Abstract
Many patients with Wegener's granulomatosis (WG) have anti-neutrophil cytoplasmic antibodies (c-ANCA). Aside from being a diagnostic marker, these autoantibodies may play roles in disease pathogenesis. Proteinase 3 (PR3) is the primary target of c-ANCA in WG patient sera. Of 60 c-ANCA-positive patients, 10 patients were selected for detailed humoral epitope analysis, contingent upon serum availability, using samples with positive levels of anti-PR3 by enzyme-linked immunosorbent assay (ELISA). Sequential epitope specificities of anti-PR3 antibodies detected by screening the maximally overlapping solid-phase octapeptides of PR3 showed seven major common antigenic targets bound by WG patient sera. These include novel and previously identified sequential PR3 epitopes bound by c-ANCA. B cell epitope prediction algorithms identified all or part of the seven defined epitopes. Several epitopes share sequence and structural proximity with functional sites, including the catalytic triad and proposed binding sites of other potential proteins [PR3 complementary peptide and soluble endothelial protein C receptor (sEPCR)]. Epitope 4 (VVLGAHNVRTQ) had the highest binding prevalence (90%) and epitope 2 (AQPHSRPYMAS) has the highest average reactivity of the antigenic regions. Epitope 4 includes the interaction site between sEPCR and PR3 which may serve as an important interaction to down-regulate inflammation. Epitopes 3, 5 and 7 are in direct proximity to amino acids that form the catalytic triad of the protein. c-ANCA targets both unique and previously known sequential PR3 peptides. This information may prove useful in understanding anti-PR3-mediated disease pathogenesis in systemic vasculitides.
Keywords: epitope mapping, PR3, vasculitis, Wegener's granulomatosis
Introduction
Cytoplasmic anti-neutrophil antibodies (c-ANCA) are found in the sera of approximately 90% of all active and/or untreated Wegener's granulomatosis (WG) patients [1,2]. This disease appears to attack the vessels of the respiratory tract and kidneys preferentially, resulting in necrotizing, granulomatous inflammatory lesions. While c-ANCA is very useful for diagnosis, the variation of level with treatment and course of disease has not proved to be a strong predictor of relapse [3–6]. An important characteristic of c-ANCA is their specificity. Approximately 90% of all c-ANCA WG patients bind one antigen, proteinase 3 (PR3) [7,8]. Reports describing patients with active WG demonstrate that in 70–80% of cases c-ANCA are directed toward PR3 [9].
Pathogenic mechanisms of anti-PR3 in the disease process of ANCA-associated vasculitis, particularly in WG, are probably varied and are still not understood fully. PR3 is a multi-functional serine protease stored as an active enzyme within azurophilic granules of polymorphonuclear neutrophils (PMNs), together with neutrophil elastase and cathepsin G [10]. PR3, also named myeloblastin, is involved in controlling the growth and differentiation of myeloid cells and has antibiotic activity. It has been described as a proteolytic protein, degrading other enzymes and structural proteins such as extracellular matrix and some collagens [11]. A proposed trigger for initiation of an immune response against PR3 is the targeting of an anti-sense complementary peptide of PR3 that is cross-reactive with normal PR3 and bears a close resemblance to microbial antigens [12]. PR3 on the surface of activated neutrophils has also been shown to bind to soluble endothelial protein C receptor (sEPCR). This sEPCR has key anti-inflammatory and anti-coagulant properties, and interruption of EPCR–PR3 interaction by anti-PR3 may upset this important balance and lead to vascular inflammation [13,14]. Far from their role being one of simple degradation, proteases such as PR3 play a role in cross-talk between leucocytes and endothelial cells [15]. Affinity-purified PR3-ANCA bind to expressed PR3 and activate endothelial cells. Neutrophils stimulated by ANCA in suspension undergo respiratory burst (superoxide response) and release proteases such as PR3, which at high concentration damages endothelial cells after prolonged exposure.
c-ANCA has been shown to react with neutrophils to release oxygen radicals and PR3, both with destructive properties. Another way in which c-ANCA might induce damage is through the interaction with PR3 itself, causing interference with PR3-inhibitor binding, thus activating PR3 [16]. c-ANCA may also interfere with PR3 proteolytic activity, allowing other destructive enzymes to continue unchecked [16–18]. PR3 is essential for immune complex-mediated neutrophil activation in vivo by cleaving anti-inflammatory progranulin into its inactive form, and both PR3 and elastase deficiency diminishes neutrophil infiltration into the site of inflammation [19]. How these interactions are realized at the level of fine epitope specificity remains to be elucidated. Studies that determine the exact pathogenic potential of epitope specificity either by influencing the known active or catalytic sites or by interference with the binding of regulatory proteins to PR3 are not yet available.
