Abstract
The genesis and progression of diabetes occur due in part to an uncontrolled inflammation profile with insulin resistance, increased serum levels of free fatty acids (FFA), proinflammatory cytokines and leucocyte dysfunction. In this study, an investigation was made of the effect of a 3-week moderate exercise regimen on a treadmill (60% of VO2max, 30 min/day, 6 days a week) on inflammatory markers and leucocyte functions in diabetic rats. The exercise decreased serum levels of tumour necrosis factor (TNF)-α (6%), cytokine-induced neutrophil chemotactic factor 2 alpha/beta (CINC-2α/β) (9%), interleukin (IL)-1β (34%), IL-6 (86%), C-reactive protein (CRP) (41%) and FFA (40%) in diabetic rats when compared with sedentary diabetic animals. Exercise also attenuated the increased responsiveness of leucocytes from diabetics when compared to controls, diminishing the reactive oxygen species (ROS) release by neutrophils (21%) and macrophages (28%). Exercise did not change neutrophil migration and the proportion of neutrophils and macrophages in necrosis (loss of plasma membrane integrity) and apoptosis (DNA fragmentation). Serum activities of creatine kinase (CK) and lactate dehydrogenase (LDH) were not modified in the conditions studied. Therefore, physical training did not alter the integrity of muscle cells. We conclude that moderate physical exercise has marked anti-inflammatory effects on diabetic rats. This may be an efficient strategy to protect diabetics against microorganism infection, insulin resistance and vascular complications.
Keywords: cytokines, macrophages, neutrophils, physical training
Introduction
Evidence from clinical and experimental studies supports the notion that inflammation plays an important role in the genesis and progression of diabetes [1–4]. Pathogenesis of diabetic complications include the deleterious effects of advanced glycation end products (AGEs), insulin resistance, impaired vasodilatory response and leucocyte (neutrophils, monocytes/macrophages and lymphocytes) dysfunction [4]. These complications are due in part to uncontrolled inflammation of the diabetic state with increased free fatty acid serum (FFA) levels [2], overproduction of endothelial growth factors, proinflammatory cytokines and acute phase proteins [3]. These mediators lead to inappropriate cell activation, and thus tissue injury and impaired wound healing, and contribute towards increased susceptibility of diabetics to invasive microorganisms [2,3]. Additionally, it has been demonstrated that insulin resistance is associated with a state of chronic low-grade inflammation, and mediators such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 have been identified as being involved in the disorder in insulin-receptor signalling [5].
Aerobic exercise has been recommended for the management of diabetes in order to improve insulin sensitivity and vascular health [6–8]. Recent studies with non-diabetic people have suggested that the beneficial effects of exercise are mediated partly by control of serum levels of FFA [9] and cytokines such IL-6, TNF-α, IL-1β and IL-8 [10,11]. A moderate physical exercise programme increases the efficiency of immune function and decreases serum levels of markers of inflammation in non-diabetic states [12]. However, effects of moderate-intensity aerobic exercise on plasma levels of pro- and anti-inflammatory cytokines and FFA and blood leucocyte (neutrophils and macrophages) number, viability and function in exercise and diabetes have not been reported.
The effects of a 3-week exercise programme (60% of VO2max, 30 min/day, 6 days a week) on the serum levels of C-reactive protein (CRP), FFA, cytokine-induced neutrophil chemotactic factor 2 alpha/beta (CINC)-2αβ, IL-1β, TNF-α, IL-6, IL-10 and IL-1ra were investigated in streptozotocin-induced diabetic rats. The number of resident macrophage and neutrophil glycogen-induced migration to peritoneum was evaluated. The proportion of cells with signs of necrosis and apoptosis and the amount of reactive oxygen species (ROS) released by these cells was also measured. We also evaluated the serum activities of the skeletal muscle enzymes creatine kinase (CK) and lactate dehydrogenase (LDH).
