Abstract
Toll-like receptor (TLR) signalling shapes dendritic cell (DC) responses by inducing co-stimulatory molecule up-regulation and cytokine secretion while TLR regulatory proteins inhibit this process. We aimed to determine if gene expression of TLRs and TLR regulatory proteins underpins the functionally different lipopolysaccharide (LPS) responses of DCs from murine Peyer's patches (PP) and spleen and of murine bacteria-conditioned bone-marrow-derived cells. Isolated spleen and PP DCs were analysed for basal expression of TLRs by flow cytometry and real time quantitative reverse transcription polymerase chain reaction (qRT-PCR). The DCs were stimulated with LPS to determine cytokine secretion by enzyme-linked immunosorbent assay and expression of TLR regulatory proteins by qRT-PCR. In vitro results were confirmed following in vivo intraperitoneal LPS injection. In addition, changes in gene expression of TLR regulatory proteins were assessed in bacteria-conditioned bone-marrow-derived cells. Results indicated that surface expression of TLR2 and TLR4 on PP DCs was decreased compared with spleen DCs. The PP DCs secreted a limited profile of cytokines compared with spleen DCs following LPS stimulation. In vivo LPS exposure up-regulated sigirr, tollip and tmed1 messenger RNA in PP DCs, but not spleen DCs. Similar gene expression changes were observed in bacteria-conditioned bone-marrow-derived cells. Therefore, functionally different LPS responses in PP and spleen DCs reflect their characteristic expression of TLRs and TLR regulatory proteins. Differential regulation of TLR signalling was also evident in bacteria-conditioned bone-marrow-derived cells indicating that bacterial signalling may be a mechanism for inducing altered gene regulation in PP DCs.
Keywords: dendritic cell, Peyer's patch, toll-like receptor
Introduction
Dendritic cell (DC) responses vary depending on the invading organism and the tissue from which they are derived. Systemic exposure to antigen induces robust immune responses and protective immunity whereas oral administration induces tolerance either through anergy1 or active suppression.2–5 How systemic and gut-associated DCs are able to mediate such dichotomous responses remains elusive.
Recognition of commensal and pathogenic bacteria occurs through germline encoded pattern recognition receptors. Toll-like receptors (TLRs) are a family of pattern recognition receptors that recognize a diversity of conserved molecules. The expression pattern of TLRs shapes the ability of DCs to respond to stimulation and may be important in the regulation of responses in gut-associated areas compared with systemic areas.
As TLR signalling initiates a cascade of inflammatory cytokine and chemokine secretion, negative regulators of TLR signalling are crucial to prevent excessive inflammation and limit collateral tissue damage following TLR activation. Negative regulators exist at each level of TLR signalling. At the plasma membrane, regulators exist to prevent the recruitment of adaptor molecules. Cytoplasmically, regulators inhibit signal transduction at each stage of signal amplification and also function in feedback loops preventing further activation to inflammatory cytokines.6,7 The pattern of negative TLR regulatory protein expression may play an important role in limiting the responses of DCs to TLR ligands, and may represent a level of control in gut-derived DCs that has not yet been investigated.
Previously, we demonstrated that the differentiation of bone marrow precursors to DCs is modified in the presence of commensal bacteria. Bacteria-conditioned bone-marrow-derived cells secreted high interleukin-10 (IL-10) and induced FoxP3+ T cells.8 These characteristics are similar to those associated with gut-derived DCs.9,10
The purpose of the present study was to analyse DCs from peyer's patches (PP) and spleen for their expression of TLRs and TLR regulatory proteins both in vitro and in vivo to understand the molecular mechanisms maintaining the hypo-responsiveness of PP DCs. Differences were observed between freshly isolated PP and spleen DCs in TLR expression. Cytokine secretion and TLR regulatory gene expression also differed between spleen and PP DCs in response to LPS stimulation. In addition, bacteria-conditioned bone marrow-derived cells displayed similar TLR regulatory protein gene expression to PP DCs. Taken together, these data indicate that increases in gene expression of TLR regulatory proteins underpins the regulation of PP DCs to TLR activation, and that this may be the result of exposure to TLR signalling in progenitor cells.
Materials and methods
Mice
Six- to 16-week-old BALB/c mice were purchased from Harlan (Oxon, UK). Animals were housed in a conventional animal facility. All procedures in animals were approved by the University College Cork, National University of Ireland ethics review board.
Spleen cell preparation
Spleens were harvested into RPMI-1640 (Invitrogen, Dun Laoghaire, Ireland) and mechanically disrupted to form a single-cell suspension. Cells were cultured in complete media containing RPMI-1640+, l-glutamine, 10% fetal calf serum, 1% sodium pyruvate, 1% penicillin/streptomycin, 1% vitamins, 1% non-essential amino acids and 0·01% 2-mercaptoethanol. All reagents were from Invitrogen, except fetal calf serum (Sigma, Irvine, UK).
Peyer's patch preparation
The PP were excised from the small intestine and incubated in 2·4 U/ml Dispase II (Roche Diagnostics GmbH, Penzbeg, Germany) in phosphate-buffered saline (PBS; pH 7·2; Invitrogen) for 20 min at 37°. EDTA (0·01 m; Sigma-Aldrich, Steinheim, Germany) was added and tissue was disrupted by continuous pipetting for 5 min. Excess complete medium was added and the cell suspension was filtered over a 70-μm filter. Cells were washed and re-suspended in complete medium.