Given the established pathogenic potential of PR3 in WG, this study seeks to detail the specific interactions between c-ANCA and PR3 by defining the humoral epitopes that are targeted most commonly within c-ANCA positive patient sera. Understanding these specific interactions may help to elucidate potential aetiological triggers of c-ANCA production and define pathogenic targets involving these autoantibodies that promote overt WG clinical disease.
Materials and methods
Patients
This work was conducted with appropriate Institutional Review Board approval from the Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center. A database search of the Oklahoma Clinical Immunology Serum Repository (Oklahoma City, Oklahoma) was performed to identify c-ANCA-positive patients diagnosed with WG fulfilling the 1990 American College of Rheumatology criteria with sufficient available coded sera. Serum samples from frequency-matched, unaffected individuals were used as controls.
Testing for ANCA- and PR3-specific autoantibodies
Indirect immunofluorescence to determine c-ANCA titre and pattern was performed in a College of American Pathologists/Clinical Laboratory Improvement Amendments (CAP/CLIA) approved laboratory on each patient sample prior to deposition of the sample into the repository. The presence of PR3 antibody was verified by a commercial PR3 antibody enzyme-linked immunosorbent assay (ELISA) (INOVA Diagnostics, Inc., San Diego, CA, USA), according to the manufacturer's protocol. Briefly, sera were diluted 1:100 with sample diluent and incubated for 30 min in a 96-well microtitre plate coated with PR3. After washing, the samples were incubated for 30 min with a prediluted anti-human immunoglobulin (Ig)G horseradish peroxidase-conjugated antibody. After washing, the tetramethylbenzidine chromogen substrate was added and the samples were incubated for 30 min before adding a stop solution. The optical density (OD) was read at 450 nm (Dynex Technologies Inc., Chantilly, VA, USA). Absorbance values were converted to units of reactivity. Interassay variability was taken into consideration and reactivity units above 3·8, which was 3 standard deviations (s.d.) greater than normal control binding, were considered positive.
Solid-phase peptide synthesis and autoantibody assays
The PR3 published sequence (P24158), comprising 256 amino acids, was used to construct all possible overlapping octapeptides of the protein. We follow the amino acid numbering scheme for the PR3 sequence by Campanelli et al. [20]. Specifically, amino acids −27 to −3 represent the signal peptide sequence, amino acids −2 to −1 the pro-peptide sequence, amino acids 1–222 the mature form of the protein and amino acids 223–229 the C-terminal domain. The sequence we used differed from the published sequence (Swiss-Prot accession number: P24158, accessed on 7 November 2007) at amino acid residues 92, 108 and 109, which have since been identified as polymorphic residues [20–22]. Amino acid differences consisted of isoleucine to valine at residue 92, threonine to serine at residue 108 and serine to valine at residue 109. None of these amino acids were within identified antigenic regions. Peptides constructed with the alternate amino acids were also constructed and tested as below. None of these alternate peptides were recognized as antigenic by patients or control sera.
The peptides were synthesized on polyethylene solid-phase supports by using Fmoc side-chain protection chemistry and arranged in a 96-well microtitre plate format (Chiron Mimotopes Pty Ltd, Clayton Victoria, Australia), as described previously [23,24]. In addition, positive control peptides were synthesized on each plate by using an octapeptide with known reactivity to our positive control sera.
Solid-phase peptides were then tested for antibody reactivity using a modified ELISA procedure that has been described previously [23,24]. Assay steps were executed by adding a solid-phase support containing a synthesized octapeptide into each well of a microtitre plate. Plates were sealed within plastic containers for subsequent incubation steps. The octapeptides were blocked with 3% low-fat milk in phosphate-buffered saline (PBS) at room temperature for 1 h and then incubated with patient sera containing primary antibodies for 3 h. Following four washes with 0·05% Tween in PBS, the peptides were incubated for 3 h with 1:10 000 dilutions of the alkaline phosphatase-labelled secondary antibody, anti-human IgG whole molecule (Jackson Immunoresearch Laboratories, West Grove, PA, USA). After washing, the peptides were incubated in a para-nitrophenyl phosphate solution to induce a colour change if antibody–peptide interactions were present. Absorbance was measured using a micro-ELISA plate reader (Dynex Technologies Inc.) at dual wavelengths of 410 nm and 490 nm. Positive controls were developed and normalized to an OD of 1·0 to standardize the results across assays for all patients and controls. Octapeptide sequences that bound at least 3 s.d. above the normal mean and that were bound by sera from at least 70% of the patients were considered to be common and significant antigenic targets.