Materials and methods
Animals
Male Wistar rats (180 ± 20 g) were kept in a room with an inverted 12-h light/dark cycle under standardized conditions of temperature and humidity. Forty animals were divided into four groups (10 animals per group): (i) control; (ii) control exercise; (iii) diabetic; and (iv) diabetic exercise. The exercise groups were subjected to a moderate physical training programme on a treadmill. Non-exercise rats were handled daily to mimic the handling conditions to which the exercise rats were subjected. A standard animal laboratory chow (52% carbohydrate, 21% protein and 4% lipids; Nuvilab CR1 Nuvital, Curitiba, Paraná, Brazil) and water were given ad libitum. The experiment was approved by the Ethical Committee of the Cruzeiro do Sul University (183/2008) and followed the Guidelines for Care and Use of Laboratory Animals.
Induction of diabetes
The experimental type 1 diabetes was induced by peritoneal injection of 65 mg/kg body weight streptozotocin dissolved in citrate buffer (pH 4·2). At 48 h after streptozotocin injection, the diabetic state was confirmed by blood glucose levels above 200 mg/dl, estimated with the aid of a glucose meter (Accu-Chek Active, Roche Diagnostics GmbH, Mannheim, Germany). Blood samples were obtained from a cut at the tip of the animal's tail. The physical training was initiated 7 days prior to the induction of diabetes.
Physical training protocol
The exercise protocol used in this study has been described previously [13]. The study was divided into two parts: the preliminary phase (1 week of duration) and the main experiment (3 weeks of duration). Briefly, in the first week of the preliminary experiments, the rats were adapted to the treadmill. The adaptation consisted of 15 min of exercise at a speed of 0·3 km/h.
After that, all the animals were subjected to a test of maximal effort to determine the maximal oxygen consumption (VO2max). The initial treadmill speed was 0·3 km/h. At 3-min intervals, the speed was increased by 0·3 km/h. The maximal speed was reached when the animal was able to run for at least 1·3 min at the same speed and unable to run at an immediately higher speed. The resulting VO2max values were used to define the moderate training programme. Briefly, this programme consisted of running on a treadmill (Millennium Inbramed, Porto Aleger, Brazil) for 30 min a day at an intensity of up to approximately 60% VO2max, 6 days a week for 3 weeks. At the end of each week, the animals were subjected to a test of maximal effort to determine the maximal oxygen consumption (VO2max) and the moderate running exercise for the next week's training programme.
The non-exercise group remained in their cages. At the end of the training programme the rats were allowed to rest for 2 days without physical exercise, after which the samples were collected (blood, neutrophils and macrophages). Neutrophil and macrophage functions were analysed immediately. Plasma was collected and stored at –80°C before determining cytokines, FFA levels and enzyme activities.
Determination of creatine kinase (CK) and lactate dehydrogenase (LDH) serum activity
Serum CK and LDH activities are markers of muscle injury. CK and LDH activities were measured according to the methods established by Oliver[14] and Zammit and Newsholme, respectively [15].
Determination of plasma IL levels
Plasma levels of IL-6, IL-1β, TNF-α, IL-8 and IL-1ra were determined based on the enzyme-linked immunosorbent assay (ELISA) using a DuoSet Kit (Quantikine, R&D Systems, Minneapolis, MN, USA), following the manufacturer's instructions.
Determination of serum non-esterified fatty acid levels
Plasma FFA levels were measured using an enzymatic spectrophotometric method (Wako Chemical, Neuss, Germany), following the manufacturer's instructions [16].
Determination of serum CRP levels
C-reactive protein was determined by a highly sensitive immunoturbidimetric method (Bioclin Diagnostics, São Paulo, Brazil), according to the manufacturer's instructions.
Preparation of peritoneal neutrophils
Neutrophils were obtained by intraperitoneal (i.p.) lavage with 40 ml sterile phosphate-buffered saline (PBS), 3 h after the i.p. injection of 10 ml of sterile type II oyster glycogen solution (1%) in PBS. This treatment induces a substantial migration of neutrophils to the intraperitoneal cavity, with little contamination by macrophages (less than 2%). The peritoneal fluid was centrifuged (400 g for 10 min), washed twice with PBS and cells were counted in a Neubauer chamber using an optical microscope (Alphaphot-2, Nikon, Japan) and trypan blue solution (at 1% in PBS) [17].
