Abstract
Evolving models of immune tolerance have challenged the view that the response of the maternal immune system to environmental or fetal antigens must be suppressed or deviated. CD8 T cells play a central role in the immune response to viruses and intracellular pathogens so the maintenance of both the number and function of these cells is critical to protect both the mother and fetus. We show that the numbers of maternal CD8 T cells in both the spleen and the uterine draining lymph nodes are transiently increased at mid-gestation and this correlates with enhanced CD8 T-cell proliferation and an increased relative expression of both pro-survival and pro-apoptotic molecules. In transgenic mice bearing T-cell antigen receptors specific for the male HY or allo-antigens, the transgenic CD8 T cells retain the ability to proliferate and function during pregnancy. Moreover, anti-HY T-cell receptor transgenic mice have normal numbers of male pups despite the presence of CD8 T cells at the maternal–fetal interface. These data suggest that pregnancy is a dynamic state in which CD8 T-cell turnover is increased while the function and ending size of the CD8 T-cell compartment are maintained.
Keywords: apoptosis, CD8/cytotoxic T cells, proliferation, reproductive immunology
Introduction
The classical model of immunology states that the semi-allogeneic fetus should be recognized as non-self and rejected. In this context, explanations of maternal tolerance evoked immune suppression or deviation. Some studies have demonstrated non-specific pregnancy-induced alterations in maternal immune function.1–3 In addition, evidence of suppression of antigen-specific T-cell function has been observed in some transgenic animals.4–6 However, other studies have found no decrease or alteration in maternal immune function.7–9 An alternative to the classical model of immunity, the Danger Model, proposes that an obligate down-regulation of the maternal immune system is not necessary, and immune responses to fetal antigens could be generated in abnormal pregnancy.10–12 An extension of this model suggests that the pregnancy-associated disease pre-eclampsia may be related to immune cell activation in response to abnormal placentation, and argues that maternal immune function is not impaired.12
During pregnancy, changes in the organs of the maternal immune system in addition to physiological adaptations in the cardiovascular,13 renal14 and endocrine systems15 have been described. For example, the size and cellularity of the thymus decreases in late pregnancy,16 and it has been proposed that this is related to maternal progesterone.17 Splenic hypertrophy has been observed during normal gestation in both humans18 and mice,19,20 but the spleen then returns to pre-pregnant size in the post-partum period. In mice, the uterine draining lymph nodes are enlarged in both syngeneic and allogeneic pregnancies.21,22 We have previously observed lineage-specific alterations in the proliferation and death of immune cell subsets during gestation.23
The size of the peripheral T-cell compartment is controlled by homeostatic mechanisms that balance the export of new T cells from the thymus plus the proliferation of peripheral T cells in response to MHC-presenting self-peptides with cell death.24,25 In addition, the profound expansion of T cells that occurs in response to infection is followed by contraction once the pathogen is cleared to return T-cell numbers to pre-infection levels.26 T-cell proliferation and death are influenced by signalling through the T-cell antigen receptor,27 co-stimulatory molecules,28,29 cytokines,30,31 soluble hormones,32 and other cell surface interactions.33,34
CD8 T-cell function protects the mother and fetus from infections during pregnancy, but at the same time may be subject to regulation to maintain fetal tolerance. The effects of pregnancy on CD8 T cells have been studied in T-cell receptor transgenic (TCR) systems and, depending on the model system used, deletion,35 tolerance,5 or ignorance of antigen-specific cells36 have been reported. As homeostatic mechanisms contribute to the functional capacity and diversity of the T-cell pool, understanding this process in the maternal immune system during normal pregnancy will probably provide critical insight into the interpretation of these studies.
To characterize the effects of normal gestation on the CD8 T-cell pool, we determined the total number, proliferation and apoptosis of these cells in the spleen and the uterine draining lymph nodes during pregnancy. We then investigated whether the expression of regulatory molecules controlling T-cell proliferation and death varied during pregnancy in CD8 T cells. Finally, fetal-antigen-specific transgenic models were used to characterize the outcomes of pregnancies and the function of CD8 T cells. The results of these studies suggest that normal homeostatic mechanisms operate during pregnancy and challenge the classical model of immune suppression driving maternal tolerance.
Materials and methods
Mice and breeding
Eight- to ten-week-old C57BL/6 (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 TCR transgenic anti-HY Rag1−/− (Matahari)37 mice were obtained from P. Matzinger (National Institutes of Health, Bethesda, MD), C57BL/6 pUBI-GFP (GFP)38 mice were gifts from P. Marack (University of Colorado Health Sciences center, Denver, Colorado), and C57BL/6 TCR transgenic Ld (2C)6 mice were obtained from T. Hansen (Washington University, St. Louis), and bred onto the C57BL/6 Rag1−/− background (2C Rag1−/−). C57BL/6 CD45.1 (B6 CD45.1), C57BL/6 Rag1−/−, and BALB/c Rag1−/− mice were bred and maintained in our animal colony. For timed pregnancies, single females were seasoned in empty male cages to induce oestrus. After 2 days, males were introduced and overnight mating was allowed. The next morning female mice were visually examined for the presence of a copulation plug, which was denoted as day 0 of pregnancy. All animals were maintained under specific pathogen-free conditions and used in accordance with the Institutional Animal Care and Use Committee at the University of Vermont and in accordance with The Association for Assessment and Accreditation of Laboratory Animal Care.
Antibodies
The following monoclonal antibodies (mAbs) were purchased from BD Biosciences (San Jose, CA): phycoerythrin (PE) -conjugated anti-TCR-β, fluorescein isothiocyanate (FITC) -conjugated anti-CD4, allophycocyanin (APC) -conjugated anti-CD8, FITC-conjugated anti-Vβ8.3, PE-conjugated anti-Vβ8.1/8.2, APC-conjugated anti-TCR-β, biotin-conjugated anti-CD45.1, and FITC-conjugated anti-bromodeoxyuridine (BrdU). PE-Texas Red (PETR) -conjugated anti-CD4, PECy5.5-conjugated anti-CD8, and PECy5.5-conjugated anti-streptavidin were purchased from Invitrogen Corporation/Caltag Laboratories (Carlsbad, CA). The FITC-conjugated anti-12-2′-deoxy-uridine-5′-triphosphate (dUTP) was purchased from Roche Applied Science (Indianapolis, IN).
In vivo bromodeoxyuridine assay
A BrdU incorporation assay was performed as described previously.39 Briefly, pregnant mice and corresponding unmated controls were given four intraperitoneal injections of 1 mg BrdU in 100 μl phosphate-buffered saline (PBS) at 24, 20, 15 and 1 hr before killing. Spleens and uterine draining lymph nodes were harvested, cells were counted, and non-specific antibody binding was blocked by treatment with 0·5 μm Fcγ III/II receptor (CD16/CD32) (BD Biosciences). Cells were then stained with mAbs against CD4, CD8, and TCR-β, fixed with 1% methanol-free formaldehyde, and permeabilized with 0·01% Tween-20 in 1% formaldehyde overnight. Cells were treated with 50 U/ml of deoxyribonuclease I (Sigma-Aldrich, St Louis, MO) in buffer containing 0·15 m NaCl, 4·2 mm MgCl2 (Sigma-Aldrich) at pH 5·0 for 15 min at 37° and incubated with anti-BrdU-FITC for 30 min. BrdU incorporation was detected by flow cytometry (BD LSRII; BD, San Jose, CA) and quantified using FlowJo software analysis (Tree Star, Inc., Ashland, OR).
