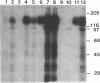
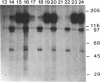
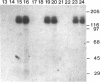
Abstract
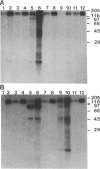
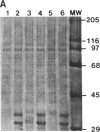
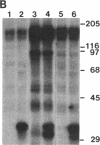
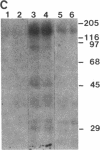
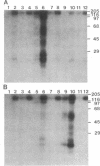
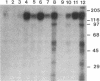
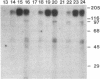
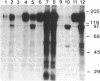
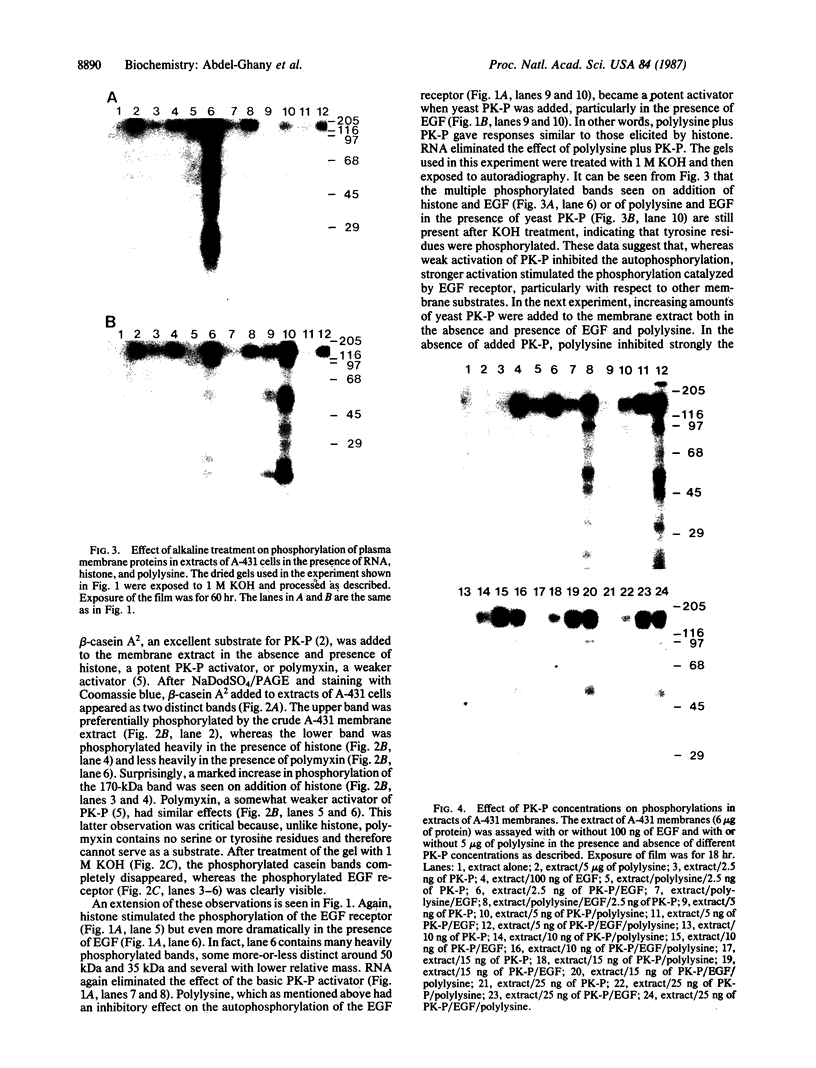
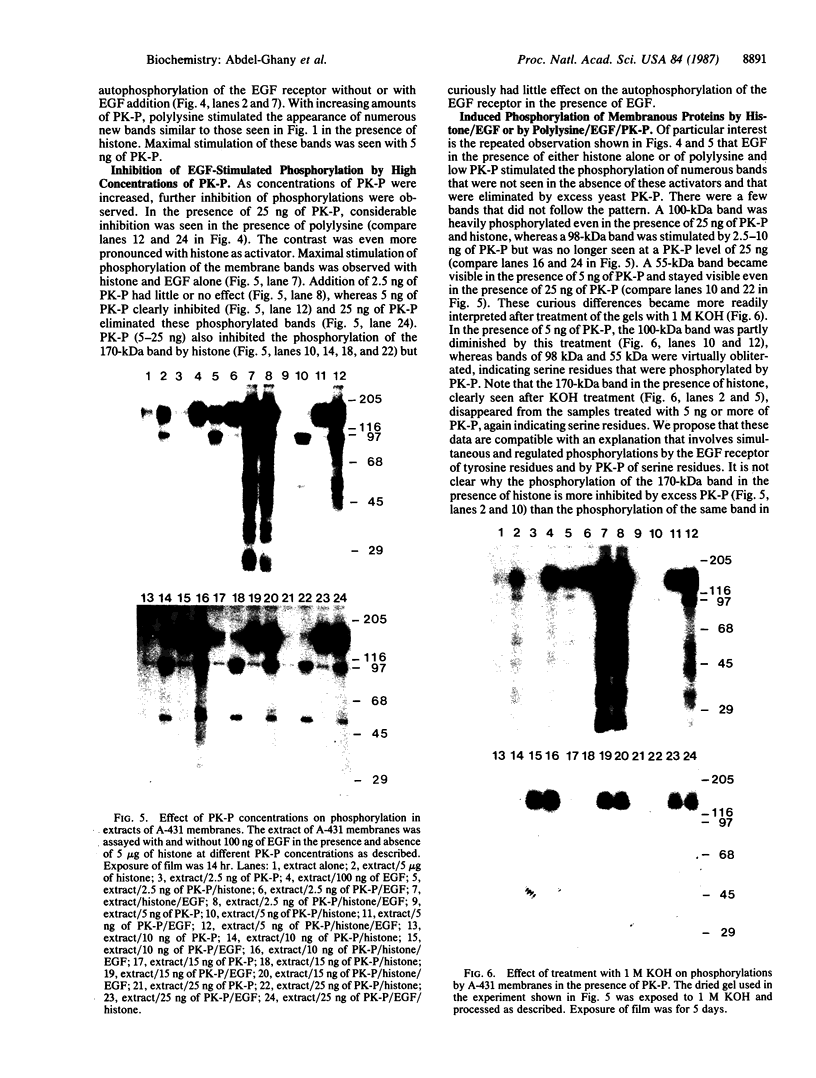
Protein kinase P (PK-P) activated by histones or certain other basic compounds has been purified previously from yeast [Yanagita, Y., Abdel-Ghany, M., Raden, D., Nelson, N. & Racker, E. (1987) Proc. Natl. Acad. Sci. USA 84, 925-929]. It is shown here that PK-P is present in solubilized membranes of A-431 carcinoma cells where it changes the epidermal growth factor (EGF) receptor kinase activity. Polylysine, a weak PK-P activator, inhibited the autophosphorylation of the EGF receptor both in the absence and presence of EGF. Increased PK-P activity induced by histone 1, a potent activator, gave rise to increased autophosphorylation of the EGF receptor as well as phosphorylation at tyrosine residues of numerous other endogenous membrane components. The stimulation by histone was particularly striking in the presence of EGF. A similar stimulation was achieved with polylysine and EGF on addition of yeast PK-P. However, addition of yeast PK-P in the presence of histone 1 markedly inhibited the EGF-stimulated phosphorylation of endogenous membrane proteins. We conclude from these results that the effect of PK-P on the EGF receptor takes place in three phases: at low levels PK-P inhibits the autophosphorylation, at intermediate levels it stimulates the autophosphorylation as well as the EGF-dependent phosphorylation of numerous other membrane proteins, and at high levels it inhibits the phosphorylation of these proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., Nakamura S., Navarro J., Racker E. A membrane-bound human placental protein kinase activated by endogenous polypeptides. Biosci Rep. 1983 Mar;3(3):275–282. doi: 10.1007/BF01122460. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany M., Riegler C., Racker E. A placental polypeptide activator of a membranous protein kinase and its relation to histone 1. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7388–7391. doi: 10.1073/pnas.81.23.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S., Raymond W. E., Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984 Feb 25;259(4):2051–2054. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Platelet-derived growth factor mimics phorbol diester action on epidermal growth factor receptor phosphorylation at threonine-654. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4080–4084. doi: 10.1073/pnas.82.12.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Navarro J., Abdel Ghany M., Racker E. Inhibition of tyrosine protein kinases by halomethyl ketones. Biochemistry. 1982 Nov 23;21(24):6138–6144. doi: 10.1021/bi00267a018. [DOI] [PubMed] [Google Scholar]
- Nemenoff R. A., Kwok Y. C., Shulman G. I., Blackshear P. J., Osathanondh R., Avruch J. Insulin-stimulated tyrosine protein kinase. Characterization and relation to the insulin receptor. J Biol Chem. 1984 Apr 25;259(8):5058–5065. [PubMed] [Google Scholar]
- Parries G., Hoebel R., Racker E. Opposing effects of a ras oncogene on growth factor-stimulated phosphoinositide hydrolysis: desensitization to platelet-derived growth factor and enhanced sensitivity to bradykinin. Proc Natl Acad Sci U S A. 1987 May;84(9):2648–2652. doi: 10.1073/pnas.84.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Abdel-Ghany M., Sherrill K., Riegler C., Blair E. A. New protein kinase from plasma membrane of Ehrlich ascites tumor cells activated by natural polypeptides. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4250–4254. doi: 10.1073/pnas.81.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita Y., Abdel-Ghany M., Raden D., Nelson N., Racker E. Polypeptide-dependent protein kinase from bakers' yeast. Proc Natl Acad Sci U S A. 1987 Feb;84(4):925–929. doi: 10.1073/pnas.84.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]