Flow cytometry
Using a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer, the surface phenotype of cells was determined using four-colour staining with the following antibodies: fluorescein isothiocyanate-conjugated (-FITC) or allophycocyanin-conjugated CD11c (HL3), phycoerythrin-conjugated (-PE) CD80 (16-10A1) (BD-Pharmingen, Oxford, UK); TLR2-PE (6C2), TLR4/MD2-PE-Cy7 (MTS510) (eBioscience, San Diego, CA) and major histocompatibility class II (MHCII)-FITC (M5/114) (Miltenyi Biotec, Bergisch Gladbach, Germany). Available antibodies for SIGIRR (single immunoglobulin and toll-interleukin 1 receptor; polyclonal) (abcam, Cambridge, UK) were insufficient for flow cytometric staining. Antibodies for flow determination of SOCS1 (suppressor of cytokine signalling 1) and TOLLIP (toll interacting protein) were not commercially available. Appropriate isotype-matched controls were used for each antibody. Cells were stained on ice in the dark for 30 min in PBS with 0·5% bovine serum albumin (Sigma-Aldrich) and washed once before analysis.
Dendritic cell isolation
For the autoMACS separation, spleen and PP were prepared to a single-cell suspension as above. CD11c+ cells were isolated using CD11c+ microbeads as per the manufacturer's instructions (Miltenyi Biotec). Recovered cells were re-suspended in complete medium and stimulated as described.
For the flow sorting, CD11c+ magnetic antibody cell sorter (MACS) separated cells were further purified following staining with CD11c and MHCII antibodies using a BD FacsVantage SE (BD, San Jose, CA).
Cell stimulations
Due to the inherent variability of LPS we performed a dose–response investigation of LPS (10 μg/ml, 1 μg/ml, 100 ng/ml, 10 ng/ml) on autoMACS-separated spleen CD11c+ cells to determine a concentration that induced high levels of cytokine secretion with no loss of cell viability. From these experiments we determined the optimal LPS concentration for stimulation to be 1 μg/ml. The autoMACS-separated PP and spleen CD11c+ cells were plated at 1 × 106 cells/ml in complete medium and stimulated for 5 hr or 18 hr with 1 μg/ml LPS (Escherichia coli 0111.B4; Sigma, Steinheim, Germany), 5 ng/ml phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml ionomycin (Io) (Sigma) or left unstimulated.
Electrochemical enzyme-linked immunosorbent assay
Supernatants from stimulations were collected and analysed for secreted proteins using the mouse pro-inflammatory 7 plex tissue culture kit measuring IL-6, IL-1β, IL-10, keratinocyte chemoattractant (KC), interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), and IL-12p70 by MesoScale Discovery (Gaithersburg, MD), as per the manufacturers instructions.
In vivo LPS administration
Groups of eight, 6-week-old BALB/c mice were intraperitoneally injected with either 4 mg/kg LPS (E. coli 0111.B4; Sigma) or 400 μl PBS (pH 7·2; Invitrogen). Animals were culled 5 hr later and organs were harvested. Cells were pooled from two mice for DC isolation.
Direct cell real time quantitative reverse transcription–polymerase chain reaction
Cells were harvested into Sidestep Lysis and Stabilization Buffer (Agilent Technologies, Amsterdam, the Netherlands) and stored at −70°. Complementary DNA (cDNA) transcripts were generated using 1 μl of lysate and random nonamers with the AffinityScript QPCR cDNA Synthesis kit (Stratagene). Synthesis conditions used were 25° for 5 min, 42° for 45 min, 95° for 5 min and 4° for 5 min. Polymerase chain reaction (PCR) primers and probes were designed using the Universal ProbeLibrary Assay Design Centre (https://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp). β-Actin was used as housekeeping gene to correct for variability in the initial amount of starting total RNA. Amplification reactions contained 1 μl cDNA and reaction components as per the manufacturer's instructions (LightCycler 480 Probe Master Roche Diagnostics GmbH, Mannheim, Germany). All reactions were performed in duplicate using 384-well plates on the LightCycler 480 System using the thermal cycling conditions as per the manufacturer's instructions (Roche Diagnostics GmbH). The 2−ΔΔCt method11 was used to calculate relative changes in gene expression determined from real-time quantitative reverse transcription PCR experiments. The mathematical average of all samples was used as a calibrator control for analysis of differential gene expression (Table 1).
Table 1.