TUNEL assay to detect apoptosis
The terminal deoxynucleotidyl-transferase (TdT)-mediated dUTP nick-end labelling (TUNEL) assay was used to detect apoptotic cells by flow cytometry as described previously.39 Spleen and uterine draining lymph node cells were treated with 0·5 μm Fcγ III/II receptor and surface-stained with the same mAbs as in the BrdU assay. Cells were then fixed with 1% formaldehyde, permeabilized with 70% ethanol, and incubated with 10 U of terminal deoxynuclotidyl transferase (TdT) and 6·25 μm FITC-dUTP in 1 × TdT reaction buffer with 2·5 mm cobalt chloride (all from Roche Applied Science) for 1 hr at 37°. TUNEL-positive cells were quantified by flow cytometry and FlowJo software analysis.
Fluorescence-activated cell sorting and reverse transcription quantitative polymerase chain reaction of sorted cells
Individual spleens and pools of uterine draining lymph nodes (two femoral and two para-aortic nodes from three mice) were isolated from unmated controls and pregnant mice on gestational days 5, 8 and 15. Non-specific antibody binding was blocked by treatment with 0·5 μm anti-Fcγ III/II receptor mAb (BD Biosciences) and cells were incubated with mAbs against CD4, CD8 and TCR-β for 30 min. Aliquots of 10–30 million cells were sorted using the 70-μm tip of the BD FACSAria cell sorter (BD Biosciences). Nucleated cells were selected by forward- and side-scatter gating, and aggregates were eliminated by doublet discrimination. Cells were first selected for TCR-β expression, and then CD4− CD8+ cells were collected at > 90% purity. Total RNA was extracted from at least 500 000 cells using Trizol reagent (Invitrogen Corporation) following the manufacturer's guidelines. Samples were quantified by UV absorbance at 260 nm on a NanoDrop spectrophotometer (ThermoScientific, Willmington, DE) and RNA integrity was tested using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA).
The iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) was used to synthesize complementary DNA (cDNA) from 250 ng of RNA template using a mix of random hexamers and oligo-dTs. From each sample, cDNA was used to amplify the following target genes: interleukin-15 receptor (IL-15R) α chain (Il15r, forward 5′-CTGACACCATCCCAAACAA CTTCTG-3′, reverse 5′-GCTGCCTTGATTTGATGTACCAGG-3′); IL-7R (Il7r, forward 5′-ACCCAAGAATCAAGGAGGATGG-5′, reverse 5′-GGCTAAGATGACCAACAAAAACAC-3′); tumour necrosis fac-tor receptor superfamily member 6 (Fas, forward 5′-AACAAAGTCCCAGAAATCGCCTATG-3′, reverse 5′-TCCTGTCTCCTT TTCCAGCACTT-3′); and tumour necrosis factor superfamily member 6 (Fasl, forward 5′-CGGTGGT ATTTTTCATGGTTCTGGT-3′, reverse 5′-TACTGGGGTTGGCTATTTGCTTTTC-3′). For quantification cDNA was also amplified for two housekeeping genes: hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1, forward 5′-CAGTCCCAGCGTCGTGAT-3′, reverse 5′-CAAGTCTTTCAGTCCTGTCCATAA-3′); and succinate dehydrogenase complex, subunit A, flavoprotein (Sdha, forward 5′-ATGCCAGGGAAGATTACAA AGTGC-3′, reverse 5′-GTAACCTTGCCAGTCTTGATGTCC-3′). Each reaction used 1 μl cDNA, 150 nm of the forward and reverse primers, and 12·5 μl Power Sybrgreen Master Mix (Applied Biosystems, Carlsbad, CA) in a 25-μl reaction. The reactions were performed on an ABI Prism 7000 (Applied Biosystems) using an initial denaturation of 10 min at 95°, 40 cycles of 15 seconds at 95° and 60 seconds at 60°, followed by a melt curve analysis to ensure that only the correct product was amplified. Polymerase chain reaction (PCR) amplicons for all genes were checked for correct size on 2% agarose gels.
Standard curves were generated for all the target genes as well as the housekeeping genes using a single sample, which was serially diluted over the working range of the assay. Using these standard curves, the relative quantities of each sample were determined. Housekeeping genes were validated using the geNorm program40 to confirm that their levels were stable throughout gestation. Dividing by the geometric mean of the housekeeping genes normalized relative target messenger RNA (mRNA) expression. Each sample was run in triplicate and averaged. Negative water controls were run for each primer set in the real-time PCR. In each primer set at least one primer was designed over an exon–exon junction or spanned an intron region.
Tissue preparation for flow cytometry
Cells were isolated from the spleen and uterine draining lymph nodes of pregnant mice and unmated controls by mechanical dissociation. For unmated controls, the uterus was removed by cutting at the cervix and ovaries and then the uteri from four mice were pooled together. For pregnant mice, the uterus was removed, each uterine horn was bisected, and fetal units were removed. Placentas were peeled away from the decidual attachment sites and two placentas from each uterine horn were pooled together. After cutting the uterus or placenta into small pieces they were subjected to enzymatic digestion for 20 min at 37°. An adaptation of a previously published method41 was then used in which each sample was treated with 200 U/ml hyaluronidase (Sigma, St Louis, MO), 0·2 mg/ml DNAse I (Sigma), and 0·28 U/ml Liberase Blendzyme 3 (Roche, Indianapolis, IN) in Hanks’ balanced salt solution containing 10% bovine serum albumin (Sigma). After digestion, samples were pressed through 100-μm mesh and then passed through a magnetic antibody cell sorter (MACS) pre-separation filter (Miltenyi Biotec, Inc., Auburn, CA) to remove large debris. CD8 T cells were enriched from the uterus and placenta using MACS positive selection with CD8 microbeads according to the manufacturer's instructions (Miltenyi Biotec, Inc.). Cells were then washed twice with phosphate-buffered saline (PBS), and then resuspended in PBS–0·1% bovine serum albumin. Non-specific antibody binding was blocked by treatment with 0·5 μm Fcγ III/II receptor (CD16/CD32) (BD Biosciences) and cells were incubated with mAbs against CD8 and TCRVβ8.3 for 30 min. The proportion of CD8 T cells recovered from each tissue was determined by flow cytometry (BD LSRII; BD) and quantified using FlowJo software analysis (Tree Star, Inc.).
In vivo killing assay
Donor spleens were harvested from naive 12-week-old B6 CD45.1 female mice and B6 GFP male mice. Single cell suspensions were generated under sterile conditions from the spleens and red blood cells were lysed using BD PharmLyse per manufacturer's guidelines (BD Biosciences). Samples were then passed through a MACS pre-separation filter (Miltenyi Biotec, Inc.) to remove debris. Splenocytes were counted and 5 × 105 cells from each donor mouse (female B6 CD45.1 and male B6 GFP) were combined. These donor cells were washed with sterile PBS twice and then resuspended in a total volume of 150 μl in sterile PBS.