Cell viability assay (proportion of necrotic cells)
Viability of neutrophils and macrophages was assessed using a fluorescence activated cell sorter (FACS) Calibur Cytometer (Becton Dickinson Systems, San Jose, CA, USA). The percentage of viable cells in each sample was determined using propidium iodide stain (solution at 0·05% in PBS). Ten thousand events were analysed per sample. Fluorescence of the propidium iodide was measured using the FL2 channel (orange–red fluorescence = 585/42 nm).
Proportion of cells with DNA fragmentation
DNA fragmentation was analysed by flow cytometry after DNA staining with propidium iodide. The presence of detergent in the solution permeabilizes the cells, which promptly incorporate the dye into DNA. Briefly, after the incubation period, the cells were centrifuged at 1000 g for 15 min at 4°C. The resulting pellet was resuspended carefully in 300 µl hypotonic solution containing 50 µg/ml propidium iodide, 0·1% sodium citrate, and 0·1% Triton X-100. The cells were then incubated for 2 h at 4°C. Fluorescence was measured and analysed as described above.
Lucigenin-enhanced chemiluminescence assay
Lucigenin (1 mM) was added to neutrophil (2·5 × 106 cells/ml) or macrophage (2·5 × 106 cells/ml) incubation medium. Immediately afterwards, cells were treated with phorbol myristate acetate (PMA) (54 ng/ml). ROS release was monitored for 20 min. The assays were run in PBS supplemented with CaCl2 (1 mM), MgCl2 (1·5 mM) and glucose (10 mM), at 37°C, in a final volume of 0·3 ml [18].
Statistical analysis
Comparisons were made using one-way analysis of variance (anova) and Tukey's test. The significance was set at P < 0·05. The values are presented as mean ± standard deviation of 10 animals per group.
Results
The 3-week physical training programme did not modify the serum activities of CK and LDH. Thus, the proposed exercise did not cause marked muscle lesion with consequent inflammation in either the diabetic or control group (Table 1). Exercise decreased the serum levels of proinflammatory cytokines in the diabetic group compared with those of diabetic sedentary rats (Fig. 1). The reducing effect of exercise was of 34% for IL-1β (P = 0·011), 9% for CINC-2αβ (P = 0·02), 86% for IL-6 (P ≤ 0·001) and 6% for TNF-α (P = 0·023). There was no difference in serum levels of IL-10 and IL-1ra between control and diabetic rats (data not shown). Exercise decreased the serum levels of CRP and FFA in diabetic rats compared with those of the diabetic sedentary group (Fig. 2). The reducing effect of exercise was of 41% for CRP (P ≤ 0·001) and 40% for FFA (P = 0·034).
Table 1.
Serum activities of creatine kinase (CK) and lactate dehydrogenase (LDH) in sedentary control, sedentary diabetic, exercised control and exercise diabetic rats.
| LDH (U/l) Mean ± s.e.m. | CK (U/l) Mean ± s.e.m. | |
|---|---|---|
| Sedentary – control | 724 ± 15 | 297 ± 37 |
| Exercise – control | 951 ± 280 | 290 ± 75 |
| Sedentary – diabetic | 943 ± 520 | 248 ± 25 |
| Exercise – diabetic | 1296 ± 355 | 230 ± 68 |
The values are presented as mean ± standard deviation of 10 animals per group; s.e.m.: standard error of the mean.
Fig. 1.