Peptide construction and anti-peptide ELISAs
The sequence encoding amino acids 7–17 (AQPHSRPYMAS, epitope 2) was synthesized on a branching polylysine backbone (MAP™) (Molecular Biology Proteomics Facility of Oklahoma) to be used as the antigen source for confirmation ELISAs [25]. Microtitre plates were coated overnight with 1 µg synthesized peptide/well, washed, and then blocked with 0·1% bovine serum albumin for 1 h. Sera samples were diluted to 1:100 and 1:1000 and incubated for 3 h at room temperature. After washing with PBS, alkaline phosphatase-conjugated anti-human IgG diluted at 1:10 000 (Jackson Immunoresearch Laboratories, West Grove, PA, USA) was added for 3 h at room temperature. After washing with PBS, the samples were incubated with para-nitrophenyl phosphate tablets (Sigma Chemical Co., St Louis, MO, USA) dissolved in 1 mg/ml glycine buffer. Samples were read at dual wavelengths of 410 nm and 490 nm (Dynex Technologies Inc.) and the OD standardized to a common positive control peptide.
Structural analysis of PR3 epitopes
The Protein Data Bank was used to identify the coordinates for the crystal structure of PR3 (PDB code 1FUJ) as defined by Fujinaga et al. [26]. These coordinates were used to calculate tertiary structure solvent exclusion surface areas by using the BALL View 1·1·1 program [27] and surface areas were calculated using a solvent probe radius of 1·5 Å. We then identified the location and surface availability of our defined epitopes.
B cell epitope prediction
The Immune Epitope Database and Analysis resource (http://www.immuneepitope.org) was accessed to determine B cell epitope predictions for the published sequence of PR3. Two algorithms were utilized in this study: the Bepipred linear epitope prediction tool, which predicts the location of linear B cell epitopes using a combination of a hidden Markov model and a propensity scale method [28], and the ElliPro algorithm that predicts antibody epitope based on a protein antigen three-dimensional structure [29]. Percentage estimates that determine if the number of amino acid that matches with the B cell epitope predictions are attributed to chance (based on a probability of a match for any amino acid at 0·25) were then computed.
Results
Anti-PR3 levels do not correlate with c-ANCA levels in this treated patient collection
We identified 60 patients with c-ANCA-positive sera stored within the Oklahoma Clinical Immunology Serum Repository. Of these patients, 60% were European American, 13·3% African American, 3·3% Native American, 1·7% Hispanic and 6·7% reported as ‘other’ or ‘mixed’ ancestry. Ethnic identity was not provided for 15% of the patients. The mean age was 51 years (±16·53) and ranged from 13 to 88 years. Nearly half (45%) the patients were male. ELISAs were performed on the sera from all 60 patients to determine anti-PR3 reactivity. Only 30 (50%) patients had sera with highly positive levels (≥ 3·8 units of reactivity) of anti-PR3 antibodies. This low level of PR3 detection may have been influenced by suppressed disease activity or medication use. A strong correlation was present between the levels of anti-PR3 reactivity and c-ANCA titres (r = 0·549, P < 0·0001 by Spearman's correlation).
For detailed epitope analysis, we identified 10 patients who had high concentrations of anti-PR3 and had sufficient sera available for sequential epitope analysis. Seven were men and three were women, with an average age of 49 (±10·9) years. These patients had varying levels of organ involvement in that some had upper respiratory, lower respiratory and renal involvement, whereas others had more limited disease. Although some patients had multiple serial serum samples available, the earliest available sample date with sufficient sera, detectable c-ANCA titres and positive anti-PR3 reactivity were selected for further testing.
Seven common antigenic regions of PR3 are defined
Sera were tested from 10 anti-PR3-positive WG patients using a modified, solid-phase ELISA to measure reactivity to the maximally overlapping octapeptides of PR3. Representative binding from the serum of two different anti-PR3-positive patients is shown in Fig. 1a and b. The binding of a representative unaffected control individual is shown in Fig. 1c. Mean patient binding of all 10 patients is shown in standard deviations above the mean binding of the eight negative controls (Fig. 2).
Fig. 1.

Sequential humoral antigenic determinants of proteinase 3 (PR3). Humoral epitope binding profiles of two representative patient samples are presented in (a) and (b). A representative control serum sample binding is presented in (c). Negative control samples have minimal background binding to the PR3 octapeptides (average optical density = 0·179).
Fig. 2.

Epitope delineation and characterization using three-dimensional structure modelling. (a) Standard deviations above normal control binding for the mean of all 10 patient samples. Epitopes 2–7 are assigned a reference colour for distinction in part b. (b) Three-dimensional structural representation of proteinase 3 illustrates the surface location of epitopes 2–7 and their relation to the catalytic triad (light blue), with epitope 7 overlapping the triad.