Twelve-week-old Matahari mice were mated to same strain males as described above. On day 3 or day 8 post-coitus Matahari mice were injected with donor cells by intraperitoneal (i.p.) injections. Control unmated mice also received the same donor cells by i.p. injection. As additional controls, naive B6 and Rag1−/− mice also received i.p. injections of donor splenocytes. One week later mice were killed, blood was collected by cardiac puncture and spleens were harvested. Tissues were then processed as described above for flow cytometry. Because Matahari mice express the CD45.2 antigen, the presence of injected donor female cells was visualized by staining with antibodies against the congenic CD45.1 antigen. GFP+ male donor cells were detected by GFP auto-fluorescence. The proportions of positive cells were quantified by flow cytometry and FlowJo software analysis.
Data analysis and statistics
Raw data collected on the flow cytometer were analysed using FlowJo software by determining a lymphocyte gate by forward- and side-scatter then gating on CD8+ TCR-β+ (double-positive) cells. The proportion of CD8 T cells positive for BrdU or TUNEL staining was then determined by histogram analysis. In an effort to normalize our results across experiments we corrected for the proportion of cells falling in the initial lymphocyte gate, which gave us a conservative but more reproducible estimate of CD8 T-cell numbers (no. of CD8 T cells = total no. of cells × % lymphocyte gate × % CD8+ TCR-β+). The number of proliferating or apoptotic cells was then calculated by multiplying the number of CD8 T cells by the proportion of these cells that were BrdU+ or TUNEL+, respectively (no. of BrdU/TUNEL+ CD8 T cells = total no. of CD8 T cells × % BrdU/TUNEL+).
Data were analysed graphically and statistical analysis was performed using GraphPad Prism 4 (GraphPad Software, La Jolla, CA). For analysis of flow cytometric data, groups of mice from each gestational day were compared with the unmated controls using one-way analysis of variance (anova) with Dunnett's multiple comparison test. For analysis of splenic reverse transcription quantitative PCR (RT-QPCR) data, one-way anova with Dunnett's post-test was also used. For analysis of syngeneic and allogeneic pregnancies in 2C mice, groups of mice were compared with each other using one-way analysis of variance (anova) with Newman–Keuls post-test. In all cases, P-values of < 0·05 were considered significant.
Results
CD8 T-cell numbers are transiently increased in normal murine pregnancy
Maintenance of the size of the CD8 T-cell compartment contributes to the generation of robust CD8 T-cell responses needed to protect both the mother and fetus from infection. Previous studies have suggested that the T-cell compartment is altered during pregnancy; however, a detailed analysis of CD8 T-cell numbers has not been reported. To investigate the effects of pregnancy on global CD8 T-cell homeostasis, cell number, proliferation and death were analysed over the course of syngeneic murine gestation. This model minimized the effects of fetal-antigen-specific responses in the CD8 T-cell compartment. The number of CD8+ T cells undergoing proliferation during a 24-hr period was determined using in vivo BrdU incorporation. Apoptosis was analysed using the TUNEL assay, with a representative flow cytometric analysis shown (Fig. 1a).
Figure 1.
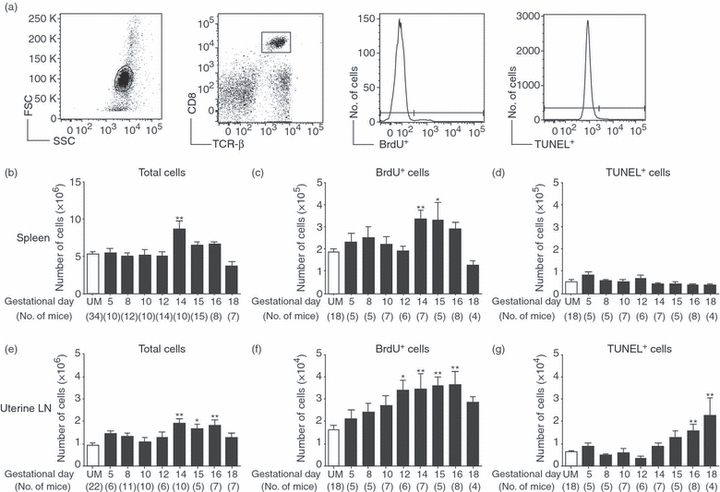
Increased proliferation of CD8 T cells during normal pregnancy. Pregnant and unmated (UM) C57BL/6 mice were injected with bromodeoxyuridine (BrdU) for the 24 hr before death, and parallel samples from each mouse were analysed for proliferation by BrdU incorporation, and apoptosis by the terminal deoxynucleotidyl-transferase (TdT)-mediated dUTP nick-end labelling (TUNEL) assay. (a) Representative analysis of flow cytometric data from a day 12 pregnant mouse. Left to right: forward versus side scatter plot of whole spleen, gating of CD8+ TCR-β+ cells, histogram analysis for the proportion of BrdU+ cells, and histogram analysis for the proportion of TUNEL+ cells. (b) Numbers of CD8 T cells in the spleen were calculated by multiplying the total number of cells counted by the proportion of CD8+ TCR-β+ cells obtained by flow cytometry. (c) Number of splenic CD8+ T cells that were BrdU+ on each gestational day. (d) Number of splenic CD8+ T cells positive for TUNEL staining throughout pregnancy. (e) Numbers of CD8 T cells in the uterine draining lymph nodes were calculated by multiplying the total number of cells counted by the proportion of CD8+ TCR-β+ cells obtained by flow cytometry. (f) Number of uterine draining lymph node CD8+ T cells that were BrdU+ on each gestational day. (g) Number of uterine lymph node CD8+ T cells positive for TUNEL staining throughout pregnancy. In each graph symbols and error bars depict mean and standard error of the mean (SEM). Numbers in parenthesis represent the total number of mice analysed at each time-point. Statistical significance was determined by one-way analysis of variance with Dunnett's post-test using UM controls for comparison *P < 0·05, **P < 0·01.
To analyse the systemic effect of pregnancy on CD8 T-cell homeostasis, the spleen was used as a model organ. The number of splenic CD8+ T cells was similar to unmated (UM) mice on each day of gestation analysed except day 14 when CD8 T-cell number was transiently elevated (Fig. 1b). The number of proliferating CD8 T cells in the spleen was comparable to unmated controls during early and mid-gestation (Fig. 1c). On gestational days 14 and 15, the number of BrdU+ CD8 T cells was significantly elevated, but on day 16 the number of proliferating CD8 T cells returned to the UM level. In the spleen, there was no significant difference in the number of apoptotic CD8 T cells between pregnant and unmated mice at any gestational day examined (Fig. 1d). These data suggested that systemically, CD8 T cells retain their capacity for proliferation during pregnancy. This elevated proliferation, combined with no detectable increase in apoptosis, may have contributed to the increased number of CD8 T cells on day 14 of pregnancy.
