCL did not affect the overall expression of MHC class II, we sought to determine if differences in endocytosis or the distribution of class II within the endocytic network might account for the defects in exogenous antigen presentation observed in the LAMP-2-deficient B-LCL. We first examined the ability of the LAMP-2-deficient DB.DR4 and wild-type 7C3.DR4 to endocytose a model exogenous antigen, FITC-albumin and observed that uptake of the FITC-albumin after 120 min was not substantially different between DB.DR4 and 7C3.DR4 cells (Fig. 3a). In data not shown, we also observed the persistence of the FITC-albumin at 8 hr in both DB.DR4 and 7C3.DR4 cells while a small amount of this labelled protein was detected in some of the LAMP-2-deficient DB.DR4 cells even at 24 hr, suggesting a slight reduction in the degradation of this molecule in some LAMP-2-negative cells. These results suggest that the absence of LAMP-2 in the Danon B-LCL does not substantially affect the internalization of exogenous proteins or their trafficking along the endocytic pathway.
Figure 3.
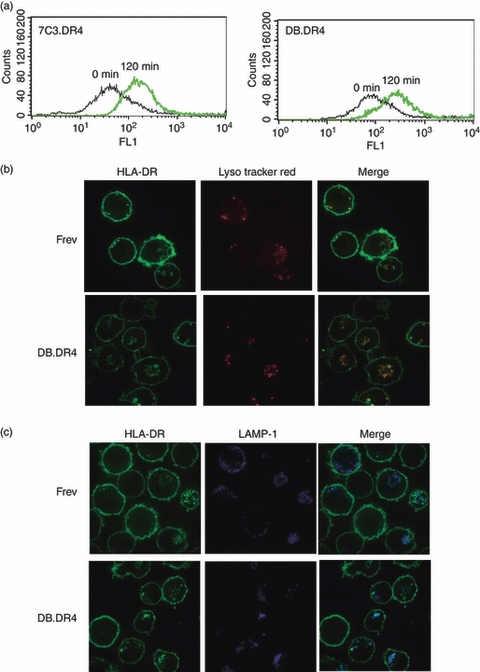
Endocytosis and MHC class II distribution in Danon and wild-type B-LCL. (a) Wild-type 7C3.DR4 or LAMP-2-deficient DB.DR4 B-LCL were incubated with fluorescein isothiocyanate (FITC) -albumin for 0 or 120 min at 37° and uptake was measured by flow cytometry. Data are representative of two independent experiments. (b) Wild-type Frev or LAMP-2-deficient DB.DR4 B-LCL were incubated with LysoTracker Red at 37° overnight to label acidic organelles, permeabilized, and then incubated with FITC-conjugated L423 antibody to detect HLA-DR. The results are representative of three independent experiments. (c) Wild-type Frev or LAMP-2-deficient DB.DR4 B-LCL were first permeabilized and then incubated with FITC-conjugated L423 antibody to detect HLA-DR and AlexaFluor647-conjugated-LAMP-1 Ab to detect LAMP-1. The results are representative of two independent experiments.
We next asked whether MHC class II molecules were similarly distributed within the endosomal/lysosomal network of wild-type and LAMP-2-deficient B-LCL. After assembly in the endoplasmic reticulum, MHC class II molecules are targeted to endosomal/lysosomal compartments for peptide loading. Antigenic peptides bind to MHC class II molecules in the MIIC, an acidic compartment resembling a mature endosome or prelysosome. Using LysoTracker Red to mark acidic organelles such as late endosomes and lysosomes, these compartments were detected in both LAMP-2-deficient DB.DR4 and wild-type Frev cells (Fig. 3b). While the majority of MHC class II molecules localized to the cell surface in both DB.DR4 and Frev, greater co-localization of intracellular class II proteins in the LysoTracker Red+ compartments was observed in the LAMP-2-deficient DB.DR4 cells compared with Frev (Fig. 3b). These findings suggest a potential difference in the intracellular distribution of class II molecules in the absence of LAMP-2. We detected MHC class II in late endosomes/lysosomes in both DB.DR4 or Frev cells as measured by LAMP-1 staining (Fig. 3c); yet there appeared to be slightly more class II in larger LAMP-1+ vesicles in DB.DR4 cells. In wild-type Frev cells, intracellular class II was co-localized with LAMP-2 as well as LAMP-1 (data not shown). MHC class II molecules were not abundant in early endosomes in either wild-type or LAMP-2-deficient cells as detected by staining for co-localization with the early endosome antigen, EEA-1 (data not shown). These results suggest that in LAMP-2-deficient cells, a greater number of MHC class II molecules may transit through or be retained in a mature endosome or lysosome-like compartment compared with wild-type B cells.