We identified seven sequences of the PR3 protein that were commonly antigenic among the 10 patients (Table 1). Sequences were defined as commonly antigenic when bound on average at least 3 s.d. above the normal mean and when bound by sera from at least 70% of the patients. These seven epitopes include amino acids 7–17 (epitope 2), amino acids 24–31 (epitope 3), amino acids 56–66 (epitope 4), amino acids 79–86 (epitope 5), amino acids 118–125 (epitope 6) and amino acids 172–179 (epitope 7) (Table 2). In addition, one epitope was bound in the signal peptide sequence of the protein spanning amino acids −27 to −18 (epitope 1). Of the 10 patients, nine had sera that bound to the octapeptide spanning amino acids 56–66 (epitope 4: VVLGAHNVRTQ) (Table 1). Of similar significance, the sequence from amino acids 7–17 (epitope 2: AQPHSRPYMAS) displayed the highest average binding reactivity of any region of the protein (Figs 1a and 2).
Table 1.
Patient demographics and epitope specificities of individuals tested for anti-proteinase 3 (PR3) humoral epitopes.
| Epitope |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number | Age | Ancestry | Sex | c-ANCA | PR3 units | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 1 | 64 | EA | M | 540 | 16 | ++ | +++ | − | ++ | − | − | − |
| 2 | 39 | NA | F | 20 | 16 | ++ | ++ | + | ++ | ++ | + | + |
| 3 | 47 | EA | F | 540 | 129 | +++ | +++ | + | +++ | + | +++ | + |
| 4 | 53 | EA | M | 540 | 106 | − | − | − | − | − | + | − |
| 5 | 49 | EA | F | 540 | 231 | ++ | +++ | + | ++ | + | − | ++ |
| 6 | 65 | Other | M | 540 | 80 | + | + | + | ++ | + | + | + |
| 7 | 54 | EA | M | 60 | 133 | +++ | +++ | +++ | ++ | + | ++ | + |
| 8 | 30 | EA | M | 180 | 29 | − | − | − | + | + | − | + |
| 9 | 61 | Unknown | M | 180 | 69 | + | + | + | ++ | ++ | + | ++ |
| 10 | 27 | EA | M | 180 | 43 | ++ | + | ++ | ++ | + | + | + |
Targeting of epitopes by each patient in terms of standard deviations (s.d.) above the normal average binding. The binding of each epitope is defined by a scale where (−) represents binding < 3 s.d. above PR3-negative control responses, (+) represents binding between 3 s.d. and 5 s.d. above controls, (++) binding between 5 s.d. and 7 s.d. and (+++) binding > 7 s.d. EA: European American; NA: Native American; C-ANCA: cytoplasmic anti-neutrophil antibodies.
Table 2.
Common, major humoral antigenic targets of proteinase 3 (PR3) autoantibodies.
| % probability occur by chance |
||||||
|---|---|---|---|---|---|---|
| Epitope | Sequence | Amino acids | % Patients | pI | Ellipro | Bepipred |
| 1 | MAHRPPSPAL | −27 to −18 | 80% | 9·52 | 0·000095% | 0·000095% |
| 2 | AQPHSRPYMAS | 7 to 17 | 80% | 8·80 | 3·43% | 0·76% |
| 3 | PGSHFCGG | 24 to 31 | 70% | 7·12 | 63·29% | 2·73% |
| 4 | VVLGAHNVRTQ | 56 to 66 | 90% | 9·73 | 0·76% | 28·67% |
| 5 | FLNNYDAE | 79 to 86 | 80% | 4·09 | 0·42% | 32·15% |
| 6 | PVPHGTQC | 118 to 125 | 70% | 7·12 | 0·04% | 2·73% |
| 7 | CFGDSGGP | 172 to 179 | 80% | 3·80 | 100% | 0·43% |
% Probability presents the likelihood that the amount of identity between the epitope of this study and the predicted epitope by a select algorithm would occur by chance. Therefore, epitopes 1, 2 and 6 would have been potentially predicted by both algorithms, while the others may have been predicted by one or the other algorithm. pI: isoelectric point.
The sequence for epitope 2 was selected for confirmatory analysis based upon the high prevalence of patients with that antibody specificity and upon the higher intensity of binding compared to the binding of the other epitopes that were detected with the solid-phase peptide assay. Patient serum binding to epitope 2 was examined by constructing the AQPHSRPYMAS peptide on a polylysine (MAP) backbone and screening the serum samples with a peptide ELISA format. Of the 10 samples, eight were positive for antibodies to this specificity (Table 1), which correlates perfectly with the solid phase epitope mapping results.
PR3 epitopes are located on the molecular surface
To visualize the location of the seven significant and common epitopes, to determine surface availability of these epitopes and to visualize the proximity of these epitopes to functional regions of the protein, we referred to the crystal structure model of PR3 determined by Fujinaga et al. [26]. Using this model, it appears that epitopes 3, 5 and 7 are in direct proximity to amino acids that form the catalytic triad of the protein (His44, Asp91 and Ser176) (Fig. 2). The interaction site between sEPCR and PR3 that is located at amino acids 53–64 on PR3 consists primarily of the sequence identified as epitope 4. Epitope 1 is found in the signal peptide sequence that is not part of the crystal structure.