The presence of the fetus and placenta create a unique local environment, which could influence CD8 T-cell proliferation and apoptosis in the uterine draining (para-aortic and femoral) lymph nodes. During early and mid-pregnancy, the numbers of CD8 T cells in the uterine draining nodes were similar to unmated controls (Fig. 1e). There was a significant elevation in the number of draining lymph node CD8 T cells on gestational days 14, 15 and 16 followed by a return to the UM level by day 18. No increase in CD8 T-cell numbers was observed in mesenteric lymph nodes isolated from the same mice (data not a shown). The number of proliferating CD8 T cells in the uterine draining lymph nodes was similar to unmated controls in early pregnancy (Fig. 1f), was significantly elevated from day 12 to 16, and then declined to the UM level on day 18. A low number of CD8 T cells was undergoing apoptosis during early and mid-pregnancy and was similar to UM mice (Fig. 1g). However, on gestational days 16 and 18 the number of apoptotic CD8 T cells was significantly higher than in UM controls. Hence, in the uterine draining lymph nodes, enhanced proliferation during mid-gestation contributed to an increased number of CD8 T cells. This was followed by elevated apoptosis in late gestation and returned cell number to the unmated level. These data suggested that differences in the environments of the spleen and draining lymph nodes affect CD8 T-cell proliferation and apoptosis.
Pregnancy increases the expression of both pro-survival and apoptotic genes in CD8 T cells
The differences in the proliferation and death between CD8 T cells in the spleen and uterine draining lymph nodes during pregnancy could result from altered expression of the molecules that regulate these processes. CD8 T-cell proliferation is initiated by signalling through the T-cell antigen receptor, co-stimulatory molecules, and the common γ-chain containing cytokine receptors for IL-742 and IL-1543 whereas apoptosis is mediated by the death receptors such as Fas and pro-apoptotic members of the Bcl-2 protein family.25,44 Expression of IL-7Rα chain mRNA in CD8 T cells isolated from the spleens of pregnant mice on gestational days 5 and 8 was similar to that in UM mice. However, IL-7Rα expression was significantly elevated above the UM level on day 15 of gestation (Fig. 2a). CD8 T cells isolated from pooled uterine draining lymph nodes expressed similar levels of IL-7Rα chain mRNA compared with spleen cells, except on gestational day 8 when the relative expression was slightly higher. The relative expression of the IL-15Rα chain was comparable in unmated and pregnant mice on each gestational day examined and was similar in CD8 T cells sorted from both the spleen and uterine draining lymph nodes (Fig. 2b). This suggested that increased expression of the IL-7R on day 15 of pregnancy may render CD8 T cells more sensitive to IL-7 and contribute to their increased proliferation.
Figure 2.
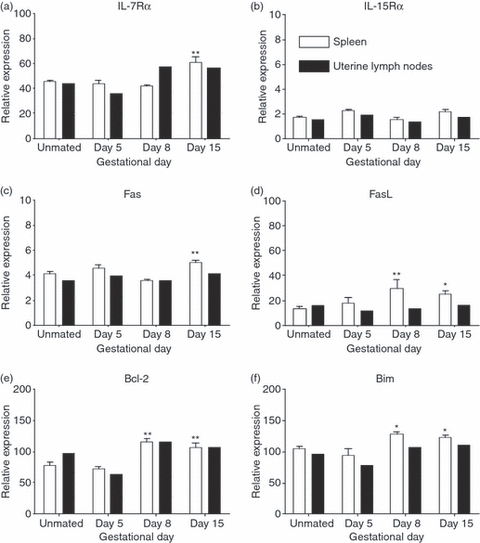
Increased expression of cytokine receptor and pro-apoptotic genes during pregnancy. CD8 T cells were sorted using fluoresence-activated cell sorting from single spleens or pooled uterine draining lymph nodes (two or three mice) from unmated (UM) controls and day 5, 8 and 15 pregnant mice. RNA was prepared by Triazol extraction and analysed by quantitative reverse transcription polymerase chain reaction. Relative expression was determined by standard curve analysis and normalized by dividing by the geometric mean of two housekeeping genes. Shown is the relative expression of (a) interleukin-7 receptor α-chain (IL-7Rα), (b) IL-15Rα, (c) Fas, (d) Fas ligand (FasL), (e) Bcl-2, and (f) Bim in CD8 T cells from the spleen and uterine draining lymph nodes during pregnancy. Open bars represent spleen cells and filled bars are pooled uterine lymph node cells. Statistical significance of spleen samples was determined by one-way analysis of variance with Dunnett's post-test using UM controls for comparison *P < 0·05, **P < 0·01.
Fas-mediated cell death is an important mechanism for limiting the size of the peripheral T-cell pool45 and may be involved in regulating fetal-antigen-specific CD8 T cells at the maternal–fetal interface.46 The relative expression of Fas in splenic CD8 T cells was similar to unmated controls on gestational days 5 and 8 but was significantly elevated on day 15 (Fig. 2c). Pooled uterine draining lymph node CD8 T cells expressed similar amounts of Fas as UM controls on each of the gestational days examined and in contrast to splenic cells, Fas expression was not elevated on gestational day 15. Compared with UM mice, the expression of Fas ligand (FasL) in splenic CD8 T cells was elevated on both day 8 and day 15 of pregnancy (Fig 2d). In contrast, the relative expression of FasL in pooled uterine draining lymph node CD8 T cells was similar to that in UM controls on the gestational days examined. This regulation of FasL expression may alter the level of Fas-mediated cell death and contribute to the regulation of CD8 T-cell numbers during pregnancy.
Apoptosis of T cells is also initiated through a mitochondria-dependent pathway that is regulated by pro- and anti-apoptotic members of the Bcl-2 family. To determine if pregnancy altered the levels of Bcl-2 family proteins in CD8 T cells, the relative expression of the pro-survival Bcl-247 and pro-apoptotic Bim48 mRNA were analysed by RT-QPCR. In the spleen, the expression of Bcl-2 was significantly higher than in UM controls on days 8 and 15 of gestation (Fig. 2e). Similar to Bcl-2, Bim expression was significantly elevated in splenic CD8 T cells on both day 8 and day 15 of pregnancy (Fig. 2f). On the gestational days examined, pooled CD8 T cells from the uterine draining lymph nodes expressed levels of Bcl-2 and Bim comparable to spleen cells and were similar in pregnant and unmated mice. Collectively, these data suggest that during pregnancy, CD8 T-cell homeostasis is a dynamic process where in the spleen increased levels of both pro- and anti-apoptotic signals contribute to enhanced levels of proliferation and death. However, in the uterine draining lymph nodes the mechanism(s) regulating these processes may involve other signalling molecules.
Antigen-specific CD8 T cells are functional during pregnancy
In addition to altering the rate of basal homeostatic proliferation and death in CD8 T cells, pregnancy may modify the response of antigen-specific CD8 T cells. To analyse fetal-antigen-specific CD8 T cells, we used TCR transgenic Matahari mice that bear a TCR specific for the male minor histocompatibility antigen HY peptide Uty. These mice were maintained on a Rag1−/− background to prevent the use of endogenous TCR-α or TCR-β chains and to allow examination of an enlarged population of fetal-antigen-specific CD8 T cells. As in the previous experiments, we determined CD8 T-cell proliferation by BrdU incorporation and apoptosis by TUNEL assay. Representative flow cytometric gating of CD8+ Vβ8.3+ T cells from a day 12 pregnant mouse is shown (Fig. 3a).
Figure 3.