Efficient presentation of an endogenous antigen by Danon B-LCL
Biochemical analysis of MHC class II ligands from human B-LCL revealed the presentation of epitopes from endogenous membrane antigens as well as exogenous protein antigens.37 Presentation of these endogenous antigens requires proteolytic processing to yield peptides that efficiently bind to MHC class II molecules within the endosomal/lysosomal compartments of APC. The presence of HLA-DRαβ dimers at the cell surface of Danon B-LCL suggested these class II molecules may acquire peptides from a source other than exogenous antigen. The ability of the LAMP-2-deficient DB.DR4 to present antigenic peptides derived from an endogenous transmembrane protein was evaluated using an HLA-DR4-restricted T-cell hybridoma that recognizes an epitope from MHC class I HLA-A alleles.26 DB.DR4 cells were capable of efficiently activating the HLA-A-specific T cells to an extent slightly greater than the wild-type 7C3.DR4 cells (Fig. 4). A murine B cell CH27 transfected with HLA-DR4 (CH27.DR4) was only recognized by the HLA-A-specific T cells when pulsed with the HLA-A52–70 peptide before the addition of T cells (Fig. 4), confirming the specificity of these T cells for the HLA-A epitope. These results suggest that while MHC class II-restricted exogenous antigen presentation was impaired in the absence of LAMP-2 in the DB.DR4 cells, the presentation of an endogenous transmembrane protein in the context of class II could be readily detected.
Figure 4.
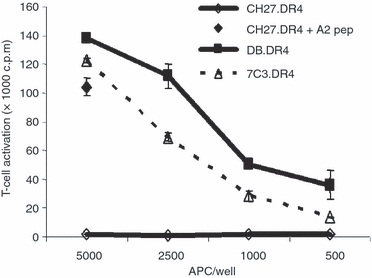
Efficient presentation of an endogenous antigen by Danon B-LCL. Variable numbers of DB.DR4 or 7C3.DR4 cells were cultured with HLA-A52–70-specific, HLA-DR4-restricted T cells to measure MHC class II presentation of endogenous HLA-A. As a control, the HLA-DR4 transfected murine B cell CH27.DR4 was left untreated or incubated with 10 μm HLA-A52–70 peptide and then cultured with HLA-A52–70-specific, HLA-DR4-restricted T cells. Data are representative of three independent experiments, and the error bars indicate the mean T-cell activation ± the standard deviation.
Reduction in MHC class II-peptide binding in Danon B-LCL
The ability of the LAMP-2-deficient DB.DR4 cells to functionally present exogenously added synthetic peptides was determined using HLA-DR4-restricted T cells. In contrast to wild-type B-LCL, DB.DR4 cells failed to efficiently present to T cells a variety of high-affinity and low-affinity peptides,24,25,38 including an epitope from the autoantigen glutamate decarboxylase GAD273–28539 (Fig. 5a), HSA64–76 (Fig. 5b), κI188–203 (Fig. 5c), or κII145–159 (Fig. 5d). However, incubation of DB.DR4 cells with either very high concentrations of synthetic peptide (100 μm instead of 10 μm) or with peptides for prolonged periods of time (16 hr instead of 4 hr) before co-culture with epitope-specific T cells resulted in reduced but detectable MHC class II-restricted peptide presentation (Fig. 5 and data not shown). T-cell activation in response to exogenous peptides and DB.DR4 cells was reduced consistently when compared with MHC class II presentation by wild-type B-LCL. These results were in stark contrast to the efficient activation of T cells recognizing the endogenous HLA-A52–70 epitope (Fig. 4) using DB.DR4 cells as the APC, suggesting that in the absence of LAMP-2, a different repertoire of peptides is selected for display by MHC class II molecules.