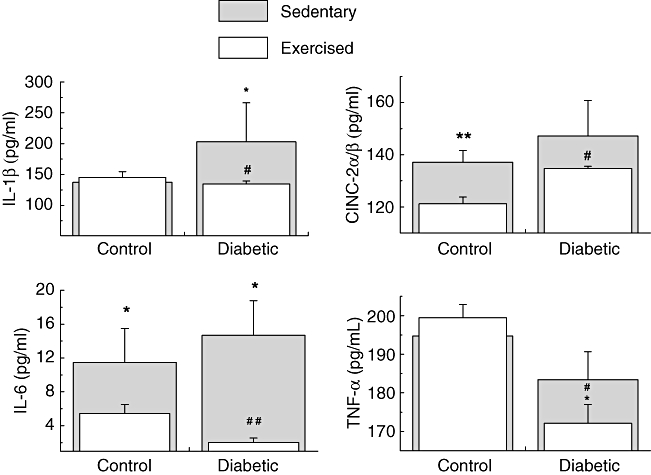
Serum concentrations of interleukin (IL)-1β, cytokine-induced neutrophil chemotactic factor 2 alpha/beta (CINC)-2-α/β, IL-6 and tumour necrosis factor (TNF)-α (pg/ml) in sedentary controls, sedentary diabetic, exercise control and exercise diabetic rats. The values are presented as mean ± standard deviation of 10 animals per group. *P < 0·05; **P < 0·01; ***P < 0·001 for comparison with the sedentary control group. #P < 0·05; ##P < 0·01; ###P < 0·001 for comparison with the sedentary diabetic group.
Fig. 2.
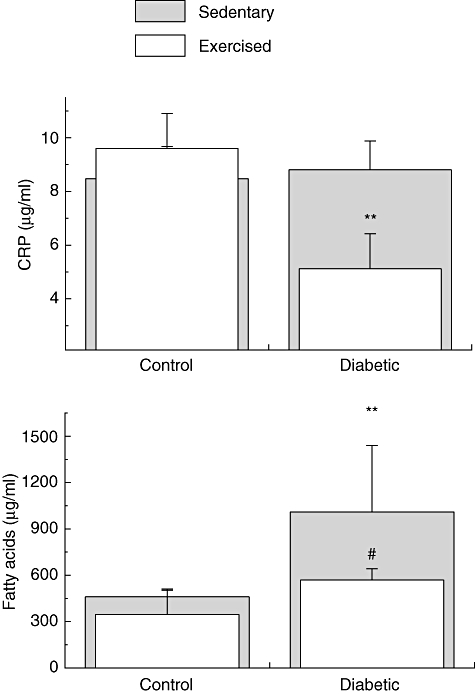
Serum concentrations of C-reactive protein (CRP) (µg/ml) and free fatty acids (FFA) (µg/ml) of sedentary controls, sedentary diabetic, exercise control and exercise diabetic rats. The values are presented as mean ± standard deviation of 10 animals per group. CRP: *P < 0·05; **P < 0·01; ***P < 0·001 for comparison with the sedentary control group. #P < 0·05; ##P < 0·01; ###P < 0·001 for comparison with the sedentary diabetic group.
Neutrophils and macrophages from non-exercise diabetic rats spontaneously released higher amounts of ROS when compared with non-exercise control rats (Fig. 3). Exercise decreased the spontaneous release of ROS by neutrophils and macrophages from diabetic rats compared with exercise control rats. The decreasing effect of exercise on superoxide release was 21% for neutrophil superoxide release (P ≤ 0·044) and 27% for macrophage (P ≤ 0·039). There was no difference in PMA-stimulating ROS release by neutrophils and macrophages between diabetic exercise and non-exercise rats (Fig. 3).
Fig. 3.