PR3 humoral epitopes have basic average isoelectric points
The characteristics of the individual epitopes found in this study are summarized in Table 2. The average isoelectric point (pI) of 8·18 for the peptides comprising the seven common antigenic regions is significantly higher than the pI of 6·45 for the non-antigenic region peptides (t = −3·05, P = 0·003). Amino acid analysis comparing differences in the usage of amino acids between the seven common identified humoral epitopes and the remaining sequences showed no significant difference between antigenic and non-antigenic regions.
Defined PR3 epitopes are identified by several B cell epitope prediction algorithms
Comparing our experimental results with the Bepipred linear epitope prediction tool, we have identified six of the 10 predicted epitopes being similar to our study, with epitope 1 being identical to the predicted first epitope (MAHRPPSPAL). The other predicted epitopes (VGGHEAQPHSRP, MRGNPGSHF, VRTQEPTQQH, QLPQQDQPVPHG, GDSGGPL) have parts containing similar amino acid sequences from epitopes 1–4, 6 and 7. Furthermore, using the ElliPro algorithm, we have found four predicted epitopes containing, again, the entire epitope 1 and parts of epitopes 4–6 (containing at least six similar amino acids). Thus, by utilizing both B cell epitope prediction tools, similarities were seen between predicted epitopes in all the seven identified epitopes in our study. Among all the identified epitopes, the percentage probabilities matching B cell epitope predictions were attributed by chance was seen to be the least for epitope 1 (0·000095%) and varies for the other identified epitopes between the two B cell epitope prediction algorithms used in this study (Table 2).
Discussion
Despite its role as a diagnostic hallmark of WG, work remains to understand the precise binding regions of c-ANCA to PR3 and the potential role of these specificities in inflammation and/or disease. Our study defined antigenic regions of the PR3 protein bound commonly by c-ANCA from WG patient sera. These regions included amino acids −27 to −18, 7–17, 24–31, 56–66, 79–86, 118–125 and 172–179. Several of these epitopes are in parts of the PR3 protein with functional or regulatory activities, leading to multiple potential mechanisms for the disruption of normal PR3 function.
Several groups have examined previously the binding of WG patient sera to epitopes generated for PR3 [30–32]. Humoral epitope maps from these studies, compared to this work, are illustrated in Fig. 3. These studies are all similar to our study in their examination of autoantibody specificity using multiple overlapping peptides to represent the PR3 sequence [33]. While the methods to examine these interactions varied, several regions of the protein have been identified consistently. The studies by Williams et al. and van der Geld et al. had the most similarity to the epitopes defined by our study. These included overlaps within epitopes 2, 3, 4, 6 and 7. These data continue to support the importance of antibodies that bind directly or bind within close proximity to the known active site or catalytic triad of the protein (His44, Asp91 and Ser176).
Fig. 3.
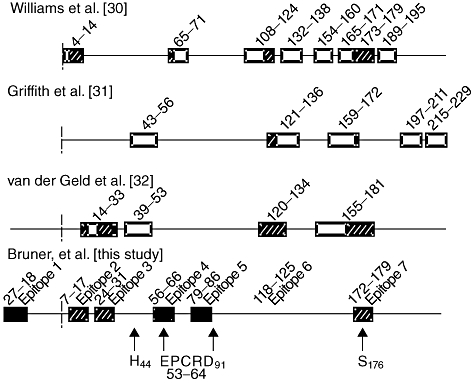
Summary of humoral epitope mapping of proteinase-3 (PR3) from this work and published literature. Humoral epitope mapping results of four major studies are shown, including this work. Solid boxes represent epitopes unique to this study, striped boxes represent epitopes that correlate between this study and previous PR3 humoral epitope mapping efforts, and empty boxes represent epitopes defined within previous studies that were not determined as significant within this study. The comparison studies include [39–41]. Dashed lines delineate the location where the pro-peptide sequence ends and the mature PR3 protein sequence begins. Solid arrows denote the catalytic triad (H44, D91 and S176) and the interaction site [endothelial protein C receptor (EPCR)] within epitope 4 of PR3. Parts of the complementary peptide sequence of PR3 (amino acids 87–172) are located within the numbered epitope boxes across all four studies.
Human PR3 is designed genetically as a pre-pro-protease, which means that theoretically the molecule can occur at least in three forms; that is, in the unprocessed pre-proform, the proform and as mature enzyme. Interestingly, we identified the signal peptide portion of PR3 (epitope 1: amino acids −27 to −18) as commonly antigenic not seen previously or evaluated in the earlier studies. Mature PR3 is a highly folded protein, stabilized by four disulphide bridges and two asparagine-linked glycosylations. A prerequisite of the binding of PR3 to other molecules is its accessibility [34]. Using the three-dimensional crystal structure defined by Fujinaga et al. [26], we found that all the antigenic epitopes that occur within the mature form of the protein have distinct sections of surface availability. Epitopes 2 and 6 have been shown to be more independent compared to the other identified epitopes in this study, which could be attributed to their location on the conformational structure. The other epitopes are located at or near established critical areas such as the catalytic site or the site of interaction between PR3 and sEPCR [33]. The close proximity of epitopes 3, 5 and 7 to the catalytic triad could provide a potential mechanism by which anti-PR3 antibodies might alter the normal proteolytic properties of PR3, thus perpetuating the disease process through epitope spreading, a common feature of other autoimmune diseases.