Figure 5.
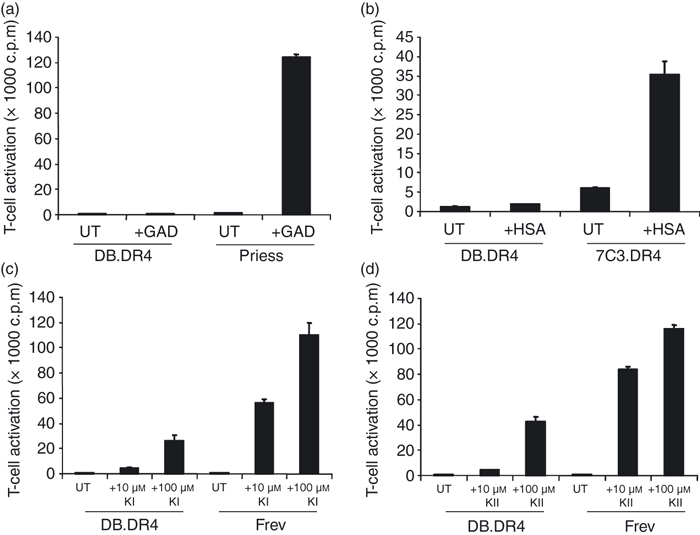
LAMP-2-deficient DB.DR4 cells fail to efficiently present exogenous peptides to CD4+ T cells. (a) LAMP-2-deficient DB.DR4 or wild-type Priess cells were left untreated (UT) or incubated with 10 μm exogenous GAD273–285 peptide for 4 hr and then cultured with GAD273–285-specific T cells to measure MHC class II presentation. (b) DB.DR4 or wild-type 7C3.DR4 cells were left untreated (UT) or incubated with 10 μm exogenous HSA64–76 peptide for 4 hr and then cultured with HSA64–76-specific T cells to measure MHC class II presentation. (c, d) DB.DR4 or wild-type Frev cells were left untreated (UT) or incubated with 10 μm or 100 μm exogenous κI188–203 peptide (c) or κII145–159 peptide (d) for 4 hr and then cultured with κI188–203-specific or κII145–159-specific T cells, respectively, to measure MHC class II presentation. Data in (a–d) are representative of three independent experiments, and the error bars indicate the mean T-cell activation ± the standard deviation.
To determine whether LAMP-2-deficient DB.DR4 cells differentially bind exogenous peptides, a capture ELISA was used to biochemically measure the amount of peptide bound to HLA-DR4 on DB.DR4 cells compared with wild-type 7C3.DR4 cells. DB.DR4 and 7C3.DR4 express equivalent levels of HLA-DR4 (Fig. 2c), and the expression of endogenous IgG κ in 7C3.DR4 does not interfere with the measurement of the binding of the biotinylated κI188–203 peptide to HLA-DR4. At physiological pH, the binding of 100 μm biotinylated κI188–203 peptide to HLA-DR4 from DB.DR4 cells was reduced approximately twofold compared with 7C3.DR4 (Fig. 6a). Relatively similar differences in peptide-binding to HLA-DR4 were also detected at lower peptide concentrations (data not shown). As antigenic peptides bind to MHC class II molecules in acidic compartments such as mature endosomes and lysosomes,10 the binding of biotinylated κI188–203 to HLA-DR4 on DB.DR4 and 7C3.DR4 cells at pH 5·5 was also evaluated in this assay. Overnight incubation of the cells at low pH improved the binding of 100 μm biotinylated κI188–203 to HLA-DR4 from both DB.DR4 and 7C3.DR4, but peptide-binding to DB.DR4 remained approximately two-fold less compared with 7C3.DR4 (Fig. 6a). The binding of peptides to DB.DR4 cells was also evaluated using strepavidin-HRP in Western blots to detect the formation of biotinylated κI188–203 peptide–HLA-DR4 complexes at pH 5·5 in DB.DR4 cells compared with 7C3.DR4 cells. Biotinylated κI188–203 peptide-HLA-DR4 complexes were detected in DB.DR4 cells after 4 hr of peptide-incubation only at pH 5·5, and the numbers of these complexes were reduced compared with wild-type 7C3.DR4 cells (data not shown). Overall, these results suggest that in cells lacking LAMP-2, class II protein binding to exogenously added peptides was impaired or limited particularly at neutral pH. Peptide binding to these class II molecules could be restored in part by exposure to low pH.
