Abstract
Interleukin-2 (IL-2) is one of the most studied cytokines driving T-cell proliferation, activation and survival. It binds to the IL-2 receptor consisting of three chains, the α (CD25), β and common γ (γc). The binding of the CD25 chain to IL-2 is necessary to expose high-affinity binding sites for the β and γc chains, which, in turn, are responsible for downstream signalling. A high level of soluble CD25 (sCD25) has been associated with a poor prognosis in patients with non-Hodgkin's lymphoma. The function and source of origin of this soluble receptor is not well investigated. In the present study we hypothesized that T regulatory (Treg) cells may release CD25 to act as a decoy receptor for IL-2, thereby depriving T-effector cells of IL-2. Peripheral blood from patients with B-cell malignancies (n = 26) and healthy controls (n = 27) was investigated for the presence and function of FoxP3+ Treg cells and sCD25 by multi-colour flow cytometry and enzyme-linked immunosorbent assay. Further, the proliferative capacity of T cells was evaluated with or without the presence of recombinant sCD25. The results demonstrate that Treg cells from patients had lower CD25 expression intensity and that they released CD25 in vitro. Further, high levels of Treg cells correlated with sCD25 plasma concentration. Recombinant sCD25 could suppress T-cell proliferation in vitro. In conclusion, the release of sCD25 by Treg cells may be a mechanism to deprive IL-2 and thereby inhibit anti-tumour T-cell responses.
Keywords: B-cell malignancy, CD25, interleukin-2 receptor α, immune escape mechanism, T regulatory cell
Introduction
Interleukin-2 (IL-2) is one of the most studied cytokines driving T-cell proliferation and survival. IL-2 binds to the IL-2 receptor consisting of three separate chains, the α (CD25), β and common γ (γc) chains. The β and γc chains can form a low-affinity receptor but the binding of CD25 chain to IL-2 is necessary to expose high-affinity binding sites for the β and γc chains. CD25 lacks intracellular signalling domains and it is therefore the β and γc chains that are responsible for downstream signalling.1 A high level of soluble CD25 (sCD25) is a common phenomenon in patients with B-cell malignancies and has been associated with a poor prognosis in patients with non-Hodgkin's lymphoma.2–5 The function and source of origin of this soluble receptor is not well investigated. A decade ago, two studies showed that both malignant B cells and activated T cells may be responsible for its release and sCD25 was considered a sign of immune activation.6,7 However, it was recently demonstrated that T regulatory (Treg) cells can release CD25 upon activation.8 The Treg cells, defined as FoxP3-expressing CD4+ T cells, are important regulators of autoimmunity and tumour immunity and also restrain immune responses post infection. They inhibit their target cells in both contact-dependent and -independent manners.9 Treg cells express high levels of CD25 on their surface and this marker has been widely used to detect, sort and target Treg cells in vitro and in vivo.10–12 Interleukin-2 is driving T-cell proliferation and is important to sustain the Treg-cell population as well.13 Treg cells may use CD25 to capture IL-2 from its environment, thereby depriving effector T cells of this stimulatory cytokine.14 In this study we investigated the levels of Treg cells, sCD25 and their relationship in patients with B-cell malignancies.
Materials and methods
Patient samples
Patients with B-cell chronic lymphocytic leukaemia (B-CLL, n = 12) and B-cell lymphoma (n = 14, represented by seven diffuse large B-cell lymphoma, four Hodgkin's lymphoma and three follicular lymphoma) were enrolled in the study. Written consent was obtained from all patients in concordance with the Helsinki Declaration and the study was approved by the regional ethics committee in Uppsala, Sweden (DNr: 2006:145). Peripheral blood from healthy donors (n = 27) (obtained through the blood bank at Uppsala University Hospital, Uppsala, Sweden) and patients was collected in sodium heparin-coated Vacutainers (Becton Dickinson, San Diego, CA). Plasma was collected after centrifugation and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque gradient centrifugation (Amersham Bioscience, Uppsala, Sweden). The PBMCs from healthy controls used for fluorescence-activated cell sorting (FACS) analysis and magnetic antibody cell sorting (MACS) separations were obtained from buffy coats. All samples were stored at −80° until analysis.