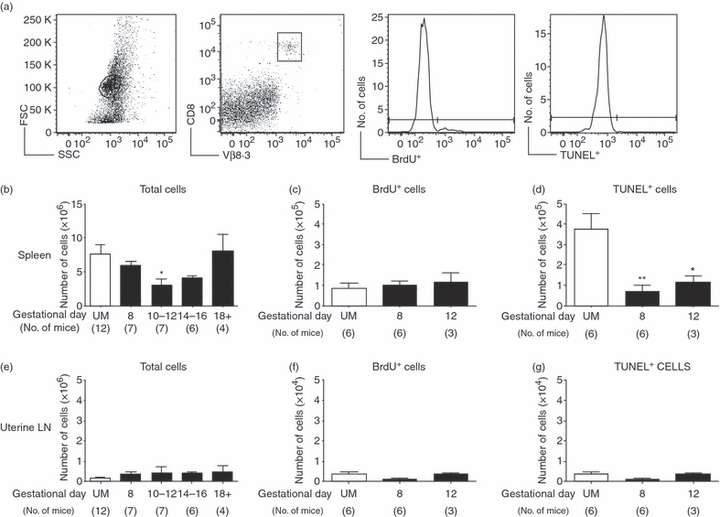
CD8 T-cell numbers in anti-HY T-cell receptor transgenic Matahari mice are maintained during pregnancy. Pregnant and unmated (UM) Matahari mice were injected with bromodeoxyuridine (BrdU) for the 24 hr before euthanasia, and parallel samples from each mouse were analysed for proliferation by BrdU incorporation, and apoptosis by the terminal deoxynucleotidyl-transferase (TdT)-mediated dUTP nick-end labelling (TUNEL) assay. In this system, transgenic CD8 T cells are identified by the Vβ8.3 T-cell receptor (TCR), and all analysis was performed on CD8+ Vβ8.·3+ cells. Cells positive for Vβ8.3 but negative for CD8 were rare and were excluded from our analysis. (a) Representative flow cytometry showing forward- versus side-scatter plot of whole spleen, gating of CD8+ Vβ8.3+ T cells, histogram analysis for the proportion of BrdU+ cells, and histogram analysis for the proportion of TUNEL+ cells. (b) Numbers of CD8 T cells in the spleen of Matahari mice were calculated by multiplying the total number of cells counted by the proportion of CD8+ Vβ8.3+ T cells obtained by flow cytometry. (c) Number of splenic CD8+ Vβ8.3+ T cells that were BrdU+ in UM and pregnant mice on days 8 and 12 of gestation. (d) Number of splenic CD8+ Vβ8.3+ T cells positive for TUNEL staining in UM mice and on days 8 and 12 of pregnancy. (e) Numbers of CD8+ Vβ8.3+ T cells in the uterine draining lymph nodes of Matahari mice were calculated by multiplying the total number of cells counted by the proportion of CD8+ Vβ8.3+ T cells obtained by flow cytometry. (f) Number of uterine lymph node CD8+ Vβ8.3+ T cells that were BrdU+ in UM and pregnant mice on days 8 and 12 of gestation. (g) Number of uterine lymph node CD8+ Vβ8.3+ T cells positive for TUNEL staining in UM mice and on days 8 and 12 of pregnancy. In each graph symbols and error bars depict mean and standard error of the mean (SEM). Numbers in parenthesis represent the total number of mice analysed from each time-point. Statistical significance was determined by one-way analysis of variance with Dunnett's post-test using UM controls for comparison *P < 0·05, **P < 0·01.
During early pregnancy there was no difference in the number of splenic CD8 T cells in pregnant Matahari mice compared to unmated controls (Fig. 3b). On days 10–12 the number of CD8+ Vβ8.3+ was significantly decreased, but by gestational day 14–16 the number of CD8 T cells was not different from UM controls. Near the end of pregnancy (day 18), CD8 T-cell number in the spleen had returned to a level similar to UM mice. In addition, after multiple pregnancies, splenic CD8 T cells persisted in Matahari mice and the number of cells was comparable to that in UM mice (data not shown). Hence, anti-HY-specific CD8 T cells manifested a markedly different pattern of splenic cell numbers compared to B6 mice.
To assess potential differences in proliferation and death between fetal-antigen-specific CD8 T cells and the CD8 population as a whole, these parameters were examined in Matahari mice during early pregnancy before changes were observed in the total CD8 population of B6 mice. In Matahari mice, the number of proliferating CD8 T cells in the spleen was similar in pregnant and UM mice (Fig. 3c). On both gestational days 8 and 12, the number of splenic CD8 T cells undergoing apoptosis was significantly decreased compared with UM controls (Fig. 3d). In the uterine draining lymph nodes, there was no significant change in the number of CD8 T cells between pregnant and unmated Matahari mice (Fig. 3e). On gestational days 8 and 12, both the number of proliferating (Fig. 3f) and apoptotic (Fig. 3g) CD8 T cells in the uterine draining nodes were similar to UM controls. These data suggested that there was no defect in the basal proliferation of anti-HY CD8 T cells during gestation and that pregnancy does not induce increased apoptosis of fetal-antigen-specific T cells.
To determine if the presence of a large population of anti-HY-specific T cells had an adverse effect on the development of male pups, Matahari breeding pairs were compared with non-TCR transgenic Rag1−/− mice for litter size, and the numbers of male and female pups weaned. In Matahari mice, the average number of live pups and the number of male and female pups at weaning were similar to Rag1−/− breeding pairs (Fig. 4a), and these results are similar to those seen in wild-type B6 mice.49 In 40 pregnant Matahari mice analysed at various gestational days, there was an average of seven viable fetuses per litter (range 1–11), and there were no resorbed fetuses in 88% (34/40) of these pregnancies (Fig. 4b). The lack of multiple resorptions in pregnant Matahari mice and the presence of normal numbers of male weanlings suggest that despite the retention of proliferative capacity, the presence of anti-HY-specific CD8 T cells did not lead to rejection of male fetuses.
Figure 4.
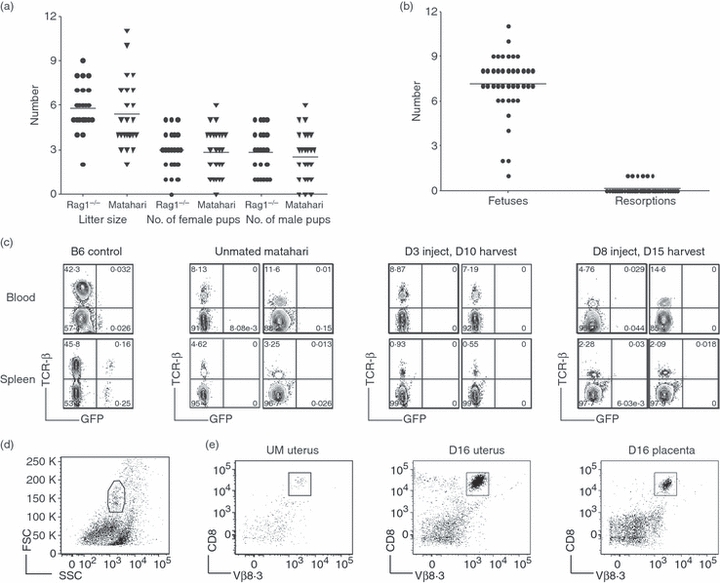
Anti-HY Matahari T-cell receptor (TCR) transgenic CD8 T cells are functional during pregnancy with no adverse effects on fetal survival. (a) Litter size and the number of male and female pups on weaning from breeding pairs of anti-HY Matahari TCR transgenic and non-transgenic Rag1−/− mice. (b) The number of fetuses and resorptions from 40 Matahari pregnancies at various gestational days. (c) CD8 T-cell activity was tested by injecting day 3 or 8 pregnant Matahari, unmated Matahari, and B6 control mice with male GFP+ spleen cells. Seven days later the presence of GFP+ cells was determined in the blood (top) and spleen (bottom) by flow cytometry. Non-transgenic B6 mice were used as controls (left most panels). Profiles for two mice from each time-point analysed are shown. Numbers refer to percentage of cells in the lymphocyte gate. (d) Representative flow cytometric gating from a Matahari day 16 uterus. (e) Isolation of CD8+ T cells from the uterus and placenta of Matahari mice. Fetal–placental units were removed and the uteri of four pregnant mice were pooled. Pooled uteri from four unmated mice were run as controls. For each pregnant mouse, two placentas from each uterine horn were pooled together (four in total). CD8 T cells were isolated from enzymatically digested uterus and placenta samples by magnetic antibody cell sorting positive selection and visualized by flow cytometry. CD8+ Vβ8.3+ T cells were purified from unmated uterus (left), day 16 uterus (middle), and day 16 placenta (right).