Gene-specific primers and probe combinations assayed by quantitative real-time polymerase chain reaction
| Gene ID | NCBI ID | Primer sequence 5′-3′ | Probe | |
|---|---|---|---|---|
| β-actin | NM_007393·3 | L | ctaaggccaaccgtgaaaag | 64 |
| R | accagaggcatacagggaca | |||
| socs1 | NM_009896·2 | L | gtggttgtggagggtgagat | 20 |
| R | cctgagaggtgggatgagg | |||
| irak3 | NM_028679·3 | L | ggacattcgaaaccaagcat | 2 |
| R | ccagtgcttctttagttgcattt | |||
| sigirr | NM_023059·3 | L | ggtggcagtcgtgactcag | 72 |
| R | acctcaggagagagcagttagc | |||
| tmed1 | NM_010744·3 | L | gctagtcttgagaccgagtacca | 26 |
| R | gctctccaaggtgaagtcca | |||
| irak1 | NM_008363·2 | L | ggatcagctccaccttcaga | 32 |
| R | cccagaagaatgtccagtcg | |||
| tollip | NM_023764·3 | L | gcagggtgttggctatgtg | 40 |
| R | cattacagcggggctgag | |||
| tlr1 | NM_030682·1 | L | tcttgctggcacccattc | 58 |
| R | catgagagttttgagcttgtgg | |||
| tlr2 | NM_011905·3 | L | ggggcttcacttctctgctt | 50 |
| R | agcatcctctgagatttgacg | |||
| tlr3 | NM_126166·4 | L | gatacagggattgcacccata | 26 |
| R | tcccccaaaggagtacattaga | |||
| tlr4 | NM_021297·2 | L | ggactctgatcatggcactg | 2 |
| R | ctgatccatgcattggtaggt | |||
| tlr5 | NM_016928·2 | L | tcatggatggatgctgagtt | 18 |
| R | tggccatgaagatcacacc | |||
| tlr6 | NM_011604·3 | L | ggtaccgtcagtgctggaa | 110 |
| R | gggttttctgtcttggctca | |||
| tlr7 | NM_133211·3 | L | gatcctggcctatctctgactc | 25 |
| R | cgtgtccacatcgaaaacac | |||
| tlr8 | NM_133212·2 | L | caaacgttttaccttcctttgtc | 56 |
| R | atggaagatggcactggttc | |||
| tlr9 | NM_031178·2 | L | gaatcctccatctcccaacat | 79 |
| R | ccagagtctcagccagcact |
Bacteria preparation
Bifidobacterium breve UCC2003 were grown anaerobically for 48 hr in de Man, Rogosa, Sharpe broth (MRS; Oxoid, Basingstoke, UK) supplemented with 5% cystein (l-cysteine HCL; Sigma) to a concentration of 1 × 109 colony-forming units (CFU)/ml. Bacteria were washed twice and re-suspended in PBS. The B. breve UCC2003 is an isolate from the University College Cork and Alimentary Health Ltd. culture collections.
Bone marrow preparation
Bone marrow was prepared as previously described12 with the following modifications. Cells were cultured in complete media as above with 200 U/ml (40 ng/ml) recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF; Peprotech, London, UK). Bone marrow cells were plated at 4 × 105 cells/ml in 100-mm Petri dishes alone or with 4 × 105 CFU/ml B. breve UCC2003 (1 : 1 ratio with bone marrow cells). Media were replenished (with or without 4 × 105 CFU/ml B. breve) on days 3 and 5. Cells were cultured for 7 days at 37° 5% CO2. At harvest, cells were routinely ≥ 90% viable as determined by trypan blue exclusion.
Statistical analysis
Means with SEM are represented in each graph. Statistical analysis was performed using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). P-values were calculated by one-way analysis of variance with Tukey post-hoc test or unpaired Student's t-test where appropriate. P-values considered as significant are indicated as ***≤0·001, **≤0·01, *≤0·05.
Results
PP DCs do not secrete TNF-α, IL-12p70 or IL-1β in response to LPS
The cytokine response from DCs isolated from spleen and PP following 18 hr of stimulation with LPS or PMA/Io revealed that PP DCs secrete a limited range of cytokines compared with spleen DCs (Fig. 1). KC, IL-6 and IL-10 were secreted in response to LPS by both spleen and PP DCs, whereas IL-12p70, TNF-α and IL-1β were secreted by spleen DCs only. In addition, PMA induced IL-12p70 and IL-10 secretion from spleen DCs, which was not observed in PP DCs.
Figure 1.
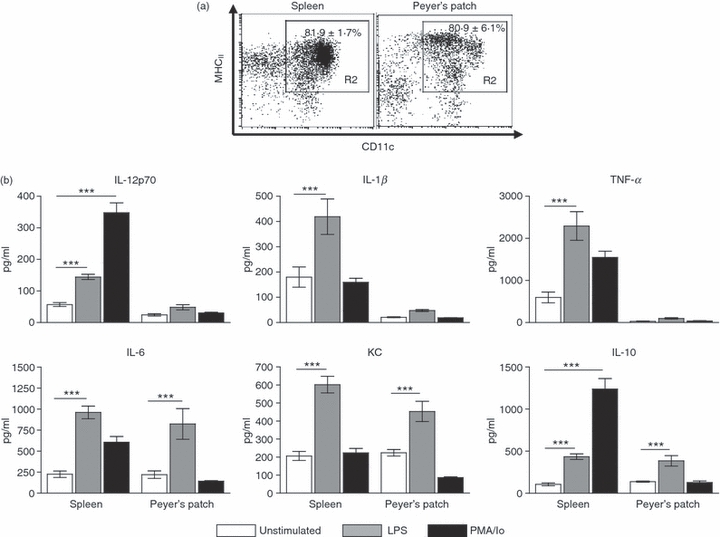
Peyer's patch dendritic cells (PP DCs) secrete a limited profile of cytokines compared with spleen DCs. (a) Spleen and PP DCs were isolated by autoMACS selection of CD11c+ cells. The purity of cells is demonstrated. Values in the upper right indicate mean ± SEM percentage of cells falling in the gate, n = 5. (b) Following CD11c+ isolation cells were cultured for 18 hr with lipopolysaccharide (LPS; 1 μg/ml) or phorbol 12-myristate 13-acetate/ionomycin (PMA/Io; 5 ng/ml PMA, 1 μg/ml Io) or left unstimulated. Supernatants were collected and interleukin-12 (IL-12) p70, IL-1β, tumour necrosis factor-α (TNF-α), IL-6, IL-10 and keratinocyte chemoattractant (KC) levels were determined by electrochemical enzyme-linked immunosorbent assay. Bars represent mean ± SEM. Significance determined by one-way analysis of variance, Tukey post-hoc test, ***P < 0·001, n = 3 to n = 9.