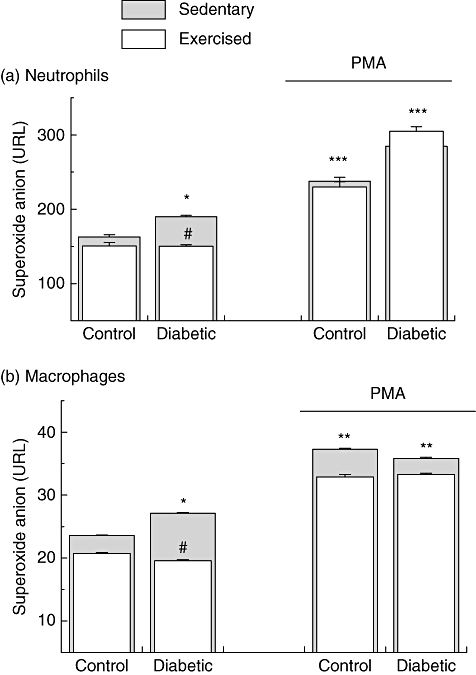
Reactive oxygen species (ROS) release by neutrophils and macrophages from sedentary controls, sedentary diabetics, exercise control and exercise diabetic rats. The measurement was carried out in basal conditions and under phorbol myristate acetate (PMA)-stimulating conditions. The values are presented as mean ± standard deviation of 10 animals per group. *P < 0·05; **P < 0·01; ***P < 0·001 for comparison with the sedentary control group. #P < 0·05; ##P < 0·01; ###P < 0·001 for comparison with the sedentary diabetic group.
Exercise did not change the oyster glycogen induced-neutrophil migration in either control or diabetic rats. The exercise diabetic group showed an increase in the number of resident macrophages compared with control rats (exercise and sedentary) (P = 0·022). Although exercise increased the number of resident macrophages in the diabetic group, no alteration was observed in the proportion of cells with signs of apoptosis and necrosis (Fig. 4). Moreover, no alteration was found in the number of neutrophils and proportion of cells with signs of necrosis and apoptosis in diabetic rats (sedentary and exercise) in comparison with the control group (Fig. 4).
Fig. 4.
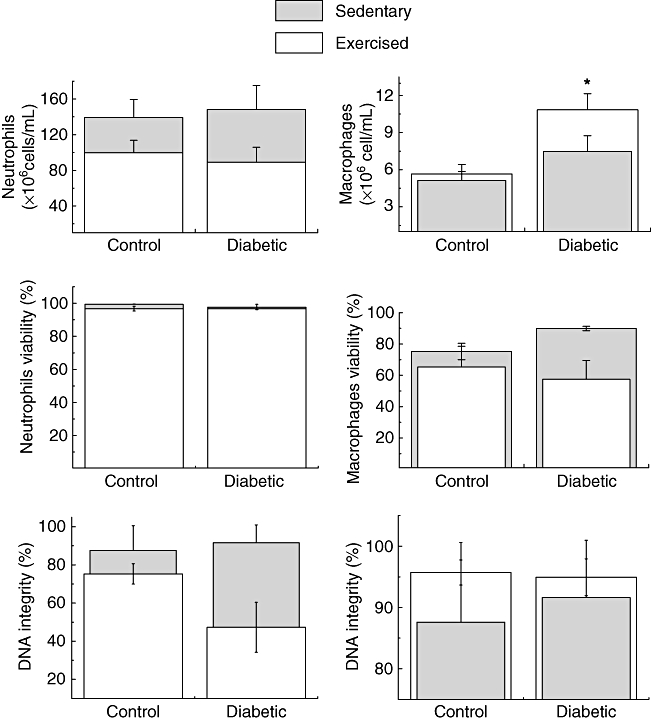
Number of macrophages and neutrophils in the peritoneal cavity and percentage of cells with signs of apoptosis (DNA fragmentation) and necrosis (loss of plasma membrane integrity) in sedentary control, sedentary diabetic, exercise control and exercise diabetic rats. The values are presented as mean ± standard deviation of 10 animals per group. *P < 0·0;, **P < 0·01; ***P < 0·001 for comparison with the sedentary control group. #P < 0·05; ##P < 0·01; ###P < 0·001 for comparison with the sedentary diabetic group.
Discussion
In this study, a 3-week moderate physical training programme on a treadmill (60% of VO2max, 30 min/day, 6 days a week) decreased the serum levels of TNF-α, CINC-2α/β, IL-1β, IL-6, CRP and FFA in diabetic rats when compared with sedentary diabetic rats. Exercise also diminished the increased responsiveness of neutrophils and macrophages from diabetic rats when compared with the control group in the basal (non-stimulated) condition, decreasing the release of ROS by these cells.