The lack of rejection of male fetuses in Matahari mice could result from the absence of cytotoxic activity by transgenic anti-HY-specific CD8 T cells. To determine if CD8 T cells in pregnant Matahari mice manifested effective responses against male cells, we designed an in vivo functional assay. Unmated and pregnant Matahari (CD45.2) mice at gestational days 3 and 8 received i.p. injections of donor splenocytes isolated from GFP-expressing male mice and congenic B6 CD45.1 female mice. As controls, non-pregnant female Rag1−/− and B6 mice received injections of the same donor splenocytes. After 7 days, the presence of male GFP+ cells in the blood and spleen was determined by flow cytometry. A population of GFP+ male cells was present in both the blood and spleen of the control B6 mice (Fig. 4c, left) and Rag1−/− mice (data not shown). In contrast, both UM Matahari mice (Fig. 4c, middle) and pregnant Matahari mice (injected on day 3 or 8 of gestation; Fig. 4c, right) contained no detectable GFP+ male cells in the spleen or blood. Both unmated and pregnant Matahari mice as well as Rag1−/− mice retained a population of CD45.1+ donor cells in the spleen and blood (data not shown). These experiments suggested that pregnancy does not impair the ability of anti-HY-specific CD8 T cells to specifically kill male target cells during pregnancy.
Alternatively, the protection of male fetuses from rejection in Matahari mice could be the result of selective exclusion of anti-HY CD8 T cells from the maternal–fetal interface. To test this possibility, T cells were isolated from pooled uteri and placentas of day 16 pregnant Matahari mice and control uteri from UM mice (Fig. 4d). A large population of CD8 T cells from pooled day 16 uteri was isolated (Fig. 4e middle panel), and there were proportionally more CD8 T cells isolated from the pregnant uteri than from the pooled uteri from UM controls (Fig. 4e). CD8 T cells could also be isolated from the pooled placentas of day 16 pregnant mice (Fig. 4e, right). These data demonstrated that anti-HY-specific T cells were not excluded from either the uterus or placenta during pregnancy.
CD8 T cells are present in similar numbers in syngeneic and allogeneic pregnancy
Allogeneic CD8 T-cell responses lead to the rejection of MHC-mismatched skin and heart grafts; however, the semi-allogeneic fetus, which expresses both maternal and paternal MHC molecules, is tolerated. Therefore, we sought to determine whether pregnancy induced a specific loss of anti-allogeneic CD8 T cells. TCR transgenic 2C Rag1−/− mice on a C57BL/6 background express MHC class I Db and contain CD8 T cells that are specific for the MHC class I molecule Ld expressed by BALB/c mice. Female 2C Rag1−/− mice were mated to syngeneic 2C Rag1−/− males or to allogeneic BALB/c Rag1−/− males and the pregnancy outcomes and number of CD8 T cells was determined. Representative gating of a day 12 pregnant mouse is shown (Fig. 5a). There were no differences in the number of viable or resorbed fetuses in pregnant 2C Rag1−/− females mated to syngeneic or allogeneic males (Fig. 5b). The number of splenic CD8+ Vβ8.1/8.2+ T cells was comparable in syngeneic and allogeneic 2C pregnancies on gestational days 12 and 16 and was similar to unmated controls (Fig. 5c). In the uterine draining lymph nodes, there was a similar number of CD8 T cells in both mating combinations on the gestational days examined and cell number was not significantly different between pregnant and UM mice (Fig. 5d). These data demonstrated that in pregnant 2C Rag1−/− mice, the fetal expression of the TCR-specific allo-antigen does not lead to deletion of CD8 T cells, and the presence of these T cells does not cause fetal demise.
Figure 5.
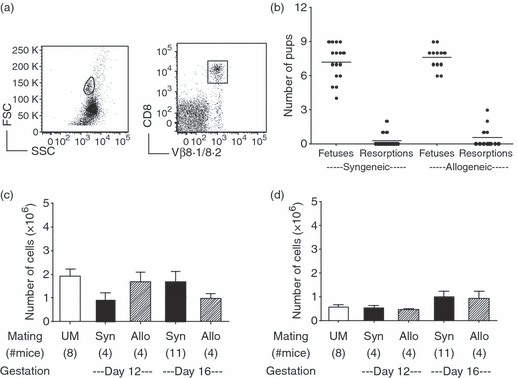
Allogeneic pregnancy does not alter the number of CD8 T cells in anti-Ld 2C transgenic mice. T-cell receptor (TCR) transgenic 2C Rag1−/− female mice were mated to either B6 Rag1−/− males for syngeneic pregnancies or to Ld expressing BALB/c Rag1−/− males for allogeneic pregnancies. Mice were analysed on gestational days 12 and 16 and compared with unmated controls. (a) Representative gating is shown from a day 12 pregnant mouse using CD8 and the clonotypic TCR Vβ8.1/8.2. (b) The number of fetuses and resorptions were counted in syngeneic and allogeneic 2C Rag1−/− pregnancies. (c) Numbers of CD8+ Vβ8.1/8.2+ T cells in the spleen. (d) Numbers of CD8+ Vβ8.1/8.2+ T cells in the uterine draining lymph nodes. Statistical significance was tested by one-way analysis of variance with Newman–Keuls post-test.
Discussion
The current observations demonstrate that during pregnancy in mice the immune system responds to changes in the maternal environment to maintain the size and function of the CD8 T-cell compartment. Splenic CD8 T-cell numbers were transiently increased at gestational day 14, which correlated with increased proliferation and expression of IL-7Rα. Although we were not able to detect a significant increase in apoptotic cells late in gestation, the relative expression of pro-apoptotic genes was up-regulated during mid- to late gestation. Collectively, these data suggest that elevated cell death might counteract enhanced proliferation to return CD8 T-cell numbers to pre-pregnancy levels. An altered balance of proliferation and death also produced a transient increase in the number of CD8 T cells in the uterine draining lymph nodes during pregnancy. This increased proliferation balanced by increased death suggests that for the immune system, pregnancy is a dynamic, rather than a static, state. These data suggest that, similar to other organ systems, the immune system undergoes reversible adaptations throughout normal gestation and argues against the global suppression of maternal T-cell proliferation during pregnancy.