Interference with the region surrounding the active site of PR3 could influence the proteolytic properties of the protein through multiple mechanisms. Anti-PR3 has the ability to inhibit the proteolytic activity of PR3 resulting in unregulated activity of the enzymes controlled normally by PR3 degradation [17]. Anti-PR3 has been shown to interfere with the ability of α-1 antitrypsin to inhibit PR3 function [18]. The epitopes defined within our analyses that are located at or proximal to the active region provide autoantibody specificity that could support these hypotheses. Together with the other identified reactive epitopes, this supplements the groundwork in identifying the inhibitory potential of each common antibody specificity in terms of surface accessibility.
Beyond interference with the active site portions of the protein, the disruption of PR3–sEPCR binding through interference of anti-PR3 could influence regulation of vascular inflammation. Aside from its anti-inflammatory role, levels of sEPCR in the plasma of WG patients respond directly to the changes of disease progression, such that levels of sEPCR increase just before relapse of disease [35,36]. Our data identified a common unique epitope (epitope 4: amino acids 56–66) that correspond to the sequence defined previously as the interaction site between PR3 and sEPCR (amino acids 53–64) [14]. Interruption of this interaction may lead to increased, unchecked inflammatory cascades.
One theory of PR3-directed autoimmunity involves the complementary peptide of PR3 (amino acids 87–172), which is encoded by the anti-sense strand of the PR3 gene [11]. Exposure of the immune system to this peptide triggers the formation of antibodies that cross-react with PR3. Pendergraft et al. [12] hypothesized that proteins that are anti-sense or complementary to the original autoantigen could be induced by infectious agents, and according to their ‘theory of autoantigen complementarity’ could induce reactivity against the original autoantigen. DNA sequences complementary to the PR3 gene are found in microorganisms, including Staphylococcus aureus, supporting the role of infectious agents as triggers of PR3 autoimmunity through molecular mimicry [37]. Our study discovered the inclusion of epitope 6 (amino acids 118–125) embedded in the middle of the sequence corresponding to the reported complementary peptide of PR3. This study, together with all three other published PR3 humoral epitope studies [30–32], identifies antigenic humoral regions in the complementary peptide sequence, although this peptide covers a large portion of the protein (amino acids 87–172).
Continued analysis of these potentially pathogenic autoantibodies is important to improve the diagnostic reliability of c-ANCA and PR3 autoantibody detection. The variation seen among all the published studies to date attempting to identify specific antigenic epitopes of WG could be explained by characteristics of the disease itself. Different patients with WG probably have PR3-ANCA that recognize different epitopes of PR3, as well as intra-individual differences, which means that there are changes in epitopes of PR3 or additional epitopes are recognized within the disease course of one patient with WG. This could possibly hold true for all forms of ANCA-associated vasculitis, thereby addressing the differences seen among the types of vessels and organs involved together with the disease severity that these patients manifest.
Our study has identified c-ANCA-targeted sequential PR3 epitopes as well as providing evidence that human anti-PR3 may interfere with interactions of PR3 with several surface accessible sites, including the catalytic triad that participates in local inflammatory responses. The importance of determining common functional epitopes lies in aiding the search for possible similar environmental triggers and providing potential therapeutic pathways in the future.
Acknowledgments
We are grateful to the University of Oklahoma Health Science Center Molecular Biology Proteomics Facility and Immune Epitope Database and Analysis resource (http://www.immuneepitope.org). We thank Tara Bruner, Shelly Biby, Scott Stewart, Catalina Lupu and Latisha Heinlen for their technical and analytical assistance. We also thank the OMRF Clinical Immunology laboratory personnel and patients. This work was supported in part by grants from the National Institutes of Health (AI47575, AR45451, AR48045, RR015577, AR48940, RR020143, AR49084, AR053483 and AI082714) and from the Lou C. Kerr Chair in Biomedical Research at the Oklahoma Medical Research Foundation.
Disclosure
The authors have no financial disclosures related to this manuscript.