An increased percentage of spleen DCs express TLR2 and TLR4 compared with PP DCs
To determine a possible mechanism for the observed altered-responsiveness of PP DCs to stimulation we sought to determine the expression of TLR receptors on the surface of DCs (Fig. 2a). CD11c+ autoMACS-separated spleen and PP cells were analysed for the expression of TLR2 and TLR4–MD2 on the CD11c+ MHCII+ population as in Fig. 1(a). Analysis of the DC population in PP revealed a decreased percentage of cells that expressed TLR2 compared with spleen DCs and a decreased percentage of cells that express the TLR4–MD2 complex. The mean fluorescent intensity (MFI) of TLR2 expression in the positive population was also significantly decreased in PP DCs compared with the spleen DCs. However, in the TLR4–MD2-positive population there was no difference in the MFI between spleen and PP DCs. We extended our observation to include the entire family of TLRs. AutoMACS-separated, flow-sorted DCs from the spleen and PP were analysed for their messenger RNA (mRNA) expression of tlr1–9 relative to each other by real-time quantitative PCR (Fig. 2b). The mRNA gene expression of tlr1, tlr2, tlr4 and tlr6 was decreased in PP compared with spleen DCs, but this change was not statistically significant. There was no difference in the expression of the other investigated TLRs.
Figure 2.
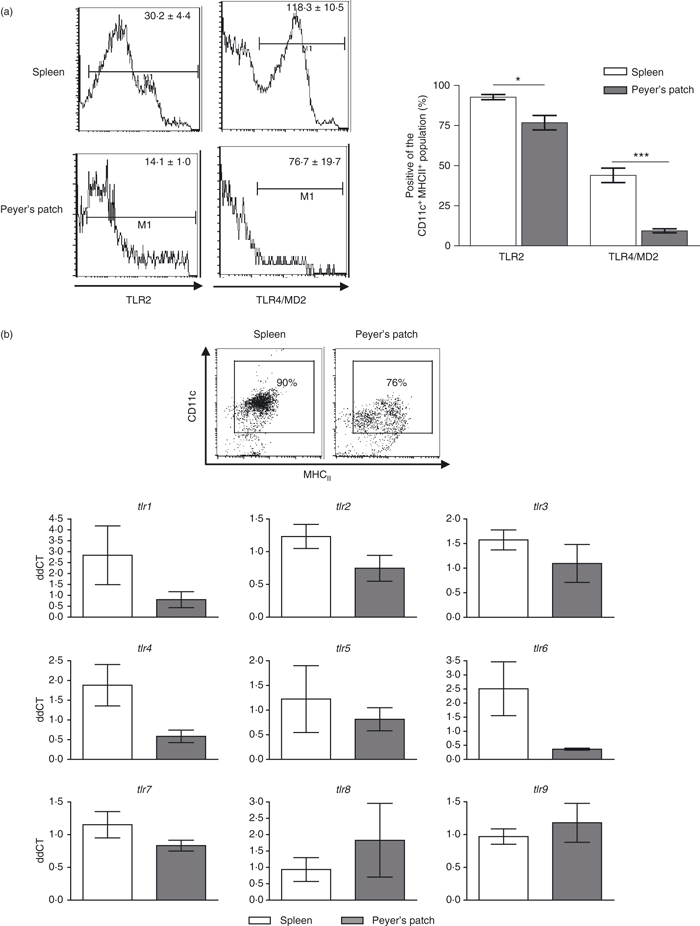
A greater percentage of spleen dendritic cells (DCs) express surface Toll-like receptor 2 (TLR2) and TLR4 than Peyer's patch (PP) DCs. (a) AutoMACS-separated CD11c+ cells from spleen and PP were gated on the expression of CD11c and MHCII as in Fig. 1(a). The expression of surface TLR2 and TLR4-MD2 was then determined on the double-positive population. Values in the upper right of the histograms indicate the mean fluorescence intensity of cells in the indicated gate, n = 3. Bars represent mean ± SEM of percentage of cells falling in the indicated gate of the CD11c+ MHCII+ population, n = 3. (b) autoMACS-separated, flow cytometrically sorted DCs were analysed for gene expression of tlr 1–9. Expression was determined as fold induction compared with β-actin housekeeper. There was no statistical difference between spleen and PP in the messenger RNA levels of any of the genes investigated. Bars represent mean ± SEM, n = 4 or n = 5. Significance determined by unpaired Student's t-test, ***P < 0·001, *P < 0·05.
TLR regulatory proteins have increased gene expression in PP DCs in vitro
As PP DCs have demonstrated a characteristic cytokine and chemokine profile in response to LPS stimulation compared with spleen DCs, we investigated the mRNA expression of several TLR regulatory proteins to determine if they were induced in PP DCs and could be responsible for regulating the modified response of PP DCs. We investigated a panel of genes (Fig. 3 and Table S1) and found three negative regulatory TLR signalling mRNAs –sigirr, tollip and tmed1, which were up-regulated in unstimulated and LPS-stimulated PP DCs compared with their counterparts from the spleen.
Figure 3.