Figure 6.
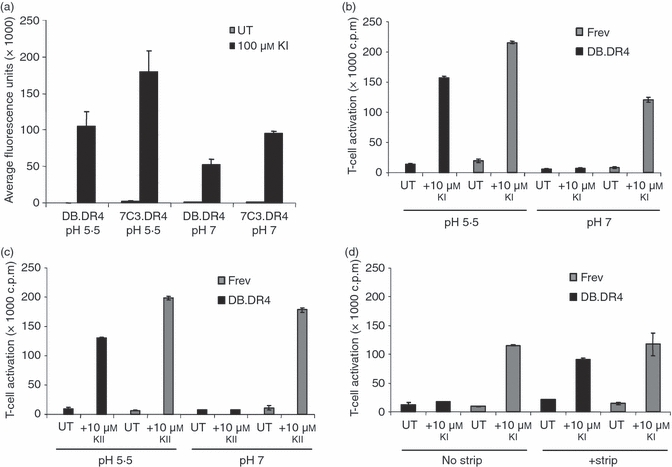
Acidic pH restores exogenous peptide presentation by LAMP-2-deficient DB.DR4. (a) LAMP-2-deficient DB.DR4 or wild-type 7C3.DR4 cells were left untreated (UT) or incubated overnight with 100 μm biotinylated κI188–203 peptide. Lysates were prepared and added to plates coated with an anti-HLA-DR4 antibody to capture HLA-DR4 molecules in the lysates. The binding of biotinylated κI188–203 peptide to captured HLA-DR4 was measured using europium-strepavidin. The results are the average of three independent experiments, and the error bars indicate the standard deviation ± between the experiments. (b, c) DB.DR4 or wild-type Frev cells were left untreated (UT) or incubated with 10 μm exogenous κI188–203 peptide (b) or 10 μm exogenous κII145–159 peptide (c) in either cell culture medium (pH 7) or in sodium phosphate/citric acid buffer (pH 5·5) for 4 hr and then cultured with κI188–203-specific or κII145–159-specific T cells, respectively, to measure MHC class II presentation. Data in (b, c) are representative of three independent experiments, and the error bars indicate the mean T-cell activation ± the standard deviation. (d) DB.DR4 or Frev cells were left untreated (UT) or treated with a NaCl/citric acid buffer to strip surface MHC class II before incubation with 10 μm exogenous κI188–203 peptide for 4 hr. Cells were then cultured with κI188–203-specific T cells to measure MHC class II presentation. Data are representative of two independent experiments, and the error bars indicate the mean T-cell activation ± the standard deviation.
Acidic pH restores exogenous peptide presentation by Danon B-LCL
Since incubating LAMP-2-deficient DB.DR4 at pH 5·5 improved the binding of biotinylated κI188–203 to HLA-DR4 on these cells, studies were designed to test whether low pH would also facilitate class II-mediated presentation of exogenous κI188–203 and κII145–159 peptides to epitope-specific T cells. DB.DR4 cells or wild-type Frev B-LCL, neither of which express endogenous IgG κ, were incubated with 10 μmκI188–203 or κII145–159 peptides at pH 5·5 for 4 hr and then co-cultured with HLA-DR4-restricted, epitope-specific T cells at physiological pH 7·2. Incubating DB.DR4 cells at acidic pH in the presence of κI188–203 or κII145–159 peptides partially restored exogenous peptide presentation such that activation of epitope-specific T cells was only minimally reduced compared with wild-type Frev cells (Fig. 6b,c). To determine whether exposure to low pH was necessary to alter class II accessibility to peptides or to directly enhance peptide-binding, additional studies were performed. Acid stripping has been used to dissociate receptor–ligand complexes including releasing endogenous ligands from the groove of MHC class I and class II molecules.36,40,41 Here, LAMP-2-deficient DB.DR4 and wild-type Frev cells were briefly exposed to acid stripping buffer before incubating with 10 μmκI188–203 or κII145–159 peptide at neutral pH for 4 hr. Following acid-stripping, both κI188–203 and κII145–159 peptides were more efficiently presented in the context of HLA-DR4 on the surface of DB.DR4 to epitope-specific T cells (Fig. 6d and data not shown). Notably, the activation of κI-specific T cells by acid-stripped DB.DR4 cells was still slightly reduced relative to levels of peptide presentation observed with untreated or acid-stripped wild-type Frev cells (Fig. 6d). These results demonstrate that the incubation of peptides with APC at low pH partially rescued class II-mediated presentation of exogenous peptides in the LAMP-2-deficient DB.DR4 cells.