References
- 1.Nolle B, Specks U, Ludemann J, Rohrbach MS, DeRemee RA, Gross WL. Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener granulomatosis. Ann Intern Med. 1989;111:28–40. doi: 10.7326/0003-4819-111-1-28. [DOI] [PubMed] [Google Scholar]
- 2.Kallenberg CG. Antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Curr Opin Rheumatol. 2007;19:17–24. doi: 10.1097/BOR.0b013e3280119842. [DOI] [PubMed] [Google Scholar]
- 3.Sanders JS, Huitma MG, Kallenberg CG, Stegeman CA. Prediction of relapses in PR3-ANCA-associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology (Oxford) 2006;45:724–9. doi: 10.1093/rheumatology/kei272. [DOI] [PubMed] [Google Scholar]
- 4.Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL. Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int. 2003;63:1079–85. doi: 10.1046/j.1523-1755.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- 5.Kerr GS, Fleisher TA, Hallahan CW, Leavitt RY, Fauci AS, Hoffman GS. Limited prognostic value of changes in antineutrophil cytoplasmic antibody titer in patients with Wegener's granulomatosis. Arthritis Rheum. 1993;36:365–71. doi: 10.1002/art.1780360312. [DOI] [PubMed] [Google Scholar]
- 6.Nowack R, Grab I, Flores-Suarez LF, Schnulle P, Yard B, van der Woude FJ. ANCA titres, even of IgG subclasses, and soluble CD14 fail to predict relapses in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2001;16:1631–7. doi: 10.1093/ndt/16.8.1631. [DOI] [PubMed] [Google Scholar]
- 7.Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 8.Jennings JG, Chang L, Savige JA. Anti-proteinase 3 antibodies, their characterization and disease associations. Clin Exp Immunol. 1994;95:251–6. doi: 10.1111/j.1365-2249.1994.tb06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berden AE, Kallenberg CG, Savage CO, et al. Cellular immunity in Wegener's granulomatosis: characterizing T lymphocytes. Arthritis Rheum. 2009;60:1578–87. doi: 10.1002/art.24576. Review. [DOI] [PubMed] [Google Scholar]
- 10.Korkmaz B, Kuhl A, Bayat B, Santoso S, Jenne DE. A hydrophobic patch on proteinase 3, the target of autoantibodies in Wegener granulomatosis, mediates membrane binding via NB1 receptors. J Biol Chem. 2008;283:35976–82. doi: 10.1074/jbc.M806754200. [DOI] [PubMed] [Google Scholar]
- 11.Preston GA, Zarella CS, Pendergraft WF, et al. Novel effects of neutrophil-derived proteinase 3 and elastase on the vascular endothelium involve in vivo cleavage of NF-kappaB and proapoptotic changes in JNK, ERK, and p38 MAPK signaling pathways. J Am Soc Nephrol. 2002;13:2840–9. doi: 10.1097/01.asn.0000034911.03334.c3. [DOI] [PubMed] [Google Scholar]
- 12.Pendergraft WF, 3rd, Preston GA, Shah RR, et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10:72–9. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- 13.Kurosawa SC, Esmon T, Stearns-Kurosawa DJ. The soluble endothelial protein C receptor binds to activated neutrophils: involvement of proteinase-3 and CD11b/CD18. J Immunol. 2000;165:4697–703. doi: 10.4049/jimmunol.165.8.4697. [DOI] [PubMed] [Google Scholar]
- 14.Kurosawa S, Stearns-Kurosawa DJ, Hidari N, Esmon CT. Identification of functional endothelial protein C receptor in human plasma. J Clin Invest. 1997;100:411–18. doi: 10.1172/JCI119548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pankhurst T, Savage CO, Little MA. Review article: leukocyte–endothelial dysregulation in systemic small vessel vasculitis. Nephrology. 2009;14:3–10. doi: 10.1111/j.1440-1797.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 17.van der Geld YM, Tool AT, Videler J, et al. Interference of PR3-ANCA with the enzymatic activity of PR3: differences in patients during active disease or remission of Wegener's granulomatosis. Clin Exp Immunol. 2002;129:562–70. doi: 10.1046/j.1365-2249.2002.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolman KM, Stegeman CA, van de Wiel BA, et al. Relevance of classic anti-neutrophil cytoplasmic autoantibody (c-ANCA)-mediated inhibition of proteinase 3-alpha 1-antitrypsin complexation to disease activity in Wegener's granulomatosis. Clin Exp Immunol. 1993;93:405–10. doi: 10.1111/j.1365-2249.1993.tb08192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu N, Westra J, Huitema MG, et al. Coexpression of CD177 and membrane proteinase 3 on neutrophils in antineutrophil cytoplasmic autoantibody-associated systemic vasculitis: anti-proteinase 3-mediated neutrophil activation is independent of the role of CD177-expressing neutrophils. Arthritis Rheum. 2009;60:1548–57. doi: 10.1002/art.24442. [DOI] [PubMed] [Google Scholar]