Flow cytometry
To analyse the level of Treg cells, PBMCs from patients and healthy controls were stained for CD3 (SK7; fluorescein isothiocyanate-conjugated), CD127 (hIL-7R-M21; phycoerythrin-conjugated), BD Bioscience, San Jose, CA, CD4 (RPA-T4; peridinin chlorophyll protein-conjugated) and FoxP3 (259D; Alexa Fluor-647-conjugated), (BioLegend, San Diego, CA). Matching isotype control antibodies were purchased from BD Biosciences and BioLegend. To analyse the phenotype of Treg cells, cells were stained for CD3 (OKT3; Pacific Blue), CD4 (RPA-T4; allophycocyanin-Cy7-conjugated), CD127, FoxP3 and CD25 (M-A251; phycoerythrin-Cy7-conjugated). Cells were analysed using FACS LSRII (Becton Dickinson) and data were analysed using FlowJo (Treestar, Ashland, OR).
Soluble CD25 enzyme-linked immunosorbent assay
The presence of sCD25 was detected with CD25/IL-2 receptor enzyme-linked immunosorbent assay (ELISA; Diaclone, Besançon, France).
Purification of cell types
The PBMCs from healthy controls and CLL patients were purified into Treg cells, T helper cells, cytotoxic T lymphocytes, B cells and monocytes by using the Miltenyi MACS separation system. Cells were cultured in 200 μl medium for 2 days at 37° in 5% CO2. The supernatants were harvested, stored at −20° and later analysed by CD25 ELISA. Cell supernatants were centrifuged before analysis to avoid contamination of cell-bound CD25.
Proliferation assay
The PBMCs (1 × 104) were cultured in 96-well plates in 200 μl RPMI-1640 supplemented with 1 mm sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mmβ-mercaptoethanol and 10% fetal bovine serum (Invitrogen, Paisley, UK). Cells were stimulated with 1 μg/ml anti-CD3 antibody (clone: OKT3, BioLegend) and 60 U/ml recombinant human IL-2 (R&D Systems Inc, Minneapolis, MN) and cultured in triplicates after the addition of 20 μl Alamar Blue (Biosource International, Camarillo, CA) per well. Cells were incubated at 37°, 5% CO2, 95% humidity. Proliferation was analysed by measuring the absorbance at 570–595 nm at day 4. Recombinant sCD25 (Diaclone) in decreasing concentrations was added to some experiments. In these, 10 U/ml IL-2 was used.
Statistical analysis
Linear regression analysis was used to analyse the relationship between factors and Mann–Whitney to analyse differences among groups. P values < 0·05*, < 0·01** and < 0·001*** were considered significant. All statistical analyses were performed using Graph Pad Prism 4 (Graph Pad Software Inc., San Diego, CA).
Results
CD25 expression on Treg cells and levels of sCD25 in plasma
The level of CD25 expression on Treg cells (defined as CD3+ CD4+ CD127low FoxP3+ lymphocytes) was investigated by multi-colour FACS analysis. In healthy donors, about 70% of the Treg cells expressed cell surface CD25. However, a significant decrease of CD25+ Treg cells was seen both in CLL patients (P = 0·0111) and in patients with B-cell lymphoma (P = 0·0121; Fig. 1a). Further, the level of CD25 on the CD25+ Treg cells was significantly decreased in the lymphoma patients (P < 0·0001; Fig. 1b). Corresponding with the loss of cell surface CD25 on patient's Treg cells, there was an elevated level of soluble CD25 in both CLL (P < 0·0001) and lymphoma patients (P = 0·0008) compared with healthy controls (Fig. 2).
Figure 1.
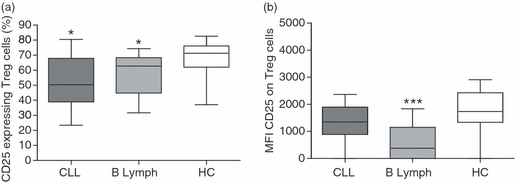
Regulatory T (Treg) cells from patients have a decreased cell surface expression of CD25. Peripheral blood mononuclear cells from patients with chronic lymphocytic leukaemia (n = 12) and B-cell lymphoma (n = 14) were stained for CD3, CD4, CD127, FoxP3 and CD25 to investigate the surface expression of CD25 on Treg cells. Patients were compared with healthy age- and gender matched controls (n = 27). Patients had a significant decrease of CD25+ Treg cells (a) and lymphoma patients had a decreased surface expression of CD25 compared with healthy controls (b). Differences among groups were analysed using the Mann–Whitney test.