Fetal-antigen-specific T cells manifested a strikingly different pattern of homeostasis compared with total CD8 T cells. In contrast to total CD8 T cells, splenic anti-HY CD8 T cells from Matahari mice declined in number at mid-gestation but exhibited a marked decrease in apoptotic cells. Moreover, anti-HY CD8 T cells maintained their proliferative capacity compared with UM mice through gestational day 12 as observed in total CD8 T cells. The reduced number of anti-HY CD8 T cells despite continued proliferation and reduced cell death suggests that these T cells traffic to other sites such as the maternal–fetal interface, but are not permanently deleted. These data are consistent with the increased proportion or number of fetal-antigen-specific CD8 T cells found in other TCR transgenic systems at multiple gestational days.4,5,36,50 In addition, studies using a different CD8 anti-HY model also found that the number of fetal-antigen-specific T cells was decreased during pregnancy, without a detectable down-regulation of the CD8 molecule.4 Collectively these data support the view that the proliferative capacity of fetal-antigen-specific CD8 T cells is not impaired during pregnancy.
Our TCR transgenic models demonstrated that during pregnancy fetal-antigen-specific CD8 T cells maintain functional capacity and can be activated without any harm to the fetus. Anti-HY CD8 T cells from Matahari mice cleared systemically injected male cells but did not reject their male fetuses despite the presence of substantial numbers of anti-HY CD8+ T cells at the maternal–fetal interface. These data contrast with reports using a different strain of anti-HY transgenic mice in which CD8 T cells were described as being deleted or rendered ‘anergic’ during pregnancy.35 These discrepancies may be attributed to the specificity of the TCR, as our mice are specific for the immunodominant HY peptide from the gene Uty and the other strain recognizes the subdominant epitope from the gene Smcy.51 Using a second TCR transgenic system, we found that maternal CD8 T-cell numbers were maintained despite fetal expression of the major histocompatibility molecules recognized by the maternal TCR transgenic CD8 T cells. Moreover, the presence of these potentially fetal-reactive T cells did not have an adverse effect on fetal survival, as allogeneic and syngeneic pregnancies had similar numbers of viable and resorbed fetuses. These data agree with and extend previous studies of allogene-specific TCR transgenic mice.6
Tissue-specific differences in immune regulation and responsiveness have been observed in the gut, liver, skin and lymphoid organs.37,52–57 However, the same suite of regulatory mechanisms is used in all tissues. Our current data suggest that CD8 T-cell homeostasis is differentially regulated in the spleen and uterine draining lymph nodes during pregnancy. T-cell homeostasis has been shown to be regulated by cell death,44,58 competition for limiting resources such as antigen-presenting cells34,59 and cytokines IL-760 and IL-15,61–63 and the presence of environmental antigen.64 In the reproductive immune system, we have previously observed tissue-specific differences in the dendritic cell populations of the uterus, uterine draining lymph nodes and spleen and that these differences were also present during pregnancy.65 Pregnancy may therefore modify the local environment but it does not fundamentally alter or suppress the tissue-specific mechanisms that maintain T-cell homeostasis.
We have observed that the proliferative capacity of CD8 T cells is maintained during pregnancy without resulting in fetal demise. During early to mid-gestation, the CD8 T-cell compartment contained equivalent numbers of cycling cells compared to the non-pregnant state. Even expansion of fetal-antigen-specific CD8 T cells was not impaired through gestational day 12. This suggests that early tolerance of the fetus does not require reduced proliferation of fetal-antigen-specific CD8 T cells. The fate of a T cell is determined by the avidity of its TCR in the context of additional signals provided by co-stimulatory molecules, cytokines and environment and may include proliferation, deletion or anergy. If the regulatory mechanisms used to maintain peripheral tolerance in the non-pregnant state also function during pregnancy to prevent fetal rejection, this obviates the need to globally suppress the maternal immune system and allows for a robust immune response in the event of an infection.
Acknowledgments
This work was supported by National Institutes of Health (NIH) T-32AI055402, NIH/National Institute of Allergy and Infectious Disease AI36333 and AI0797112, R01-HD043185, P20 RR021905, and support from the Department of Obstetrics, Gynecology and Reproductive Sciences, University of Vermont, College of Medicine. We thank Koela Ray and Chloe Adams for help with animal care, Matt Poynter for reading the manuscript, and our respective laboratories for helpful discussions.
References
- 1.Baines M, Pross H, Millar K. Effects of pregnancy on the maternal lymphoid system in mice. Obstet Gynecol. 1977;50:457–61. [PubMed] [Google Scholar]
- 2.Hamilton M, Hellström I. Altered immune responses in pregnant mice. Transplantation. 1977;23:423–30. doi: 10.1097/00007890-197705000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Parmiani G, Invernizzi G. Depression of cellular immune response during syngeneic pregnancy as measured by the graft-versus-host reaction. Transplantation. 1975;19:363–8. doi: 10.1097/00007890-197505000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jiang S, Vacchio M. Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft”. J Immunol. 1998;160:3086–90. [PubMed] [Google Scholar]
- 5.Tafuri A, Alferink J, Möller P, Hämmerling G, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 6.Rogers A, Boime I, Connolly J, Cook J, Russell J. Maternal–fetal tolerance is maintained despite transgene-driven trophoblast expression of MHC class I, and defects in Fas and its ligand. Eur J Immunol. 1998;28:3479–87. doi: 10.1002/(SICI)1521-4141(199811)28:11<3479::AID-IMMU3479>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Constantin C, Masopust D, Gourley T, Grayson J, Strickland O, Ahmed R, Bonney E. Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J Immunol. 2007;179:4383–9. doi: 10.4049/jimmunol.179.7.4383. [DOI] [PubMed] [Google Scholar]
- 8.Harrison M. Maternal immunocompetence. I. The graft-versus-host reactivity of lymphocytes from pregnant rats and the distribution pattern of 51Cr-labeled lymphocytes in pregnant mice. Scand J Immunol. 1976;5:549–58. doi: 10.1111/j.1365-3083.1976.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 9.Pavia C, Stites D. Trophoblast regulation of maternal–paternal lymphocyte interactions. Cell Immunol. 1981;58:202–8. doi: 10.1016/0008-8749(81)90161-1. [DOI] [PubMed] [Google Scholar]
- 10.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 11.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 12.Bonney EA. Preeclampsia: a view through the danger model. J Reprod Immunol. 2007;76:68–74. doi: 10.1016/j.jri.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256(4 Pt 2):H1060–5. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 14.Schobel HP. Pregnancy-induced alterations in renal function. Kidney Blood Press Res. 1998;21:274–6. doi: 10.1159/000025876. [DOI] [PubMed] [Google Scholar]
- 15.Nolten WE, Lindheimer MD, Oparil S, Ehrlich EN. Desoxycorticosterone in normal pregnancy. I. Sequential studies of the secretory patterns of desoxycorticosterone, aldosterone, and cortisol. Am J Obstet Gynecol. 1978;132:414–20. [PubMed] [Google Scholar]