- 20.Campanelli D, Melchior M, Fu Y, et al. Cloning of cDNA for proteinase 3: a serine protease, antibiotic, and autoantigen from human neutrophils. J Exp Med. 1990;172:1709–15. doi: 10.1084/jem.172.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturrock AB, Franklin KF, Rao G, et al. Structure, chromosomal assignment, and expression of the gene for proteinase-3. The Wegener's granulomatosis autoantigen. J Biol Chem. 1992;267:21193–9. [PubMed] [Google Scholar]
- 22.Bories D, Raynal MC, Solomon DH, Darzynkiewicz Z, Cayre YE. Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell. 1989;59:959–68. doi: 10.1016/0092-8674(89)90752-6. [DOI] [PubMed] [Google Scholar]
- 23.James JA, Harley JB. Human lupus anti-spliceosome A protein autoantibodies bind contiguous surface structures and segregate into two sequential epitope binding patterns. J Immunol. 1996;156:4018–26. [PubMed] [Google Scholar]
- 24.McClain MT, Ramsland PA, Kaufman KM, James JA. Anti-sm autoantibodies in systemic lupus target highly basic surface structures of complexed spliceosomal autoantigens. J Immunol. 2002;168:2054–62. doi: 10.4049/jimmunol.168.4.2054. [DOI] [PubMed] [Google Scholar]
- 25.James JA, Mamula MJ, Harley JB. Sequential autoantigenic determinants of the small nuclear ribonucleoprotein Sm D shared by human lupus autoantibodies and MRL lpr/lpr antibodies. Clin Exp Immunol. 1994;98:419–26. doi: 10.1111/j.1365-2249.1994.tb05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujinaga M, Chernaia MM, Halenbeck R, Koths K, James MN. The crystal structure of PR3, a neutrophil serine proteinase antigen of Wegener's granulomatosis antibodies. J Mol Biol. 1996;261:267–78. doi: 10.1006/jmbi.1996.0458. [DOI] [PubMed] [Google Scholar]
- 27.Moll A, Hildebrandt A, Lenhof HP, Kohlbacher O. BALL view: an object-oriented molecular visualization and modeling framework. J Comput Aided Mol Des. 2005;19:791–800. doi: 10.1007/s10822-005-9027-x. [DOI] [PubMed] [Google Scholar]
- 28.Larsen JE, Lund O, Nielsen M. Improved method for predicting linear B-cell epitopes. Immunome Res. 2006;2:2. doi: 10.1186/1745-7580-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponomarenko J, Bui HH, Li W, et al. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics. 2008;9:514. doi: 10.1186/1471-2105-9-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams RC, Jr, Staud R, Malone CC, Payabyab J, Byres L, Underwood D. Epitopes on proteinase-3 recognized by antibodies from patients with Wegener's granulomatosis. J Immunol. 1994;152:4722–37. [PubMed] [Google Scholar]
- 31.Griffith ME, Coulthart A, Pemberton S, George AJ, Pusey CD. Anti-neutrophil cytoplasmic antibodies (ANCA) from patients with systemic vasculitis recognize restricted epitopes of proteinase 3 involving the catalytic site. Clin Exp Immunol. 2001;123:170–7. doi: 10.1046/j.1365-2249.2001.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Der Geld YM, Simpelaar A, Van Der Zee R, et al. Antineutrophil cytoplasmic antibodies to proteinase 3 in Wegener's granulomatosis: epitope analysis using synthetic peptides. Kidney Int. 2001;59:147–59. doi: 10.1046/j.1523-1755.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 33.van der Geld YM, Stegeman CA, Kallenberg CG. B cell epitope specificity in ANCA-associated vasculitis: does it matter? Clin Exp Immunol. 2004;137:451–9. doi: 10.1111/j.1365-2249.2004.02572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller A, Voswinkel J, Gottschlich S, Csernok E. Human proteinase 3 (PR3) and its binding molecules: implications for inflammatory and PR3-related autoimmune responses. Ann NY Acad Sci. 2007;1109:84–92. doi: 10.1196/annals.1398.010. [DOI] [PubMed] [Google Scholar]
- 35.Stearns-Kurosawa DJ, Swindle K, D'Angelo A, et al. Plasma levels of endothelial protein C receptor respond to anticoagulant treatment. Blood. 2002;99:526–30. doi: 10.1182/blood.v99.2.526. [DOI] [PubMed] [Google Scholar]
- 36.Boomsma MM, Stearns-Kurosawa DJ, Stegeman CA, et al. Plasma levels of soluble endothelial cell protein C receptor in patients with Wegener's granulomatosis. Clin Exp Immunol. 2002;128:187–94. doi: 10.1046/j.1365-2249.2002.01803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch X, Mirapeix E. Vasculitis syndromes: LAMP-2 illuminates pathogenesis of ANCA glomerulonephritis. Nat Rev Nephrol. 2009;5:247–9. doi: 10.1038/nrneph.2009.51. [DOI] [PubMed] [Google Scholar]