Discussion
In this study, a novel mutant B-cell line from a patient with Danon disease lacking expression of the lysosomal membrane protein LAMP-2 was used to investigate the role of LAMP-2 in MHC class II-mediated antigen presentation. In the absence of LAMP-2, MHC class II presentation of exogenous antigens and peptides to CD4+ T cells was significantly impaired. This was not because of alterations in the levels of cell surface or total MHC class II molecules in LAMP-2-deficient Danon B-LCL. In wild-type and LAMP-2-deficient cells, the majority of class II molecules were expressed at the cell surface, yet some class II proteins were observed in intracellular punctuate vesicles, probably mature endosomes or pre-lysosomes. The co-localization of class II molecules in LAMP-1+ vesicles appeared greater in the LAMP-2-deficient cells. Biochemical analysis of class II molecules from Danon B-LCL revealed a reduced capacity for peptide-binding compared with class II complexes isolated from wild-type cells. Peptide-binding to class II molecules from these LAMP-2-deficient cells could be partially restored upon incubation of cells with peptides at acidic pH. Incubation of Danon B-LCL at low pH for even a brief period before the addition of peptide also partially restored T-cell recognition of the resulting peptide–MHC class II complexes on these cells. Interestingly, class II presentation of an epitope from an endogenous transmembrane protein was similarly detected in wild-type or LAMP-2-deficient Danon B-LCL. Overall, these results suggest that the absence of LAMP-2 within the endosomal/lysosomal network selectively altered class II acquisition and presentation of peptide ligands to T cells.
Danon disease is a rare, X-linked lysosomal disorder characterized by the accumulation of dense, translucent vacuoles in the cytoplasm of skeletal and cardiac muscle cells as the result of the absence of LAMP-2 protein expression.15 Preliminary electron microscopy studies have revealed the presence of vesicles with inclusions in both fibroblasts and B cells from patients with Danon disease (unpublished observations). Intracellular immunofluorescence revealed greater co-localization of class II molecules with the late endosome/lysosome marker LAMP-1 in DB.DR4 cells from a patient with Danon disease compared with wild-type cells. These vesicles appeared slightly larger and more clustered than the LAMP-1+ vesicles in wild-type cells, and stained more brightly for LysoTracker Red. Proteins associated with early endosomes (EEA1) or autophagosomes (LC3) were not detected co-localizing with these class II compartments, again suggesting that this compartment is more closely related to mature endosomes or lysosomes (data not shown). Enlarged LAMP-1+ vesicles were also detected clustered in the cytoplasm of LAMP-2-deficient neutrophils.42 Defects in phagocytosis, an important component of the innate immune response to intracellular pathogens, were observed in these neutrophils that lacked LAMP-2.
The current study is the first report of a deficiency in exogenous antigen presentation in human B cells lacking LAMP-2 expression. Treatment of a wild-type B-cell line Priess transfected with antisense complementary DNA for LAMP-2, partially reduced cellular LAMP-2 expression.19 While exogenous antigen presentation was partially diminished in these cells, class II presentation of an exogenous peptide was comparable with cells with normal LAMP-2 levels. In the current study, the complete absence of LAMP-2 protein in Danon B-LCL had a more profound effect, abolishing exogenous antigen presentation and greatly reducing exogenous peptide presentation by these cells. These results also differ significantly from a previous study involving B cells from patients with another rare hereditary immunodeficiency Chediak–Higashi syndrome (CHS).43 This syndrome results from mutations in a single gene encoding a large cytosolic protein, termed lysosomal trafficking regulator (LYST).44–46 Similar to LAMP-2-deficient Danon B cells, CHS B cells display reduced MHC class II-mediated presentation of exogenous antigen. However, in contrast to Danon B cells, addition of exogenous peptide to CHS B cells restored class II presentation to the levels observed with wild-type B cells.43 These results not only support the importance of the lysosomal network in MHC class II-mediated antigen presentation, but they also suggest that alterations in different components of the lysosomal pathway may reveal novel regulatory events in antigen presentation.