- 16.Zoller A, Schnell F, Kersh G. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology. 2007;121:207–15. doi: 10.1111/j.1365-2567.2006.02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibbetts T, DeMayo F, Rich S, Conneely O, O’Malley B. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci U S A. 1999;96:12021–6. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maymon R, Zimerman AL, Strauss S, Gayer G. Maternal spleen size throughout normal pregnancy. Semin Ultrasound CT MR. 2007;28:64–6. doi: 10.1053/j.sult.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Maroni E, de Sousa M. The lymphoid organs during pregnancy in the mouse. A comparison between a syngeneic and an allogeneic mating. Clin Exp Immunol. 1973;13:107–24. [PMC free article] [PubMed] [Google Scholar]
- 20.Mattsson R, Nilsson B, Lindahl-Kiessling K. An investigation of splenic enlargement in pregnant mice. Dev Comp Immunol. 1979;3:683–95. doi: 10.1016/s0145-305x(79)80062-2. [DOI] [PubMed] [Google Scholar]
- 21.Ansell J, McDougall C, Speedy G, Inchley C. Changes in lymphocyte accumulation and proliferation in the lymph nodes draining the pregnant uterus. Clin Exp Immunol. 1978;31:397–407. [PMC free article] [PubMed] [Google Scholar]
- 22.Hetherington C, Humber D. The effect of pregnancy on lymph node weight in the mouse. J Immunogenet. 1977;4:271–6. doi: 10.1111/j.1744-313x.1977.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 23.Norton MT, Fortner KA, Bizargity P, Bonney EA. Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol Reprod. 2009;81:457–64. doi: 10.1095/biolreprod.109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge Q, Hu H, Eisen HN, Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. Proc Natl Acad Sci U S A. 2002;99:2989–94. doi: 10.1073/pnas.052714099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamood AS, Bargatze D, Xiao Z, et al. Fas-mediated apoptosis regulates the composition of peripheral alphabeta T cell repertoire by constitutively purging out double negative T cells. PLoS ONE. 2008;3:e3465. doi: 10.1371/journal.pone.0003465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 27.Seddon B, Zamoyska R. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J Immunol. 2002;169:3752–9. doi: 10.4049/jimmunol.169.7.3752. [DOI] [PubMed] [Google Scholar]
- 28.Wu LX, La Rose J, Chen L, Neale C, Mak T, Okkenhaug K, Wange R, Rottapel R. CD28 regulates the translation of Bcl-xL via the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway. J Immunol. 2005;174:180–94. doi: 10.4049/jimmunol.174.1.180. [DOI] [PubMed] [Google Scholar]
- 29.Sharpe A, Freeman G. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 30.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mor G, Sapi E, Abrahams V, Rutherford T, Song J, Hao X, Muzaffar S, Kohen F. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J Immunol. 2003;170:114–22. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- 33.Surh CD, Tan J, Kieper WC, Ernst B. Factors regulating naive T cell homeostasis. Adv Exp Med Biol. 2002;512:73–80. doi: 10.1007/978-1-4615-0757-4_10. [DOI] [PubMed] [Google Scholar]
- 34.Dummer W, Ernst B, LeRoy E, Lee D, Surh C. Autologous regulation of naive T cell homeostasis within the T cell compartment. J Immunol. 2001;166:2460–8. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 35.Vacchio M, Jiang S. The fetus and the maternal immune system: pregnancy as a model to study peripheral T-cell tolerance. Crit Rev Immunol. 1999;19:461–80. [PubMed] [Google Scholar]
- 36.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Celli S, Matzinger P. Liver transplants induce deletion of liver-specific T cells. Transplant Proc. 2001;33:102–3. doi: 10.1016/s0041-1345(00)01926-6. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/sdendritic cell interactions in vivo. Cell Immunol. 2001;214:110–22. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 39.Norton M, Fortner K, Bizargity P, Bonney E. Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol Reprod. 2009;81:457–64. doi: 10.1095/biolreprod.109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilburgs T, Roelen DL, van der Mast BJ, et al. Differential distribution of CD4+ CD25bright and CD8+ CD28− T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl. A):S47–53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Park J, Yu Q, Erman B, Appelbaum J, Montoya-Durango D, Grimes H, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Sandau M, Winstead C, Jameson S. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–5. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- 44.Bouillet P, Metcalf D, Huang D, Tarlinton D, Kay T, Köntgen F, Adams J, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 45.Fortner KA, Budd RC. The death receptor Fas (CD95/APO-1) mediates the deletion of T lymphocytes undergoing homeostatic proliferation. J Immunol. 2005;175:4374–82. doi: 10.4049/jimmunol.175.7.4374. [DOI] [PubMed] [Google Scholar]
- 46.Vacchio MS, Hodes RJ. Fetal expression of Fas ligand is necessary and sufficient for induction of CD8 T cell tolerance to the fetal antigen H-Y during pregnancy. J Immunol. 2005;174:4657–61. doi: 10.4049/jimmunol.174.8.4657. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–95. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonney EA. Maternal tolerance is not critically dependent on interleukin-4. Immunology. 2001;103:382–9. doi: 10.1046/j.1365-2567.2001.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou M, Mellor AL. Expanded cohorts of maternal CD8+ T-cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol. 1998;40:47–62. doi: 10.1016/s0165-0378(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 51.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 52.Alpan O, Rudomen G, Matzinger P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J Immunol. 2001;166:4843–52. doi: 10.4049/jimmunol.166.8.4843. [DOI] [PubMed] [Google Scholar]
- 53.Laouar A, Manocha M, Wan M, Yagita H, van Lier RAW, Manjunath N. Cutting edge: distinct NK receptor profiles are imprinted on CD8 T cells in the mucosa and periphery during the same antigen challenge: role of tissue-specific factors. J Immunol. 2007;178:652–6. doi: 10.4049/jimmunol.178.2.652. [DOI] [PubMed] [Google Scholar]
- 54.Garner OB, Yamaguchi Y, Esko JD, Videm V. Small changes in lymphocyte development and activation in mice through tissue-specific alteration of heparan sulphate. Immunology. 2008;125:420–9. doi: 10.1111/j.1365-2567.2008.02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 56.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–42. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 57.Bonomo A, Matzinger P. Thymus epithelium induces tissue-specific tolerance. J Exp Med. 1993;177:1153–64. doi: 10.1084/jem.177.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosque A, Aguilo JI, del Rey M, Paz-Artal E, Allende LM, Naval J, Anel A. Cell cycle regulation by FasL and Apo2L/TRAIL in human T-cell blasts. Implications for autoimmune lymphoproliferative syndromes. J Leukoc Biol. 2008;84:488–98. doi: 10.1189/jlb.0108043. [DOI] [PubMed] [Google Scholar]
- 59.Troy AE, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J Immunol. 2003;170:672–6. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- 60.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 61.Lodolce J, Boone D, Chai S, Swain R, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 62.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–87. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells [see comment] Nat Immun. 2009;10:149–57. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci U S A. 1999;96:13306–11. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bizargity P, Bonney EA. Dendritic cells: a family portrait at mid-gestation. Immunology. 2009;126:565–78. doi: 10.1111/j.1365-2567.2008.02918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