Accumulating evidence supports the recommendation of regular physical activity for the prevention and treatment of diabetes and other chronic diseases that present a constant proinflammatory status [19]. The practice of regular physical activity is known to bring health benefits such as increased insulin sensitivity, glycaemic control, decrease of body weight and percentage of body fat, lower blood pressure and reduction of overall risk of vascular disease [6,8,20–22].
Increased levels of inflammatory mediators such as FFA and proinflammatory cytokines have been reported in diabetic states to be a consequence of hyperglycaemia [2]. Proinflammatory cytokines and FFA have been considered to be the link between inflammation and insulin resistance. At a molecular level, exposure of cells to TNF-α, IL-1β, IL-6 or elevated levels of FFAs stimulated inhibitory phosphorylation of serine residues of insulin receptor. This effect is involved directly in insulin resistance [5].
Additionally, abnormal levels of FFA and proinflammatory cytokines participate in the development of vascular complications and increasing susceptibility to invasive microorganisms [2,4]. FFA oxidation causes mitochondrial overproduction of ROS, with consequent activation of nuclear factor (NF)-κB and greater release of proinflammatory cytokines [2,23]. Additionally, increases in plasma FFA levels can activate immune cells directly [17,24,25]. Increased serum levels of proinflammatory cytokines also stimulate leucocytes to produce ROS and cytokines.
Neutrophils and monocytes/macrophages from diabetic patients show impaired functions such as migration to inflammatory sites, phagocytosis, release of lytic proteases, production of ROS and apoptosis [3,4,26]. The impaired responsiveness of neutrophils and macrophages of diabetics are considered part of the scenario of diabetes pathophysiology [4].
Although diabetes by itself is unable to trigger a chemiluminescent signal of the magnitude expected for classical stimuli (around three to four orders of magnitude higher than non-stimulated cells), it caused a slight increase (1·3 and 1·2 times) in the basal chemiluminescence of neutrophils and macrophages, respectively. This effect (mainly in neutrophils) supports the proposition that diabetes is an inflammatory priming condition [27,28]. Increased ROS production results in activation of NF-κb and thus transcription of proinflammatory cytokines [2,23].
The combined effect of an increment in the production of ROS, TNF-α and IL-1β in diabetic patients may intensify the inflammatory response due to increased activity of neutrophils. Therefore, the prescription of exercise for these patients may be an important therapeutic strategy to control the pathogenesis of the disease. In fact, our data demonstrated that regular physical exercise has been shown to present anti-inflammatory effects such as decreased serum levels of inflammatory mediators and reduced basal inflammatory status of neutrophils and macrophages.
Several studies have shown that physical exercise leads to an increase of serum levels of anti-inflammatory cytokine [9,11,29]. However, in the present study, exercise did not change plasma levels of IL-10 and IL-1ra, due probably to the low intensity and/or short duration of the exercise protocol employed here.
These observations lead us to conclude that physical exercise causes marked anti-inflammatory effects in diabetic rats. Therefore, physical exercise may be used as a therapeutic strategy to protect against insulin resistance and vascular and other complications in diabetic patients.
Acknowledgments
This research was supported by the Brazilian research funding agencies FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Disclosure
None of the authors have any conflict of interest.