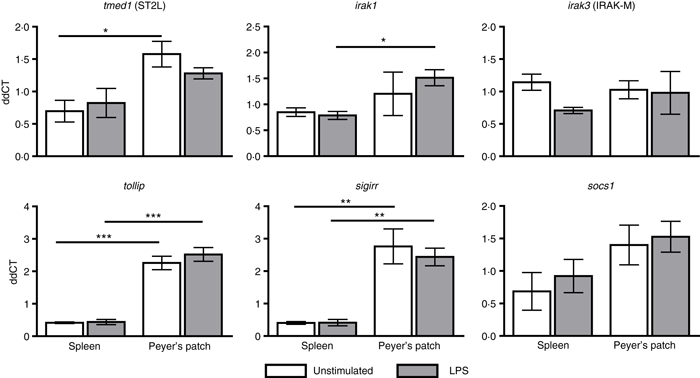
Peyer's patch dendritic cells (PP DCs) have distinct gene expression profile of Toll-like receptor (TLR) regulatory proteins compared with spleen DCs. AutoMACS-separated CD11c+ spleen and PP DCs were cultured in vitro for 5 hr with or without lipopolysaccharide (LPS; 1 μg/ml). Gene expression of tmed1, socs1, irak1, tollip, sigirr and irak3 was determined as a fold induction compared with the β-actin housekeeper. Bars represent mean ± SEM. Significance determined by one-way analysis of variance, Tukey post-hoc test ***P < 0·001, **P < 0·01, *P < 0·05, n = 3.
Attenuated co-stimulatory molecule responses in PP DCs following in vivo LPS exposure
We next investigated the response of PP and spleen DCs following in vivo LPS injection. The LPS was administered by intraperitoneal injection. Five hours later, DCs were isolated from spleen and PP to determine surface expression of MHCII and CD80 compared with PBS-injected control mice (Fig. 4). Both spleen and PP DCs exhibited a modest increase in MHCII and a significant increase in CD80 expression compared with the PBS-injected controls. Spleen DCs responded to LPS injection by up-regulating the expression of CD80 to a significantly greater degree than PP DCs.
Figure 4.
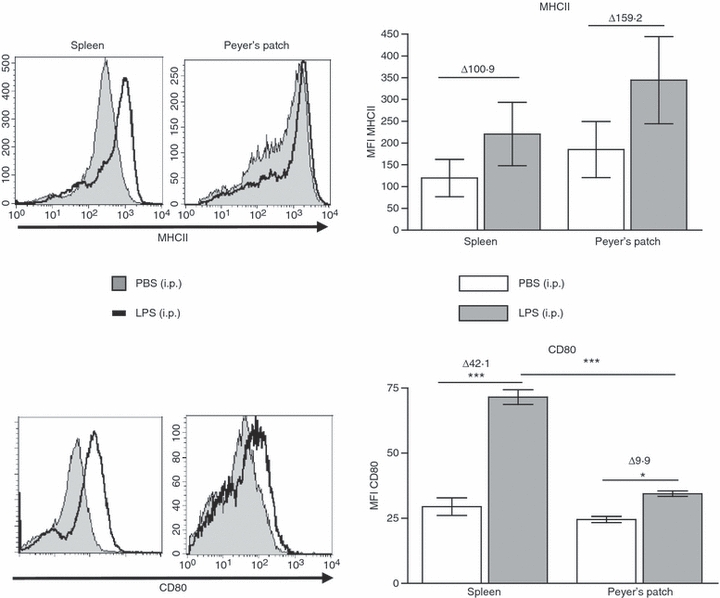
In vivo administration of lipopolysaccharide (LPS) up-regulates co-stimulatory molecules to a greater extent in spleen dendritic cells (DCs) than in Peyer's patch (PP) DCs. LPS (4 mg/kg) or phosphate-buffered saline (PBS) was administered by intraperitoneal injection. Five hours later spleen and PP were harvested and DCs were isolated by CD11c+ autoMACS separation. The DCs were stained for activation markers MHCII and CD80 and analysed by flow cytometry. Representative histograms are displayed. Bars represent mean ± SEM. Significance determined by one-way analysis of variance, Tukey post-hoc, ***P < 0·001, *P < 0·05, n = 4.
TLR regulatory protein gene expression is induced by LPS in PP DCs in vivo
Gene expression of a panel of TLR regulatory proteins was analysed in DCs isolated from PP and spleen following LPS injection and compared with PBS-injected controls (Fig. 5 and Table S1). The expression of tmed1 (ST2L), tollip and sigirr was increased following LPS injection in PP DCs, but not in spleen DCs. Expression of socs1 was also increased in PP in response to LPS, although not significantly. In vivo spleen DCs have increased gene expression of the toll-inhibitory protein interleukin-1-receptor-associated kinase 3 (irak3) compared with PBS-injected controls. The results of this in vivo gene expression analysis are similar to those gene changes observed in vitro, confirming that the in vitro data are not a result of culture conditions or an artefact.
Figure 5.

In vivo lipopolysaccharide (LPS) administration up-regulates Toll-like receptor (TLR) regulatory genes in Peyer's patch (PP) dendritic cells (DCs). The LPS (4 mg/kg) or phosphate-buffered saline (PBS) was administered by intraperitoneal injection. Five hours later spleen and PP were harvested and DCs were isolated by CD11c+ autoMACS separation. Lysates of separated cells were analysed for tmed1, irak1, sigirr, tollip, irak3 and socs1. Gene expression was determined as fold induction over β-actin housekeeper. Bars represent mean ± SEM. Significance determined by one-way analysis of variance, Tukey post-hoc, ***P < 0·001, **P < 0·01, *P < 0·05, n = 4.