Figure 2.
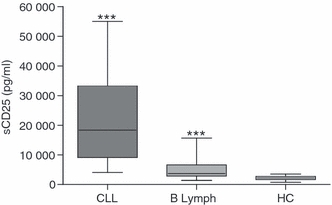
Soluble (s) CD25 is increased in chronic lymphocytic leukaemia (CLL) and lymphoma plasma. Plasma from CLL patients (n = 12), B-cell lymphoma patients (n = 14) and healthy controls (n = 27) was analysed for the presence of sCD25 using enzyme-linked immunosorbent assay. Patients displayed a significantly higher level of soluble CD25 than healthy individuals. Mann–Whitney U-test was used to calculate significant differences.
Correlations between Treg-cell levels and sCD25 in plasma
To investigate if Treg cells may be partly responsible for the high levels of sCD25 seen in patient plasma, correlation analysis of Tregs (CD3+ CD4+ CD127low FoxP3+ lymphocytes) and sCD25 was performed. In healthy individuals, no correlation existed between the level of peripheral Treg cells and plasma levels of sCD25 (Fig. 3a). There was no overall correlation of Treg cells and sCD25 patients with CLL (Fig. 3b) but lymphoma patients had a strong correlation (Fig. 3c, P < 0·0001). However, the patients with the highest sCD25 levels also had the highest Treg-cell levels in both patient groups. In Fig. 3(d–e) it is further demonstrated that sCD25 levels do not correlate with CD4+ T cells in general. Previous publications have shown that sCD25 in patients with B-cell malignancy may originate from malignant B cells, for which reason a correlation analysis between sCD25 and white blood cell count (mostly malignant B cells) on patients with CLL was performed. As can be seen in Fig. 3(f), no such correlation exists in our patient cohorts.
Figure 3.
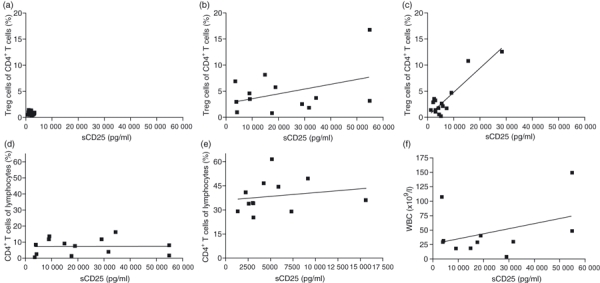
Soluble (s) CD25 levels correlates with regulatory T (Treg) cell levels in patients with B-cell lymphoma. Levels of sCD25 were correlated with Treg-cell levels using linear regression. No correlation was found between these two factors in healthy controls (a) or patients with chronic lymphocytic leukaemia (CLL) (b). In patients with B-cell lymphoma, a strong correlation was found between sCD25 and Treg-cell levels (c; P < 0·0001). The sCD25 did not correlate with the general CD4+ T cells in lymphoma (d) or CLL (e). To investigate if the high levels of sCD25 originated from malignant B cells we performed correlation analysis on sCD25 and white blood cell count. No correlation between these two factors was found (f).
Purified Treg cells release sCD25 in culture
To investigate the responsible cell type for sCD25 release, PBMCs were fractionated into Treg cells (CD4+ CD127low CD25high), CD4+ and CD8+ T cells, monocytes (CD14+) and B cells (CD20+) using the MACS system. Unfractionated PBMCs served as a control. Patient PBMCs and CD20 sorted B cells were analysed as well. Cells (1 × 10e5) were cultured for 2 days in 96-well plates. Supernatants were analysed using sCD25 ELISA. Unstimulated Treg cells produced the highest levels of sCD25 (P = 0·0286; Fig. 4a). However, upon polyclonal CD3 (OKT-3) T-cell stimulation also CD4+ T cells released sCD25 (Fig. 4b). In this experiment CD3 negative cells such as monocytes and B cells were used as negative controls. No sCD25 was detected from malignant B cells even when the cultures were increased to 2·5 × 10e5 cells (Fig. 4a).
Figure 4.