The absence of LAMP-2 did not alter the cell surface levels of MHC class II molecules, suggesting that the egress of peptide–MHC class II complexes from the endosomal network to the plasma membrane is maintained. However, MHC class II molecules from LAMP-2-deficient Danon B-LCL displayed a reduced capacity for peptide-binding at the cell surface. Binding of exogenous peptides to class II could be restored upon incubation of these cells with peptides at acidic pH. Furthermore, incubation of Danon B-LCL at low pH before the addition of peptide also partially restored T-cell recognition of the resulting peptide–MHC class II complexes on these cells. Restoration of MHC class II function in Danon B-LCL treated with a low pH buffer may facilitate the removal of some endogenous ligands from the peptide-binding groove of class II molecules. Alternatively, this low pH treatment may stabilize class II molecules in a conformation more receptive to peptide loading. These studies therefore suggest that LAMP-2 influences the repertoire of peptides binding MHC class II molecules in human B cells.
Despite deficiencies in exogenous antigen and peptide presentation, Danon B-LCL were capable of presenting an epitope from an endogenous transmembrane protein, the MHC class I molecule HLA-A, to epitope-specific CD4+ T cells. Incubation of Danon B-LCL at low pH did not enhance T-cell recognition of the HLA-A epitope and HLA-DR4 at the cell surface. Yet, endogenous peptides such as the epitope from HLA-A may bind tightly to class II molecules in the acidic LAMP-1+ vesicles detected in LAMP-2-deficient cells, and facilitate the export of these class II molecules to the cell surface. In contrast to our previous observation that LAMP-2 facilitated the MHC class II-mediated presentation of the cytoplasmic GAD antigen, the absence of LAMP-2 in Danon B-LCL did not hinder the presentation of the endogenous HLA-A epitope. The HLA-A epitope is one of the most abundant epitopes detected bound to HLA-DR4 as measured by peptide-elution studies and mass spectrometry and is probably formed during the turnover of class I A alleles in lysosomes.47 GAD is found only in the cytosol and relies on chaperone-mediated autophagy and LAMP-2A to access class II for presentation.19
In conclusion, our data support a role for LAMP-2 in the MHC class II-mediated presentation of exogenous antigens and peptides in human B cells. Peptide-binding to MHC class II on LAMP-2-deficient B cells was reduced at the cell surface yet could be restored by incubation at acidic pH. Restoration of MHC class II function in Danon B-LCL upon incubation at low pH buffer may facilitate the removal of endogenous ligands from the peptide-binding groove of MHC class II molecules or stabilize class II molecules in a conformation more receptive to peptide loading. Efficient loading of exogenous epitopes by MHC class II molecules is therefore dependent upon LAMP-2 expression in B cells. LAMP-2-deficient B cells displayed slightly enhanced presentation of an epitope derived from an endogenous transmembrane protein suggesting that LAMP-2 may control the overall repertoire of peptides displayed by MHC class II molecules on B cells and subsequently, CD4+ T-cell activation.
Acknowledgments
This work was supported by grants from the National Institutes of Health to V.L.C (T32DK007519) and J.S.B. (AI49589), from the Melanoma Research Foundation to V.L.C., and from the American Heart Association to D.Z.
Glossary
Abbreviations:
- B-LCL
B-lymphoblastoid cell line
- CHS
Chediak–Higashi syndrome
- CLIP
class II-associated invariant chain peptide
- GAD
glutamate decarboxylase
- HSA
human serum albumin
- LAMP
lysosome-associated membrane protein
- LYST
lysosomal trafficking regulator
- MFI
mean fluorescence intensity
Disclosures
The authors have no financial conflict of interest.