Bacteria-conditioned bone-marrow-derived cells up-regulate gene expression of TLR regulatory proteins in response to LPS
Previously we demonstrated that exposure of bone marrow to commensal bacteria over a 7-day culture in the presence of GM-CSF prevents DC differentiation and instead cells develop into myeloid-suppressor-like cells, which secrete high IL-10 and induce regulatory T-cell responses.8 As PP DCs have demonstrated an altered gene expression pattern of TLR regulatory proteins, we sought to investigate if these genes were similarly regulated in cells rendered tolerant through exposure to commensal bacteria (Fig. 6). Interestingly, we found that the expression of both socs1 and irak3 were up-regulated in LPS-stimulated bacteria-conditioned bone-marrow-derived cells; mRNA expression of tollip was also increased but this was not statistically significant. Other genes investigated were not found to be significantly induced (Table S2).
Figure 6.
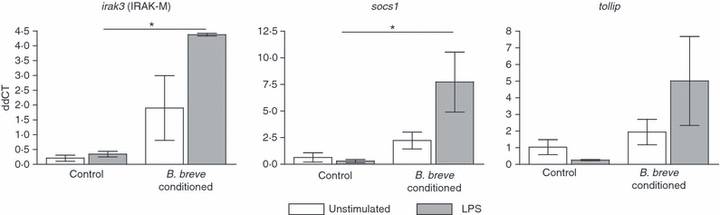
Bacteria-exposed bone marrow-derived cells induce regulatory gene up-regulation to lipopolysaccharide (LPS). Non-pathogenic Bifidobacterium breve (4 × 105 CFU/ml) were added to bone marrow cultures for 7 days in the presence of granulocyte–macrophage colony-stimulating factor. At harvest, cells were washed and re-plated with LPS (1 μg/ml) for 18 hr. Cell lysates were analysed for gene expression of regulatory proteins irak3, socs1 and tollip. Gene expression was compared with housekeeper β-actin. Bars represent mean ± SEM. Significance determined by one-way analysis of variance, Tukey post-hoc test, *P < 0·05, n = 3.
Discussion
We have demonstrated that DCs from PP regulate their response to LPS by increasing gene expression of several TLR regulatory proteins. The PP DCs were also found to have reduced expression of TLRs and to secrete a limited profile of cytokines compared with spleen DCs. Additionally, bacteria-conditioned bone-marrow-derived cells demonstrated altered expression of several TLR regulatory genes. Taken together, this suggests a fundamental regulation initiated by exposure of precursor cells to TLR ligands leading to the observed function of PP DCs.
Our investigations are limited by the purity of cells isolated by autoMACS. The purity of cells was routinely approximately 80% and therefore there is the possibility of contaminating cells influencing the results.
Peyer's patch DCs secrete a more limited profile of cytokines than spleen DCs; secreting only IL-6, IL-10 and KC in response to LPS but not IL-12p70, IL-1β or TNF-α. Previous studies have also found that PP DCs secrete IL-6 and IL-10,9,13,14 and this was found to be conducive to the generation of immunoglobulin A antibody responses.13
Altered LPS responsiveness has obvious relevance to the outcome of bacterial signalling in the gut. Whether our results can be extrapolated to other TLR ligands was not the aim of the current study. It has been reported by Cerovic et al. that intestinal DCs are only hypo-responsive to LPS stimulation, whereas challenge with other TLR ligands such as Pam3Cys and CpG elicits similar responses to those found in spleen DCs.15 Our investigation determined that PP DC responses to the mitogen PMA/Io are also dissimilar to responses elicited from spleen DCs. The PP DCs did not secrete IL-12p70 following PMA/Io stimulation, indicating that PP DCs may have altered responsiveness compared with spleen DCs to a number of different stimulators.
To account for the differences in responsiveness of spleen and PP DCs to LPS, we analysed the surface protein expression of TLR2 and TLR4 and the gene expression of tlr1–9 in freshly isolated cells. Fewer DCs from PP were found to have surface expression of TLR2 and TLR4 compared with spleen DCs. The gene expression analysis of tlr1–9 did not indicate a statistical significance in gene expression levels although there was an indication that mRNA expression of tlr1, tlr2, tlr4 and tlr6 were decreased in PP DCs compared with spleen DCs. Other groups have investigated different populations of DCs from the small intestine and colonic lamina propria to find that TLR4 expression on CD11c+ cells is present16 or absent.17,18 Our work suggests that PP DCs have reduced receptor expression for ligands on the surface of bacteria and this may be one of the mechanisms by which PP DCs regulate their responsiveness to commensal bacteria.
Immune responses in the gut to commensal bacteria that express the same ligands for TLRs as pathogenic bacteria induce tolerance rather than inflammation, there must therefore be regulation of TLR signalling beyond the level of TLR surface molecule expression. We investigated the gene expression of a panel of TLR regulatory proteins in spleen and PP DCs in vitro and in vivo in response to LPS, and found differences in several genes.
At the cell membrane, membrane-bound mediators are stationed to sequester adaptor proteins and directly interact with receptors preventing ligand binding. SIGIRR and ST2L are examples of this type of regulatory protein.19–22 Our work has demonstrated both in vitro and in vivo that tmed1 (ST2L) and sigirr mRNA expression is increased in PP DCs compared with spleen DCs, indicating that PP DCs use these molecules in their arsenal to prevent the initiation of TLR signalling.