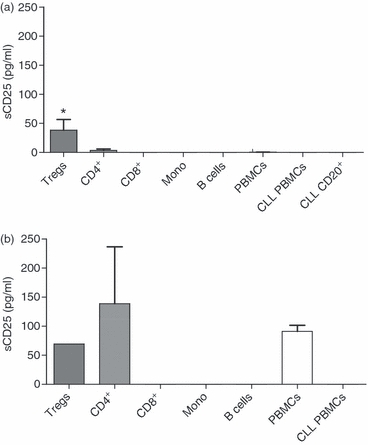
Purified regulatory T (Treg) cells release soluble CD25 (sCD25). Immune cell types were purified from peripheral blood mononuclear cells (PBMCs; n = 4) using magnetic antibody cell sorting beads. Unfractionated PBMCs, CD4+ CD127low CD25high Treg cells, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD20+ B cells, and PBMCs as well as CD20+ B cells from CLL blood were cultured for 2 days in a 96-well plate. Cells were cultured without stimulation (a) or with anti-CD3 stimulation (b). Mann–Whitney U-test was used to analyse differences among groups.
Recombinant sCD25 inhibits T-cell proliferation
Next, the role of sCD25 on stimulated T cells was analysed in proliferation assays. Healthy donor PBMCs were stimulated with anti-CD3/IL-2 and cultured with increasing levels of recombinant sCD25. In Fig. 5(a) it is demonstrated that sCD25 inhibited T-cell proliferation in a dose-dependent manner (1000 pg/ml P < 0·0001; 500 pg/ml P < 0·0001, 250 pg/ml P = 0·0007, 100 pg/ml P = 0·0121. In Fig. 5(b) we pre-incubated sCD25 (1000 pg/ml) with the IL-2 for 1 hr at 37° before adding the IL-2/sCD25 mix to stimulated T-cells, which resulted in a similar proliferation level to that seen when sCD25 is added directly into the stimulations. To investigate if the T cells in patients with B-cell malignancies were affected by suppressive mechanisms such as Treg cells and sCD25, PBMCs were stimulated with anti-CD3/IL-2 and analysed for their proliferative capacity, which was decreased compared with controls (Fig. 6a). Further, patients with > 5000 pg/ml sCD25 plasma levels had poorer T-cell proliferation than patients with low sCD25 or healthy donors, who all had low sCD25 levels (Fig. 6b; P = 0·0242 and P = 0·0105, respectively).
Figure 5.
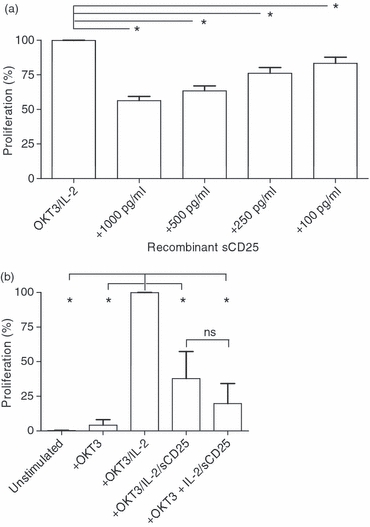
Recombinant soluble (s) CD25 inhibit T-cell proliferation. (a) Recombinant sCD25 was added to OKT-3/interleukin-2 (IL-2) stimulated peripheral blood mononumear cells in triplicates from 11 donors in an Alamar Blue assay. Cells were cultured in final concentrations of 1000 (n = 8), 500 (n = 8) 250 (n = 8) or 100 pg/ml (n = 3) sCD25. The experiment was repeated four times with similar results. (b) PBMCs from six healthy donors were unstimulated, or stimulated with anti-CD3 (OKT3), OKT3 + IL-2, OKT3 + IL-2 + sCD25 (1000 pg/ml) or OKT3 + pre-incubated IL-2/sCD25 to allow complex formation before culture. The OKT3/IL-2 T cells were significantly different from unstimulated (P = 0·002), OKT3-stimulated (P = 0·02), OKT3/IL-2/sCD25-stimulated (P = 0·0152) and OKT3 + IL-2/sCD25 complex (P = 0·02). The experiment was repeated with six additional donors with similar results. Mann–Whitney U-test was used to analyse differences among groups.
Figure 6.