References
- 1.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–5. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 3.Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. Interaction between serum amyloid A and leukocytes – a possible role in the progression of vascular complications in diabetes. Immunol Lett. 2007;108:160–6. doi: 10.1016/j.imlet.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 2006;146:443–7. doi: 10.1111/j.1365-2249.2006.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspersen CJ, Fulton JE. Epidemiology of walking and type 2 diabetes. Med Sci Sports Exerc. 2008;40:519–28. doi: 10.1249/MSS.0b013e31817c6737. [DOI] [PubMed] [Google Scholar]
- 7.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32:1531–35. doi: 10.2337/dc09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams PT. Reduced diabetic, hypertensive, and cholesterol medication use with walking. Med Sci Sports Exerc. 2008;40:433–43. doi: 10.1249/MSS.0b013e31815f38f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J Nutr. 2008;138:2293–6. doi: 10.3945/jn.108.097188. [DOI] [PubMed] [Google Scholar]
- 10.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicklas BJ, Hsu FC, Brinkley TJ, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045–52. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 13.Leandro CG, Levada AC, Hirabara SM, et al. A program of moderate physical training for Wistar rats based on maximal oxygen consumption. J Strength Cond Res. 2007;21:751–6. doi: 10.1519/R-20155.1. [DOI] [PubMed] [Google Scholar]
- 14.Oliver IY. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1995;61:116–22. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zammit VA, Newsholme EA. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem J. 1976;160:447–62. doi: 10.1042/bj1600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christmass MA, Mitoulas LR, Hartmann PE, Arthur PG. A semiautomated enzymatic method for determination of nonesterified fatty acid concentration in milk and plasma. Lipids. 1998;33:1043–9. doi: 10.1007/s11745-998-0304-9. [DOI] [PubMed] [Google Scholar]
- 17.Pereira LM, Hatanaka E, Martins EF, et al. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem Funct. 2008;26:197–04. doi: 10.1002/cbf.1432. [DOI] [PubMed] [Google Scholar]
- 18.Hatanaka E, Levada-Pires AC, Pithon-Curi TC, Curi R. Systematic study on ROS production induced by oleic, linoleic, and gamma-linolenic acids in human and rat neutrophils. Free Radic Biol Med. 2006;41:1124–32. doi: 10.1016/j.freeradbiomed.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–17. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 20.Bruce CR, Hawley JA. Improvements in insulin resistance with aerobic exercise training: a lipocentric approach. Med Sci Sports Exerc. 2004;36:1196–1. [PubMed] [Google Scholar]
- 21.Ribeiro IC, Iborra RT, Neves MQ, et al. HDL atheroprotection by aerobic exercise training in type 2 diabetes mellitus. Med Sci Sports Exerc. 2008;40:779–86. doi: 10.1249/MSS.0b013e3181632d2d. [DOI] [PubMed] [Google Scholar]
- 22.Wilund KR. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiovascular disease? Clin Sci (Lond) 2007;112:543–55. doi: 10.1042/CS20060368. [DOI] [PubMed] [Google Scholar]
- 23.Coughlan MT, Thorburn DR, Penfold SA, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–52. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins de Lima T, Gorjão R, Hatanaka E, et al. Mechanisms by which fatty acids regulate leucocyte function. Clin Sci (Lond) 2007;113:65–77. doi: 10.1042/CS20070006. [DOI] [PubMed] [Google Scholar]
- 25.Otton R, da Silva DO, Campoio TR, et al. Non-esterified fatty acids and human lymphocyte death: a mechanism that involves calcium release and oxidative stress. J Endocrinol. 2007;195:133–43. doi: 10.1677/JOE-07-0195. [DOI] [PubMed] [Google Scholar]
- 26.Alba-Loureiro TC, Pithon-Curi TC, Curi R. Reduced cytokine production by glycogen-elicited peritoneal cells from diabetic rats. Shock. 2008;30:308–0. doi: 10.1097/SHK.0b013e318164e834. [DOI] [PubMed] [Google Scholar]
- 27.Hatanaka E, Pereira Ribeiro F, Campa A. The acute phase protein serum amyloid A prime neutrophils. FEMS Immunol Med Microbiol. 2003;38:81–4. doi: 10.1016/S0928-8244(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 28.Koenderman L, Yazdanbakhsh M, Roos D, Verhoeven AJ. Dual mechanisms in priming of the chemoattractant-induced respiratory burst in human granulocytes. A Ca2+-dependent and a Ca2+-independent route. J Immunol. 1989;142:623–8. [PubMed] [Google Scholar]
- 29.Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14:837–3. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]