Signal propagation is necessary to amplify the initiating signal to trigger transcription factor nuclear mobilization and induce gene expression. This occurs through a cascade of mediators, each of which is a possible target for regulatory actions. TOLLIP is an adaptor molecule which can bind to TLR2 and TLR4 to inhibit MyD88 binding and activation.23,24 In addition, TOLLIP binds to and is phosphorylated by IRAK,24,25 suppressing its ability to function in the TLR pathway. In our experiments, tollip mRNA was increased in both resting and LPS-stimulated PP DCs in vitro and in in vivo LPS-injected PP DCs. Both the in vitro and in vivo data imply that in PP, DCs inhibit TLR signal propagation by up-regulating TOLLIP protein.
The IRAK-M binds to IRAK1/IRAK4 and prevents it from dissociating from the TLR complex26 inhibiting further downstream signal transduction. IRAK-M is highly expressed in blood lymphocytes and can also be found in the spleen but is not present in large amounts in other locations and is almost undetectable in intestinal tissue.27 Our finding that irak3 gene expression is increased in spleen DCs following LPS challenge, but not in PP DCs, is in keeping with this location-specific expression pattern, and indicates that cells from different locations use different proteins to regulate their TLR responses.
Regulation of responsiveness to inflammatory cytokines induced by TLR activation prevents excessive activation,28 and is important in preventing the induction of autoimmune diseases.29,30 Our in vivo findings indicate that socs1 gene expression is increased in PP DCs following LPS exposure. The SOCS1 protein functions to prevent signalling by IL-12 and IFNs.31 SOCS1 binds signalling proteins through their SH2 domains and targets them for ubiquitination,32,33 arresting signal transduction and the production of further cytokines leading to immune response resolution.34 We have demonstrated that PP DCs do not secrete significant quantities of IL-12p70 in response to LPS or PMA/Io. It may be through SOCS1 inhibition of IFN signalling and subsequent attenuation of TLR responsiveness that production of this inflammatory cytokine is limited.
The toll regulatory protein gene expression analysis we performed revealed several proteins which are important in LPS tolerance in the gut. Interestingly, both SIGIRR and SOCS1 have been investigated for their role in murine models of colitis. In epithelial cells and T cells, which lacked SIGIRR35,36 and SOCS137,38 respectively, mice were shown to have increased susceptibility to disease and much greater inflammatory cytokine responses. Although the influence these proteins have on the function of DCs in models of colitis has not been investigated, it can be inferred that without appropriate control of inflammatory processes, DCs would most likely propagate inflammation and exacerbate colitis. Hence, these proteins are targets for manipulation in disease states.
In addition to the altered gene regulation of TLR regulatory proteins, DCs from the PP did not up-regulate the antigen presentation molecule MHCII or the co-stimulatory molecule CD80 to the same extent as spleen DCs. This indicates another level of altered functional responses beyond cytokine production which differs between cells from the two locations. Reduced antigen presentation and co-stimulation by PP DCs would impact on the ability to induce T-cell responses and may be another mechanism by which DCs control immune responses in intestinal tissues.
We previously introduced a model system in which bone marrow progenitor cells exposed to commensal bacteria do not develop into classical DCs but instead take on the characteristics of myeloid-suppressor cells, including IL-10 secretion and the induction of regulatory T cells.8 We postulated that this MyD88-dependent tolerization of precursors may occur in vivo, contributing to the development of gut DC responses. Therefore, we analysed the gene expression of toll-regulatory proteins in this model system to determine if they were under similar regulation to PP DCs. The LPS stimulation of bacteria-conditioned bone marrow-derived cells led to an increase in irak3 that was not observed in control bone-marrow-derived DCs stimulated with LPS. As previously mentioned, IRAK-M is expressed in blood lymphocytes, and is only found at low levels in intestinal tissues but is involved in the negative regulation of TLR signalling. Interestingly, cells in this in vitro culture system have never come into contact with intestinal tissue and up-regulate irak3 in response to LPS, in a similar manner to spleen DCs. However, these cells also up-regulate expression of socs1 and tollip in response to LPS which was observed in PP DCs. While these changes were in keeping with those found in PP DCs, neither sigirr nor tmed1 were increased in bacteria-exposed bone marrow cells (Table S2). Therefore, exposure of precursor cells to bacteria induces partial overlap in gene expression responses to LPS stimulation with PP DCs. This indicates that signalling through TLRs at an early precursor stage may be involved in biasing some aspects of molecular regulation of TLR signalling and tolerance induction in PP DCs.
In conclusion, PP DCs exhibit altered regulatory gene expression in response to LPS stimulation compared with spleen DCs, which impacts on cytokine secretion and co-stimulatory molecule up-regulation. These genes may be up-regulated in response to precursor exposure to TLR ligands indicating that the commensal flora plays a role in conditioning PP DCs. Understanding the molecular regulation of PP DCs will be important for the development of strategies not only to re-establish tolerance in intestinal disease states such as inflammatory bowel disease, but also to design effective mucosal adjuvants capable of overcoming tolerance to induce protective immunity at mucosal surfaces.
Acknowledgments
The authors would like to acknowledge Grainne Hurley and Aoife Quinlan for their expert help with immunizing animals. The authors have been supported in part by Science Foundation Ireland, the Health Research Board of Ireland and the Higher Education Authority of Ireland.