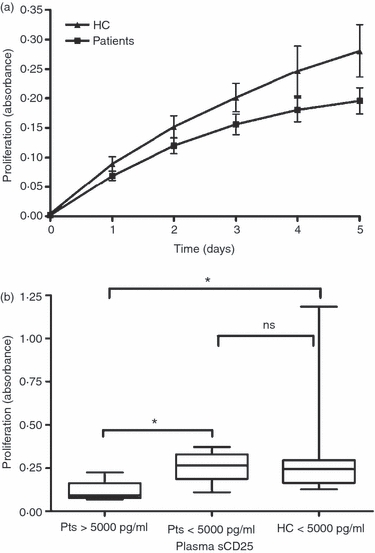
Patient T cells exhibit decreased proliferative capacity. (a) Peripheral blood mononuclear cells were stimulated with anti-CD3/interleukin-2 and cultured in triplicates in 96-well plates in Alamar Blue assays. The mean valuesof 18 patients and 23 controls are shown in the figure. (b) Healthy controls (< 5000 pg/ml) and patients divided into high (> 5000 pg/ml) and low (< 5000 pg/ml) levels of plasma sCD25 and evaluated for proliferative capacity at day 5 of stimulation. Bars represent standard error of the mean and statistic analysis was performed using Mann–Whitney U-test.
Discussion
The Treg-cell-associated molecule CD25 has been used as a detection marker of Treg cells in cancer. Because it is also expressed on activated T cells and because some Treg cells lack CD25, other markers such as FoxP3, are more appropriate.15 Because of the high content of sCD25 in patient plasma we investigated the presence of CD25+ Treg cells as well as the CD25 expression intensity on those cells. CD25+ Treg cells were decreased in cancer patients compared with healthy age- and gender-matched donors. Further, cell surface CD25 expression intensity was lower in lymphoma patients compared with the control cohort. We hypothesized that the Treg cells released CD25 as a means of T-effector-cell suppression via deprivation of IL-2. Treg cells, and other immune cells, were sorted from PBMCs and cultured to collect supernatants. We found that Treg cells released sCD25. It has been published that Tregs from healthy donors can release sCD25 post-stimulation.8 Therefore, we stimulated cells with anti-CD3/IL-2 and measured CD25 release. Under these circumstances, not only Tregs, but also the general CD4 T-cell population could release this molecule. However, the CD8+ T cells did not. In PBMCs from the CLL patients no increase of sCD25 was seen upon stimulation. However, PBMCs from the patients mainly consisted of malignant B cells, which are not affected by CD3 stimulation.
The Treg cells can suppress T cells in numerous ways. Subpopulations of Treg cells release different panels of suppressive cytokines such as transforming growth factor-β, IL-10 and IL-35, which have both direct and indirect effects on T-cell proliferation, activation status and survival.9 Interleukin-2 deprivation may be another mechanism of T-cell suppression. Because Treg cells have a much higher CD25 surface expression than other T cells they are likely to capture IL-2 from its surroundings. It is also possible that CD25 is released as a decoy receptor to block free IL-2 from binding to effector T cells. In the present study, we demonstrate that recombinant CD25 can efficiently block T-cell proliferation in a dose-dependent manner. Furthermore, if sCD25 is pre-incubated with the IL-2 before being added to the T cells then the inhibition tended to be even better but did not reach significant levels. These results confirm that sCD25 may hamper T-cell proliferation and the IL-2 bound to sCD25 will not stimulate T cells. However, Pedersen et al.8 showed that cell supernatant with high levels of sCD25 did not hamper in vitro T-cell proliferation. It is possible that sCD25 molecules found in supernatants are already loaded with IL-2 that may be produced by the cultured cells. There may also be a number of other cytokines in cultures that affect T-cell proliferation. In our study we used recombinant sCD25. The highest dose hampered, but did not block, proliferation. Since the β and γc chains of the IL-2 receptor have low affinity to IL-2, blocking of these chains as well may be needed for a complete proliferative block.1
To further investigate if Treg cells may be responsible for the high level of sCD25 in CLL and lymphoma patients we performed correlation analysis between Treg-cell level and sCD25. We showed that sCD25 indeed had a strong correlation with Treg cells in patients with B-cell lymphoma. However, this correlation was not seen in CLL patients. However, patients in both cohorts that had the highest Treg-cell levels also had the highest sCD25 concentrations in plasma. To make sure that the sCD25 was not a reflection of helper T-cell activation, we correlated the level of CD4+ T cells to sCD25 and the results showed that there was no such correlation. It is possible that a population of patients have tumour cells releasing sCD25 even if those tested in vitro did not release CD25 into the supernatants. Further, CD25 release from tumours may occur upon stimuli that we could not mimic in vitro. Therefore, we correlated the level of sCD25 to the white blood cell count in CLL patients because the majority of these cells are malignant cells. However, white blood cell count did not correlate with sCD25 levels.