References
- 1.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–92. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–7. doi: 10.1016/s0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- 3.Sant AJ, Miller J. MHC class II antigen processing: biology of invariant chain. Curr Opin Immunol. 1994;6:57–63. doi: 10.1016/0952-7915(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 4.Maric MA, Taylor MD, Blum JS. Endosomal aspartic proteinases are required for invariant-chain processing. Proc Natl Acad Sci U S A. 1994;91:2171–5. doi: 10.1073/pnas.91.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–66. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 6.Watts C. Antigen processing in the endocytic compartment. Curr Opin Immunol. 2001;13:26–31. doi: 10.1016/s0952-7915(00)00177-1. [DOI] [PubMed] [Google Scholar]
- 7.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 8.Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–6. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 9.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–65. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 10.Pieters J. MHC class II compartments: specialized organelles of the endocytic pathway in antigen presenting cells. Biol Chem. 1997;378:751–8. [PubMed] [Google Scholar]
- 11.Cuervo AM, Dice JF. Lysosomes, a meeting point of proteins, chaperones, and proteases. J Mol Med. 1998;76:6–12. doi: 10.1007/s001090050185. [DOI] [PubMed] [Google Scholar]
- 12.Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–78. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- 13.Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–45. doi: 10.1016/s0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 14.Danon MJ, Oh SJ, DiMauro S, Manaligod JR, Eastwood A, Naidu S, Schliselfeld LH. Lysosomal glycogen storage disease with normal acid maltase. Neurology. 1981;31:51–7. doi: 10.1212/wnl.31.1.51. [DOI] [PubMed] [Google Scholar]
- 15.Nishino I, Fu J, Tanji K, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–10. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Guhde G, Suter A, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–6. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 17.Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G, Blanz J, Von Figura K, Saftig P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13:3355–68. doi: 10.1091/mbc.E02-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konecki DS, Foetisch K, Zimmer KP, Schlotter M, Lichter-Konecki U. An alternatively spliced form of the human lysosome-associated membrane protein-2 gene is expressed in a tissue-specific manner. Biochem Biophys Res Commun. 1995;215:757–67. doi: 10.1006/bbrc.1995.2528. [DOI] [PubMed] [Google Scholar]
- 19.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–81. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–3. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 21.Cuervo AM, Dice JF. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci. 2000;113(Pt 24):4441–50. doi: 10.1242/jcs.113.24.4441. [DOI] [PubMed] [Google Scholar]
- 22.Majeski AE, Dice JF. Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36:2435–44. doi: 10.1016/j.biocel.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Pathak SS, Lich JD, Blum JS. Cutting edge: editing of recycling class II: peptide complexes by HLA-DM. J Immunol. 2001;167:632–5. doi: 10.4049/jimmunol.167.2.632. [DOI] [PubMed] [Google Scholar]
- 24.Pathak SS, Blum JS. Endocytic recycling is required for the presentation of an exogenous peptide via MHC class II molecules. Traffic. 2000;1:561–9. doi: 10.1034/j.1600-0854.2000.010706.x. [DOI] [PubMed] [Google Scholar]
- 25.Ma C, Whiteley PE, Cameron PM, et al. Role of APC in the selection of immunodominant T cell epitopes. J Immunol. 1999;163:6413–23. [PubMed] [Google Scholar]
- 26.Kovats S, Whiteley PE, Concannon P, Rudensky AY, Blum JS. Presentation of abundant endogenous class II DR-restricted antigens by DM-negative B cell lines. Eur J Immunol. 1997;27:1014–21. doi: 10.1002/eji.1830270431. [DOI] [PubMed] [Google Scholar]