Glossary
Abbreviations:
- CFU
colony-forming unit
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- Ig
immunoglobulin
- IL
interleukin
- irak
interleukin-1-receptor-associated kinase
- KC
keratinocyte chemoattractant
- LPS
lipopolysaccharide
- MFI
mean fluorescent intensity
- MyD88
myeloid differentiation factor 88
- PBS
phosphate-buffered saline
- sigirr
single immunoglobulin and toll-interleukin 1 receptor
- socs1
suppressor of cytokine signalling 1
- tmed1
transmembrane emp24 domain containing 1
- TNF-α
tumour necrosis factor α
- tollip
toll interacting protein
Conflict of Interest
The authors declare no financial or commercial conflict of interest. F.S. has received unrelated grants from Alimentary Health Ltd, GlaxoSmithKline Ltd., and the Procter & Gamble Company, but these facts neither influenced nor constrained the content of this manuscript.
Supporting Information
Additional Supporting information may be found in the online version of this article:
Table S1. Gene expression of CD11c+ cells.
Table S2. Gene expression of control and bacteria conditioned bone marrow-derived cells.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Chen Y, Inobe J, Weiner HL. Inductive events in oral tolerance in the TCR transgenic adoptive transfer model. Cell Immunol. 1997;178:62–8. doi: 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+ CD4+ regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 3.Millington OR, Mowat AM, Garside P. Induction of bystander suppression by feeding antigen occurs despite normal clonal expansion of the bystander T cell population. J Immunol. 2004;173:6059–64. doi: 10.4049/jimmunol.173.10.6059. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss I, Boirivant M, Kitani A. Insights into the mechanism of oral tolerance derived from the study of models of mucosal inflammation. Ann N Y Acad Sci. 2004;1029:115–31. doi: 10.1196/annals.1309.029. [DOI] [PubMed] [Google Scholar]
- 5.Mowat AM. Basic mechanisms and clinical implications of oral tolerance. Curr Opin Gastroenterol. 1999;15:546–56. doi: 10.1097/00001574-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S. Toll-like receptor signal transduction. Exp Mol Med. 2007;39:421–38. doi: 10.1038/emm.2007.47. [DOI] [PubMed] [Google Scholar]
- 7.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 8.Davies JM, Sheil B, Shanahan F. Bacterial signalling overrides cytokine signalling and modifies dendritic cell differentiation. Immunology. 2009;128:e805–15. doi: 10.1111/j.1365-2567.2009.03086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 13.Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171:3684–90. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8α+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884–90. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 15.Cerovic V, Jenkins CD, Barnes AG, Milling SW, MacPherson GG, Klavinskis LS. Hyporesponsiveness of intestinal dendritic cells to TLR stimulation is limited to TLR4. J Immunol. 2009;182:2405–15. doi: 10.4049/jimmunol.0802318. [DOI] [PubMed] [Google Scholar]
- 16.Monteleone I, Platt AM, Jaensson E, Agace WW, Mowat AM. IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function. Eur J Immunol. 2008;38:1533–47. doi: 10.1002/eji.200737909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takenaka S, Safroneeva E, Xing Z, Gauldie J. Dendritic cells derived from murine colonic mucosa have unique functional and phenotypic characteristics. J Immunol. 2007;178:7984–93. doi: 10.4049/jimmunol.178.12.7984. [DOI] [PubMed] [Google Scholar]
- 18.Uematsu S, Jang MH, Chevrier N, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–74. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 19.Wald D, Qin J, Zhao Z, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 20.Garlanda C, Riva F, Polentarutti N, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–6. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. J Biol Chem. 2005;280:25233–41. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- 22.Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O’Neill LA, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–9. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 23.Bulut Y, Faure E, Thomas L, Equils O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001;167:987–94. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002;277:7059–65. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- 25.Burns K, Clatworthy J, Martin L, et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol. 2000;2:346–51. doi: 10.1038/35014038. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 27.Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403–10. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 28.Crespo A, Filla MB, Murphy WJ. Low responsiveness to IFN-γ, after pretreatment of mouse macrophages with lipopolysaccharides, develops via diverse regulatory pathways. Eur J Immunol. 2002;32:710–9. doi: 10.1002/1521-4141(200203)32:3<710::AID-IMMU710>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanada T, Yoshida H, Kato S, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–50. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 31.Crespo A, Filla MB, Russell SW, Murphy WJ. Indirect induction of suppressor of cytokine signalling-1 in macrophages stimulated with bacterial lipopolysaccharide: partial role of autocrine/paracrine interferon-α/β. Biochem J. 2000;349:99–104. doi: 10.1042/0264-6021:3490099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang JG, Farley A, Nicholson SE, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci U S A. 1999;96:2071–6. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamizono S, Hanada T, Yasukawa H, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–8. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JG, Metcalf D, Rakar S, et al. The SOCS box of suppressor of cytokine signaling-1 is important for inhibition of cytokine action in vivo. Proc Natl Acad Sci U S A. 2001;98:13261–5. doi: 10.1073/pnas.231486498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao H, Gulen MF, Qin J, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–75. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Garlanda C, Riva F, Veliz T, et al. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–21. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- 37.Chinen T, Kobayashi T, Ogata H, et al. Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFNγ and IL-4 are involved. Gastroenterology. 2006;130:373–88. doi: 10.1053/j.gastro.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 38.Horino J, Fujimoto M, Terabe F, et al. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunol. 2008;20:753–62. doi: 10.1093/intimm/dxn033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