The high plasma levels of sCD25 may provoke the effector T cells, which are anergic or poor responders to antigen stimulation. Therefore, the proliferative capacity of T cells of patients was determined in proliferation assays. Indeed, the T cells from patients had suppressed proliferation capacity compared with T cells from healthy controls. Taken together, high levels of sCD25 have previously been observed in the plasma of patients with B-cell malignancy.2,6,7 In the present study, we demonstrate that Treg cells release sCD25 and that recombinant CD25 can block T-cell proliferation in vitro. We further show that patients with B-cell malignancies have high levels of sCD25, which correlate with the high Treg-cell levels. Release of sCD25 by Treg cells may, therefore, be a mechanism of suppressing anti-tumour responses by depriving IL-2 from effector cells.
Acknowledgments
This study was supported by The Swedish Cancer Society, The Swedish Research Council, EU FP6 ‘Apotherapy’, European LeukemiaNet, and the Medical Faculty at Uppsala University.
Disclosures
The authors indicated no potential conflicts of interest.
References
- 1.Létourneau S, Krieg C, Pantaleo G, Boyman O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol. 2009;123:758–62. doi: 10.1016/j.jaci.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn DJ, Dou QP. The role of interleukin-2 receptor alpha in cancer. Front Biosci. 2005;10:1462–74. doi: 10.2741/1631. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Encinas M, Villamayor M, Campos A, Gonzalez S, Bello JL. Tumor burden and serum level of soluble CD25, CD8, CD23, CD54 and CD44 in non-Hodgkin's lymphoma. Haematologica. 1998;83:752–4. [PubMed] [Google Scholar]
- 4.Fabre-Guillevin E, Tabrizi R, Coulon V, Monnereau A, Edhbali H, Soubeyran I, Soubeyran P. Aggressive non-Hodgkin's lymphoma: concomitant evaluation of interleukin-2, soluble interleukin-2 receptor, interleukin-4, interleukin-6, interleukin-10 and correlation with outcome. Leuk Lymphoma. 2006;47:603–11. doi: 10.1080/10428190500361029. [DOI] [PubMed] [Google Scholar]
- 5.Goto H, Tsurumi H, Takemura M, et al. Serum-soluble interleukin-2 receptor (sIL-2R) level determines clinical outcome in patients with aggressive non-Hodgkin's lymphoma: in combination with the International Prognostic Index. J Cancer Res Clin Oncol. 2005;131:73–9. doi: 10.1007/s00432-004-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso C, Candore G, Cigna D, Colucci AT, Modica MA. Biological significance of soluble IL-2 receptor. Mediators Inflamm. 1993;2:3–21. doi: 10.1155/S0962935193000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay NE, Burton J, Wagner D, Nelson DL. The malignant B cells from B-chronic lymphocytic leukemia patients release TAC-soluble interleukin-2 receptors. Blood. 1988;72:447–50. [PubMed] [Google Scholar]
- 8.Pedersen AE, Lauritsen JP. CD25 shedding by human natural occurring CD4+ CD25+ regulatory T cells does not inhibit the action of IL-2. Scand J Immunol. 2009;70:40–3. doi: 10.1111/j.1365-3083.2009.02268.x. [DOI] [PubMed] [Google Scholar]
- 9.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khazaie K, von Boehmer H. The impact of CD4+ CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–36. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Baecher-Allan CM, Hafler DA. The purification and funtional analysis of human CD4+ CD25high regulatory T cells. Curr Protoc Immunol. 2006 doi: 10.1002/0471142735.im0704bs72. Chapter 7:unit7:4B. [DOI] [PubMed] [Google Scholar]
- 12.Hartigan-O’Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Turka LA, Walsh PT. IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci. 2008;13:1440–6. doi: 10.2741/2773. [DOI] [PubMed] [Google Scholar]
- 14.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+ CD25+ Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 15.Kryczek I, Liu R, Wang G, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]