- 27.Woods A, Chen HY, Trumbauer ME, Sirotina A, Cummings R, Zaller DM. Human major histocompatibility complex class II-restricted T cell responses in transgenic mice. J Exp Med. 1994;180:173–81. doi: 10.1084/jem.180.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiraiwa A, Yamanaka K, Kwok WW, Mickelson EM, Masewicz S, Hansen JA, Radka SF, Nepom GT. Structural requirements for recognition of the HLA-Dw14 class II epitope: a key HLA determinant associated with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1990;87:8051–5. doi: 10.1073/pnas.87.20.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampson LA, Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980;125:293–9. [PubMed] [Google Scholar]
- 30.Gruneberg U, Rich T, Roucard C, Marieke van Ham S, Charron D, Trowsdale J. Two widely used anti-DR alpha monoclonal antibodies bind to an intracellular C-terminal epitope. Hum Immunol. 1997;53:34–8. doi: 10.1016/s0198-8859(97)00025-6. [DOI] [PubMed] [Google Scholar]
- 31.Cresswell P, Arunachalam B, Bangia N, Dick T, Diedrich G, Hughes E, Maric M. Thiol oxidation and reduction in MHC-restricted antigen processing and presentation. Immunol Res. 1999;19:191–200. doi: 10.1007/BF02786487. [DOI] [PubMed] [Google Scholar]
- 32.Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev. 2005;207:229–41. doi: 10.1111/j.0105-2896.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 33.Villadangos JA, Bryant RA, Deussing J, et al. Proteases involved in MHC class II antigen presentation. Immunol Rev. 1999;172:109–20. doi: 10.1111/j.1600-065x.1999.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 34.Denzin LK, Sant’Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–9. doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- 35.Ramachandra L, Kovats S, Eastman S, Rudensky AY. Variation in HLA-DM expression influences conversion of MHC class II alpha beta: class II-associated invariant chain peptide complexes to mature peptide-bound class II alpha beta dimers in a normal B cell line. J Immunol. 1996;156:2196–204. [PubMed] [Google Scholar]
- 36.Lich JD, Jayne JA, Zhou D, Elliott JF, Blum JS. Editing of an immunodominant epitope of glutamate decarboxylase by HLA-DM. J Immunol. 2003;171:853–9. doi: 10.4049/jimmunol.171.2.853. [DOI] [PubMed] [Google Scholar]
- 37.Zhou D, Blum JS. Presentation of cytosolic antigens via MHC class II molecules. Immunol Res. 2004;30:279–90. doi: 10.1385/IR:30:3:279. [DOI] [PubMed] [Google Scholar]
- 38.Wicker LS, Chen SL, Nepom GT, et al. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J Clin Invest. 1996;98:2597–603. doi: 10.1172/JCI119079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenmark A. Glutamic acid decarboxylase – gene to antigen to disease. J Intern Med. 1996;240:259–77. doi: 10.1046/j.1365-2796.1996.27859000.x. [DOI] [PubMed] [Google Scholar]
- 40.Storkus WJ, Zeh HJ, Salter RD, Lotze MT. Identification of T-cell epitopes: rapid isolation of class I-presented peptides from viable cells by mild acid elution. J Immunother Emphasis Tumor Immunol. 1993;14:94–103. [PubMed] [Google Scholar]
- 41.Avva RR, Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994;1:763–74. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 42.Beertsen W, Willenborg M, Everts V, Zirogianni A, Podschun R, Schroder B, Eskelinen EL, Saftig P. Impaired phagosomal maturation in neutrophils leads to periodontitis in lysosomal-associated membrane protein-2 knockout mice. J Immunol. 2008;180:475–82. doi: 10.4049/jimmunol.180.1.475. [DOI] [PubMed] [Google Scholar]
- 43.Faigle W, Raposo G, Tenza D, et al. Deficient peptide loading and MHC class II endosomal sorting in a human genetic immunodeficiency disease: the Chediak–Higashi syndrome. J Cell Biol. 1998;141:1121–34. doi: 10.1083/jcb.141.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbosa MD, Nguyen QA, Tchernev VT, et al. Identification of the homologous beige and Chediak–Higashi syndrome genes. Nature. 1996;382:262–5. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagle DL, Karim MA, Woolf EA, et al. Identification and mutation analysis of the complete gene for Chediak–Higashi syndrome. Nat Genet. 1996;14:307–11. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 46.Perou CM, Leslie JD, Green W, Li L, Ward DM, Kaplan J. The Beige/Chediak–Higashi syndrome gene encodes a widely expressed cytosolic protein. J Biol Chem. 1997;272:29790–4. doi: 10.1074/jbc.272.47.29790. [DOI] [PubMed] [Google Scholar]
- 47.Chicz RM, Urban RG, Gorga J, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]


